Polysorbate-Based Drug Formulations for Brain-Targeted Drug Delivery and Anticancer Therapy
Abstract
1. Introduction
2. Basic Characteristics of PSs
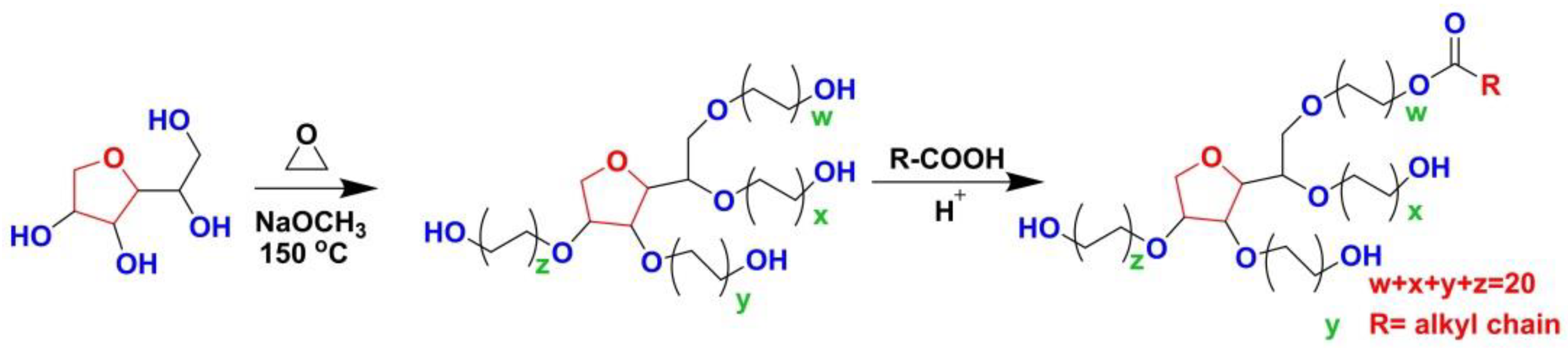
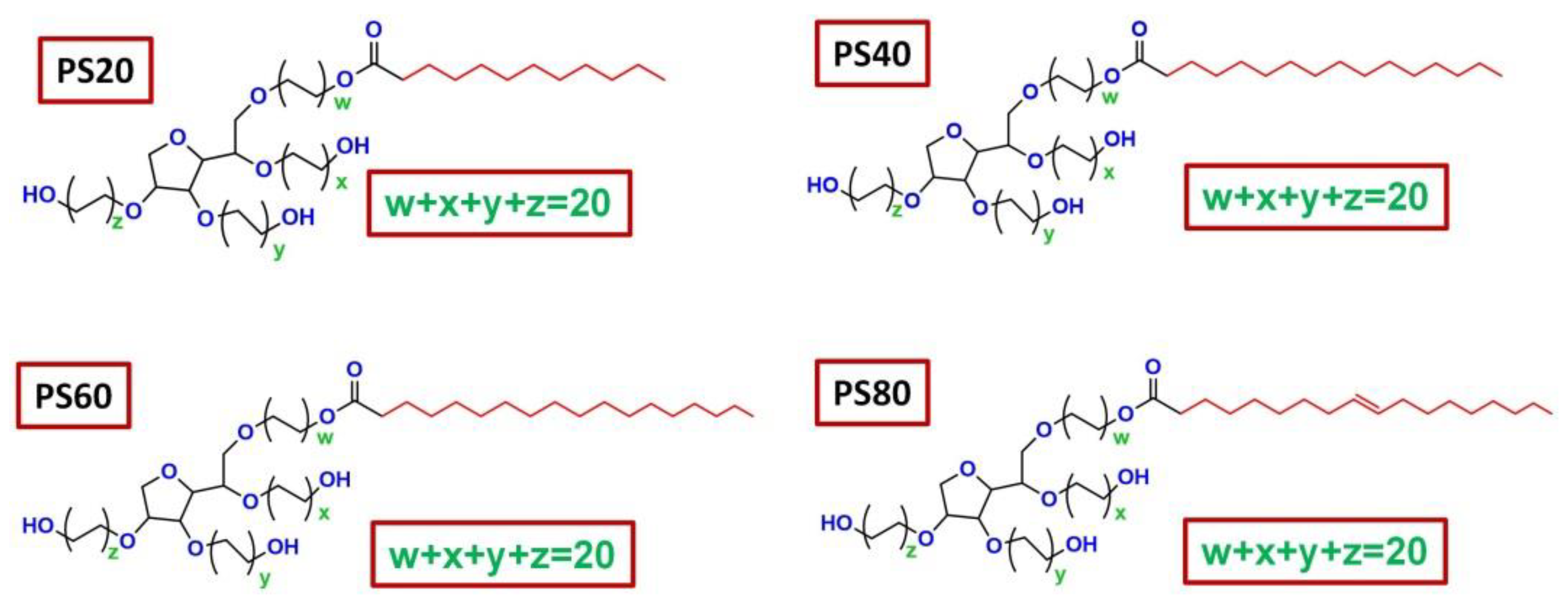
2.1. Chemical Structures and Physicochemical Properties of PSs
2.2. Biosafety Studies of PSs
2.3. P-glycoprotein (P-gp) Inhibitory Property of PS
3. PS-Based Formulations for Various Routes of Drug Administration
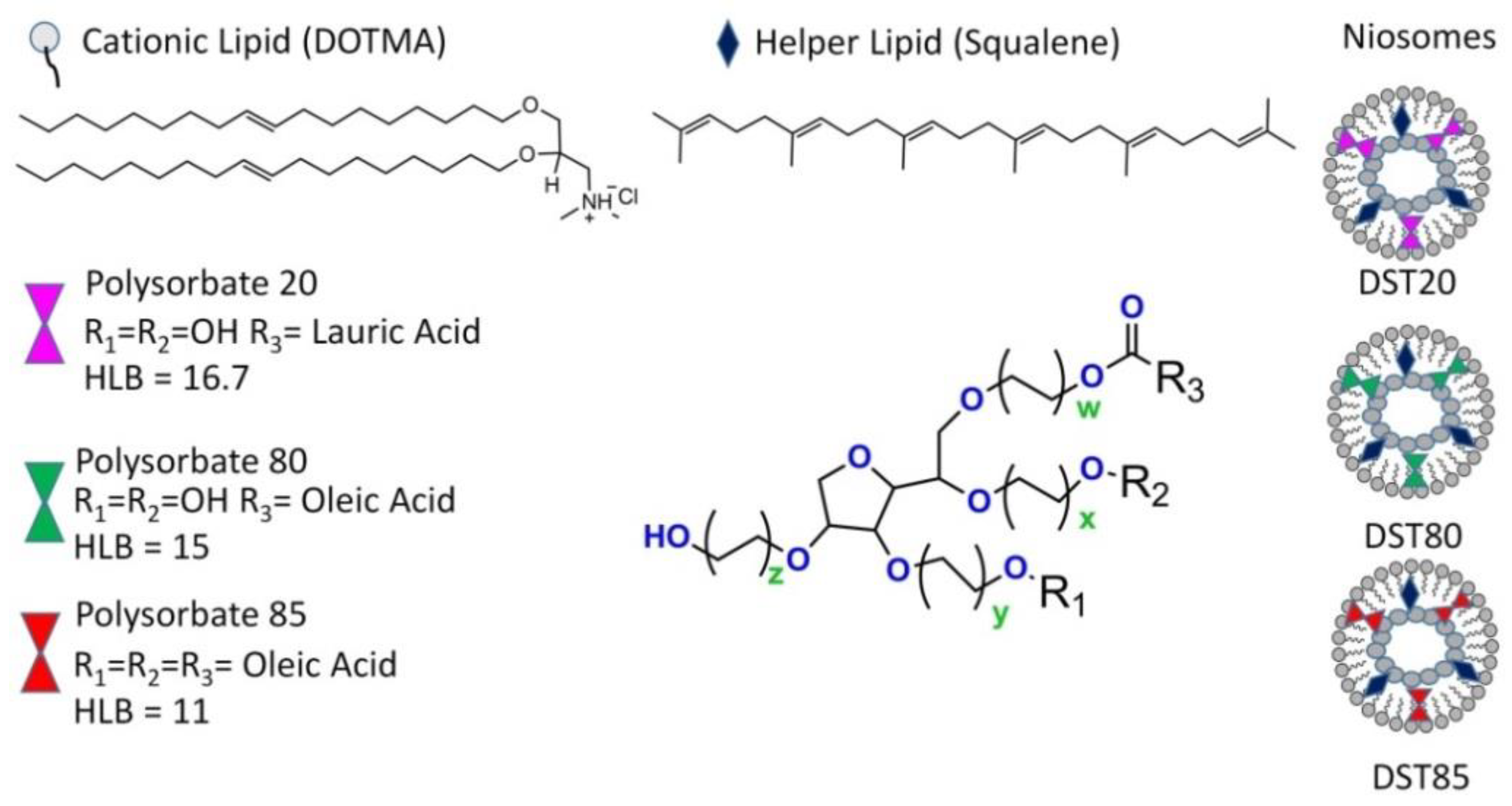
3.1. PS-Based Formulations for Ocular Delivery
3.2. PS-Based Formulations for Transdermal Delivery
3.3. PS-Based Formulations for Oral Delivery
3.4. PS-Based Formulations for Intranasal Delivery
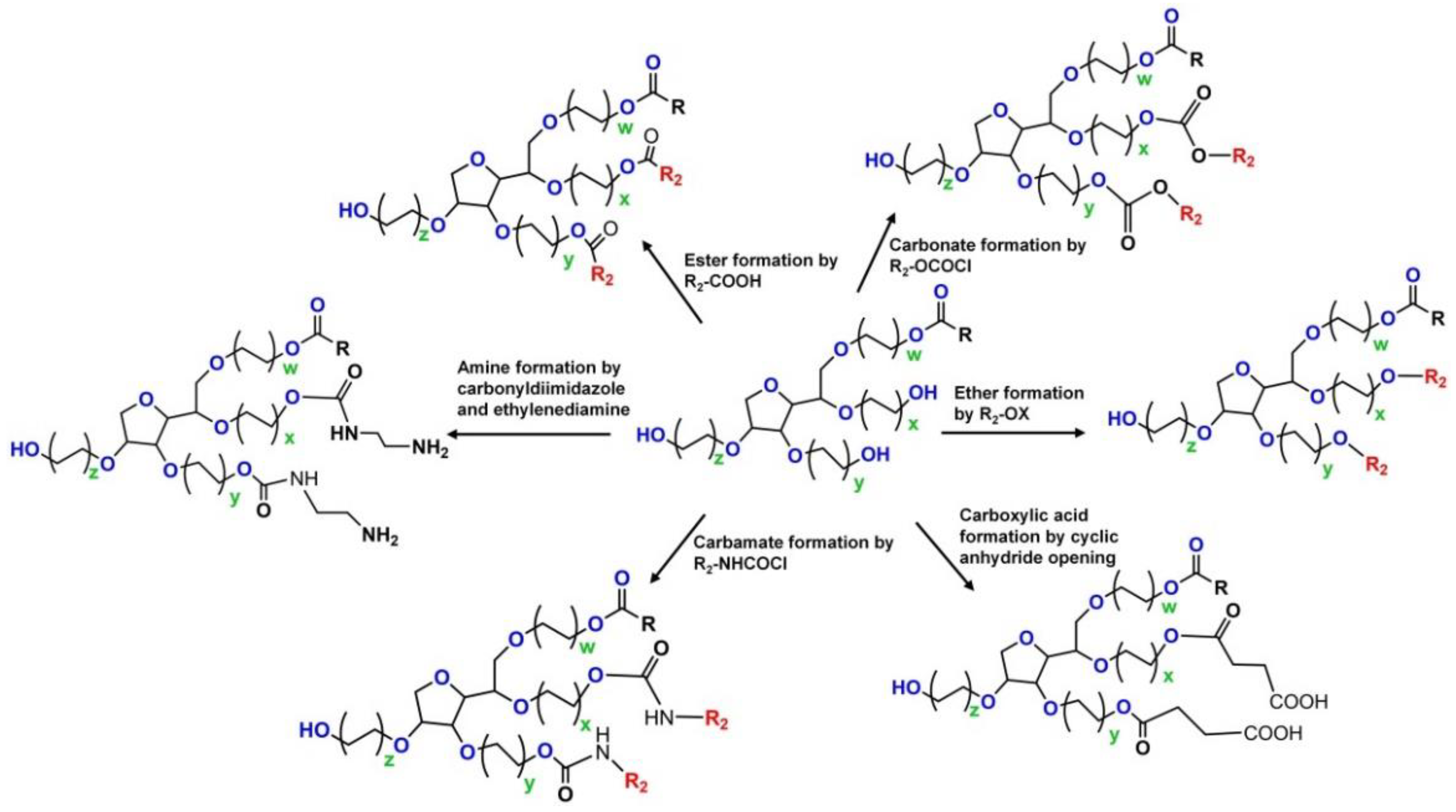
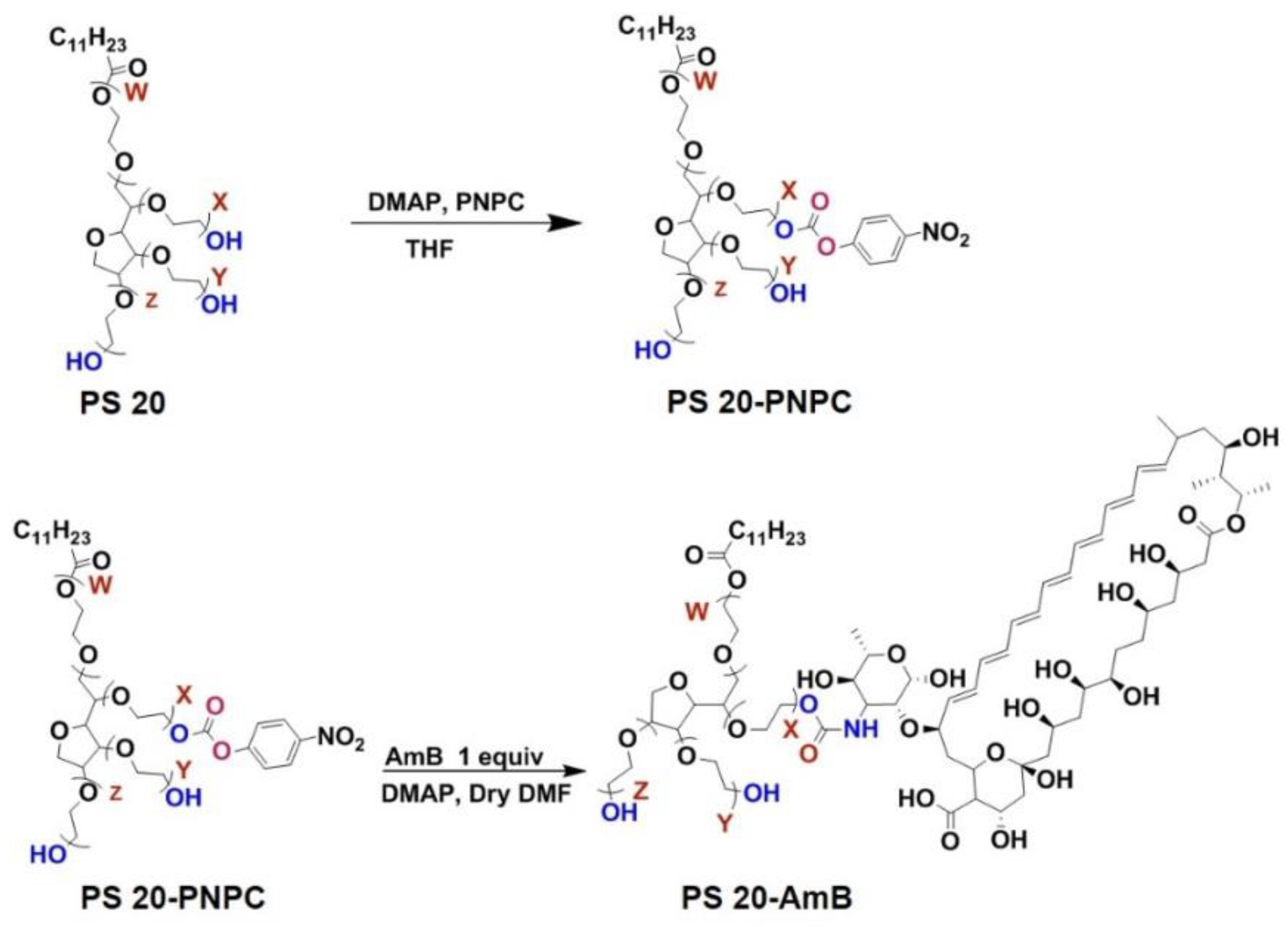
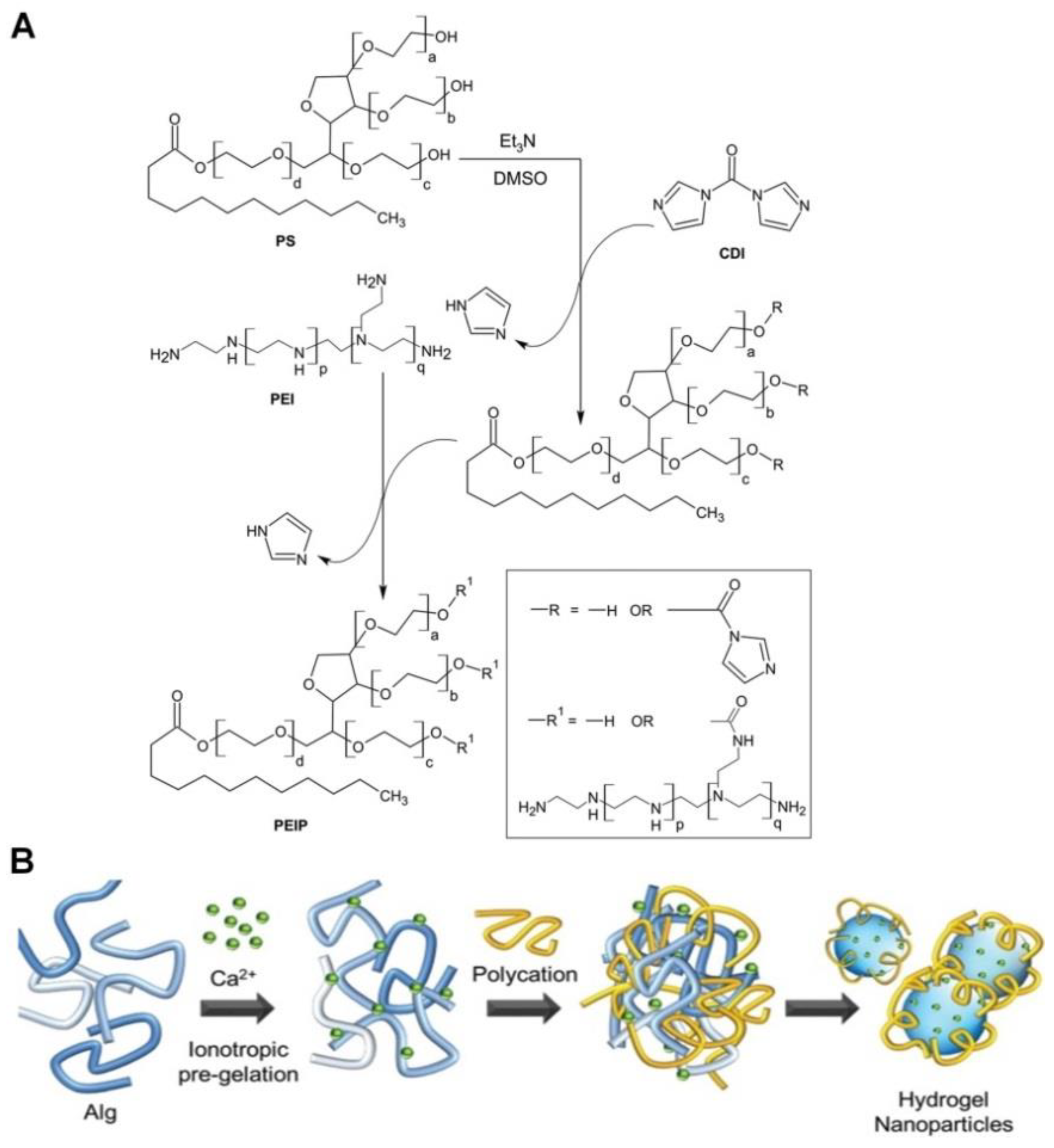
4. PS-Conjugated Drugs or Drug Carriers
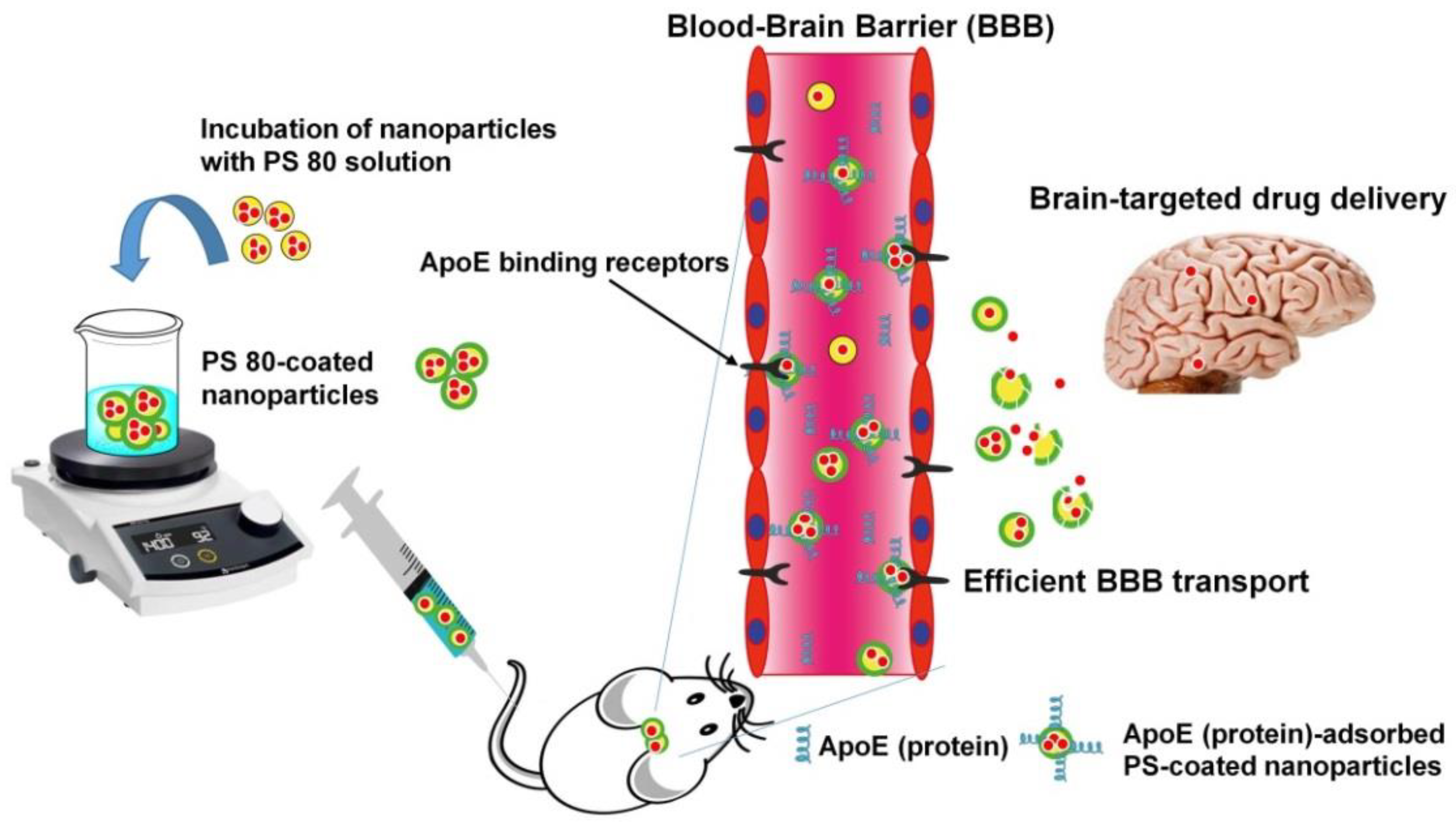
5. PS-Coated NPs for Efficient BBB Transport
| Drug Carriers | Therapeutic Agents | Diseases | Surface Coating | Study Model | Reference |
|---|---|---|---|---|---|
| PCBA | Dalargin | Analgesic | PS 80 | In vivo mice | [113] |
| PCBA | Dalargin | Analgesic | PS 80 | In vivo mice | [115] |
| PCBA | Dalargin | Analgesic | PS 80 | In vivo mice | [120] |
| PCBA | Dalargin | Analgesic | PS 80 | In vivo mice | [125] |
| PCBA | Rivastigmine | Alzheimer | PS 80 | In vivo Wistar rats | [126] |
| PLA | FITC-dextran | BBB | PS 80 | In vivo Kunming mice | [132] |
| PLGA | Acetylpuerarin | Cerebral ischaemiareperfusion injury | PS 80 | In vivo Wistar rats and Kunming mice | [133] |
| PCBA | Gemcitabine | Glioblastoma | PS 80 | In vivo rats | [134] |
| PCBA | Doxorubicin | Glioblastoma | PS 80 | In vivo white male non-inbred rats | [135] |
| PLA-b-PEG | Amphotericin B | Cryptococcal Meningitis | PS 80 | In vivo BALB/c mice | [136] |
| Iron oxide | Brain targeting | PS 80 | In vivo Sprague−Dawley rats | [137] | |
| Hyaluronic acid | Curcumin | Targeting glioma | PS 80 | In vitro B.End3 cells and G422 cells | [138] |
| PBCA | Flurophore and anti-Aβ antibody | Brine targeting/Alzheimer detection | PS 80 | In vivo mice | [139] |
| PLGA | Methotrexate-transferrin | Brain cancer | PS 80 | In vivo Wistar rats | [140] |
| PLGA | Thymoquinone | Alzheimer | PS 80 | In vivo albino mice | [141] |
| PLGA | siRNA | Traumatic brain injury | PS 80 | In vivo C57BL/6J mice | [142] |
6. Anticancer Therapy Using PS-Based Formulations
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef]
- Trucillo, P. Drug carriers: Classification, administration, release profiles, and industrial approach. Processes 2021, 9, 470. [Google Scholar] [CrossRef]
- Kaur, G.; Mehta, S.K. Developments of Polysorbate (Tween) based microemulsions: Preclinical drug delivery, toxicity and antimicrobial applications. Int. J. Pharm. 2017, 529, 134–160. [Google Scholar] [CrossRef]
- Milton, J.R.; Joy, T.K. Surfactants and interfacial phenomena. In Surfactants in Biology; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 978-0-470-54194-4. [Google Scholar]
- Uchegbu, I.F.; Vyas, S.P. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int. J. Pharm. 1998, 172, 33–70. [Google Scholar] [CrossRef]
- Tao, X.; Li, Y.; Hu, Q.; Zhu, L.; Huang, Z.; Yi, J.; Yang, X.; Hu, J.; Feng, X. Preparation and drug release study of novel nanopharmaceuticals with polysorbate 80 surface adsorption. J. Nanomater. 2018, 2018, 4718045. [Google Scholar] [CrossRef]
- Prieto, C.; Calvo, L. Performance of the biocompatible surfactant tween 80, for the formation of microemulsions suitable for new pharmaceutical processing. J. Appl. Chem. 2013, 2013, 930356. [Google Scholar] [CrossRef]
- Szymczyk, K.; Zdziennicka, A.; Jańczuk, B. Effect of polysorbates on solids wettability and their adsorption properties. Colloids Interfaces 2018, 2, 26. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.Y.; Yang, N.; Zhang, D.; Zhang, F.Y.; Gao, H.T.; Rong, W.T.; Yu, S.Q.; Xu, Q. Food emulsifier polysorbate 80 increases intestinal absorption of di-(2-ethylhexyl) phthalate in rats. Toxicol. Sci. 2014, 139, 317–327. [Google Scholar] [CrossRef]
- Ding, S. Quantitation of hydroperoxides in the aqueous solutions of non-ionic surfactants using polysorbate 80 as the model surfactant. J. Pharm. Biomed. Anal. 1993, 11, 95–101. [Google Scholar] [CrossRef]
- Porter, M.R. Handbook of Surfactants; Chapman and Hall: London, UK, 1994; Volume 0751401706, pp. 126–168. [Google Scholar]
- Wu, H.; Zhu, H.; Zhuang, J.; Yang, S.; Liu, C.; Cao, Y.C. Water-soluble nanocrystals through dual-interaction ligands. Angew. Chem. 2008, 47, 3730–3734. [Google Scholar] [CrossRef]
- Xiao, J.; Duan, X.; Meng, Q.; Yin, Q.; Zhang, Z.; Yu, H.; Chen, L.; Gu, W.; Li, Y. Effective delivery of p65 shRNA by optimized Tween 85-polyethyleneimine conjugate for inhibition of tumor growth and lymphatic metastasis. Acta Biomater. 2014, 10, 2674–2683. [Google Scholar] [CrossRef]
- Lim, E.K.; Yang, J.; Suh, J.S.; Huh, Y.M.; Haam, S. Self-labeled magneto nanoprobes using tri-aminated polysorbate 80 for detection of human mesenchymal stem cells. J. Mater. Chem. 2009, 19, 8958–8963. [Google Scholar] [CrossRef]
- Kreuter, J.; Ramge, P.; Petrov, V.; Hamm, S.; Gelperina, S.E.; Engelhardt, B.; Alyautdin, R.; von Briesen, H.; Begley, D.J. Direct evidence that polysorbate-80-coated poly(butylcyanoacrylate) nanoparticles deliver drugs to the CNS via specific mechanisms requiring prior binding of drug to the nanoparticles. Pharm Res. 2003, 20, 409–416. [Google Scholar] [CrossRef]
- Sahu, A.K.; Mishra, J.; Mishra, A.K. Introducing Tween-curcumin niosomes: Preparation, characterization and microenvironment study. Soft Matter 2020, 16, 1779–1791. [Google Scholar] [CrossRef]
- Deng, L.L.; Taxipalati, M.; Que, F.; Zhang, H. Physical characterization and antioxidant activity of thymol solubilized Tween 80 micelles. Sci. Rep. 2016, 6, 38160. [Google Scholar] [CrossRef] [PubMed]
- Hekmat, A.; Attar, H.; Seyf Kordi, A.A.; Iman, M.; Jaafari, M.R. New oral formulation and in vitro evaluation of docetaxel-loaded nanomicelles. Molecules 2016, 21, 1265. [Google Scholar] [CrossRef] [PubMed]
- Garidel, P.; Hoffmann, C.; Blume, A. A thermodynamic analysis of the binding interaction between polysorbate 20 and 80 with human serum albumins and immunoglobulins: A contribution to understand colloidal protein stabilisation. Biophys. Chem. 2009, 143, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Approved or Authorized in the United States. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html#Appendix-C (accessed on 3 September 2021).
- Jiao, J. Polyoxyethylated nonionic surfactants and their applications in topical ocular drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- El-Setouhy, D.A.; Basalious, E.B.; Abdelmalak, N.S. Bioenhanced sublingual tablet of drug with limited permeability using novel surfactant binder and microencapsulated polysorbate: In vitro/in vivo evaluation. Eur. J. Pharm. Biopharm. 2015, 94, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Yang, L.; Wang, Y.; Yu, S.; Guo, Y.; Deng, J.; Zhao, Q.; Jin, X. Formulation, development, and optimization of a novel octyldodecanol-based nanoemulsion for transdermal delivery of ceramide IIIB. Int. J. Nanomed. 2017, 12, 5203–5221. [Google Scholar] [CrossRef]
- Liu, L.; Qi, W.; Schwartz, D.K.; Randolph, T.W.; Carpenter, J.F. The effects of excipients on protein aggregation during agitation: An interfacial shear rheology study. J. Pharm. Sci. 2013, 102, 2460–2470. [Google Scholar] [CrossRef] [PubMed]
- Bam, N.B.; Cleland, J.L.; Yang, J.; Manning, M.C.; Carpenter, J.F.; Kelley, R.F.; Randolph, T.W. Tween protects recombinant human growth hormone against agitation-induced damage via hydrophobic interactions. J. Pharm. Sci. 1998, 87, 1554–1559. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, M.; Morrison, R.A.; Chong, S. Commonly used surfactant, Tween 80, improves absorption of P-glycoprotein substrate, digoxin, in rats. Arch. Pharmacal Res. 2003, 26, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Hugger, E.D.; Novak, B.L.; Burton, P.S.; Audus, K.L.; Borchardt, R.T. A comparison of commonly used polyethoxylated pharmaceutical excipients on their ability to inhibit P-glycoprotein activity in vitro. J Pharm Sci. 2002, 91, 1991–2002. [Google Scholar] [CrossRef]
- Shono, Y.; Nishihara, H.; Matsuda, Y.; Furukawa, S.; Okada, N.; Fujita, T.; Yamamoto, A. Modulation of intestinal p-glycoprotein function by Cremophor EL and other surfactants by an in vitro diffusion chamber method using the isolated rat intestinal membranes. J Pharm Sci. 2004, 93, 877–885. [Google Scholar] [CrossRef]
- Cornaire, G.; Woodley, J.F.; Saivin, S.; Legendre, J.Y.; Decourt, S.; Cloarec, A.; Houin, G. Effect of polyoxyl 35 castor oil and polysorbate 80 on the intestinal absorption of digoxin in vitro. Arzneimittelforschung 2000, 50, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, B.A. Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: Structure and degradation pathways. J. Pharm. Sci. 2008, 97, 2924–2935. [Google Scholar] [CrossRef]
- Yu, Y.; Bu, F.; Zhou, H.; Wang, Y.; Cui, J.; Wang, X.; Nie, G.; Xiao, H. Biosafety materials: An emerging new research direction of materials science from the COVID-19 outbreak. Mater. Chem. Front. 2020, 4, 1930–1953. [Google Scholar] [CrossRef]
- Safety Assessment of Polysorbates as Used in Cosmetics. Available online: https://www.cir-safety.org/sites/default/files/polysorbates.pdf (accessed on 3 September 2021).
- Kazuaki, W.; Haruyoshi, K.; Noriko, F.; Chiye, T.; Kyoko, S.; Hiroshi, A. A comparative study of the hydroxyl and saponification values of polysorbate 60 in international food additive specifications. AJAC 2014, 5, 199–204. [Google Scholar]
- Cox, S.; Sandall, A.; Smith, L.; Rossi, M.; Whelan, K. Food additive emulsifiers: A review of their role in foods, legislation and classifications, presence in food supply, dietary exposure, and safety assessment. Nutr. Rev. 2020, 79, 726–741. [Google Scholar] [CrossRef]
- Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Jose Frutos, M.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambre, C.; Leblanc, J.C.; et al. Scientific opinion on the re-evaluation of polyoxyethylene sorbitan monolaurate (E 432), polyoxyethylene sorbitan monooleate (E 433), polyoxyethylene sorbitan monopalmitate (E 434), polyoxyethylene sorbitan monostearate (E 435) and polyoxyethylene sorbitan tristearate (E 436) as food additives. EFSA J. 2015, 13, 1–74. [Google Scholar]
- Kriegel, C.; Festag, M.; Kishore, R.S.K.; Roethlisberger, D.; Schmitt, G. Pediatric safety of polysorbates in drug formulations. Children 2020, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Van Tellingen, O.; Beijnen, J.H.; Verweij, J.; Scherrenburg, E.J.; Nooijen, W.J.; Sparreboom, A. Rapid esterase-sensitive breakdown of polysorbate 80 and its impact on the plasma pharmacokinetics of docetaxel and metabolites in mice. Clin. Cancer Res. 1999, 5, 2918–2924. [Google Scholar]
- Mary, A.L. Final report on the safety assessment of polysorbates 20, 21, 40, 60, 61, 65, 80, 81, and 85. J. Am. Coll. Toxicol. 1984, 3, 1–82. [Google Scholar]
- Nelson, M.F.; Poulos, T.A.; Gongwer, L.E.; Kirschman, J.C. Preparations of carbon-l4-labeled polyoxyethylene (20) sorbitan monolaurate and their metabolic fate in rats. J. Food Sci. 1966, 31, 253–258. [Google Scholar] [CrossRef]
- Oser, B.L.; Oser, M. Nutritional studies on rats on diets containing high levels of partial ester emulsifiers. III. Clinical and metabolic observations. J. Nutr. 1957, 61, 149–166. [Google Scholar] [CrossRef]
- Czajkowska-Kośnik, A.; Wolska, E.; Chorazewicz, J.; Sznitowska, M. Comparison of cytotoxicity in vitro and irritation in vivo for aqueous and oily solutions of surfactants. Drug Dev. Ind. Pharm. 2015, 41, 1232–1236. [Google Scholar] [CrossRef]
- Arechabala, B.; Coiffard, C.; Rivalland, P.; Coiffard, L.J.; de Roeck-Holtzhauer, Y. Comparison of cytotoxicity of various surfactants tested on normal human fibroblast cultures using the neutral red test, MTT assay and LDH release. J. Appl. Toxicol. 1999, 19, 163–165. [Google Scholar] [CrossRef]
- Norris, L.; Qureshi, Z.; Bookstaver, B.; Raisch, D.; Sartor, O.; Chen, H.; Chen, F.; Bennett, C. Polysorbate 80 hypersensitivity reactions: A renewed call to action. Community Oncol. 2010, 7, 425–428. [Google Scholar] [CrossRef]
- Kicker, J.S.; Haizlip, J.A.; Buck, M.L. Hepatotoxicity after continuous amiodarone infusion in a postoperative cardiac infant. J. Pediatr. Pharmacol. Ther. 2012, 17, 189–195. [Google Scholar] [CrossRef]
- Gameiro, M.; Silva, R.; Rocha-Pereira, C.; Carmo, H.; Carvalho, F.; Bastos, M.L.; Remião, F. Cellular models and in vitro assays for the screening of modulators of P-gp, MRP1 and BCRP. Molecules 2017, 22, 600. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.J.; dos Santos, D.J.; Ferreira, M.J. P-glycoprotein and membrane roles in multidrug resistance. Future Med. Chem. 2015, 7, 929–946. [Google Scholar] [CrossRef] [PubMed]
- Dudeja, P.K.; Anderson, K.M.; Harris, J.S.; Buckingham, L.; Coon, J.S. Reversal of multidrug resistance phenotype by surfactants: Relationship to membrane lipid fluidity. Arch. Biochem. Biophys. 1995, 319, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Sawangrat, K.; Yamashita, S.; Tanaka, A.; Morishita, M.; Kusamori, K.; Katsumi, H.; Sakane, T.; Yamamoto, A. Modulation of intestinal transport and absorption of topotecan, a BCRP substrate, by various pharmaceutical excipients and their inhibitory mechanisms of BCRP transporter. J. Pharm. Sci. 2019, 108, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.L. Relationships between the hydrophilic-lipophilic balance values of pharmaceutical excipients and their multidrug resistance modulating effect in Caco-2 cells and rat intestines. J. Control. Release 2003, 90, 37–48. [Google Scholar] [CrossRef]
- Nielsen, R.B.; Kahnt, A.; Dillen, L.; Wuyts, K.; Snoeys, J.; Nielsen, U.G.; Holm, R.; Nielsen, C.U. Montmorillonite-surfactant hybrid particles for modulating intestinal P-glycoprotein-mediated transport. Int. J. Pharm. 2019, 571, 118696. [Google Scholar] [CrossRef] [PubMed]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.-H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Tamilvanan, S.; Benita, S. The potential of lipid emulsion for ocular delivery of lipophilic drugs. Eur. J. Pharm. Biopharm. 2004, 58, 357–368. [Google Scholar] [CrossRef]
- Lv, F.F.; Zheng, L.Q.; Tung, C.H. Phase behavior of the microemulsions and the stability of the chloramphenicol in the microemulsion-based ocular drug delivery system. Int. J. Pharm. 2005, 301, 237–246. [Google Scholar] [CrossRef]
- Ali, Y.; Lehmussaari, K. Industrial perspective in ocular drug delivery. Adv. Drug Delivery Rev. 2006, 58, 1258–1268. [Google Scholar] [CrossRef]
- Murdan, S.; Gregoriadis, G.; Florence, A. Non-ionic surfactant based organogels incorporating niosomes. Stp. Pharm. Sci. 1996, 6, 44–48. [Google Scholar]
- Alonso, M.J.; Sánchez, A. The potential of chitosan in ocular drug delivery. J. Pharm. Pharmacol. 2010, 55, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Desireddy, R.B.; Taraka, L.K.C.; Naga, S.G.; Sowjanya, T.; Lavanya, L.R.K. Occular drug delivery system—Emerging trends. Int. J. Pharm. Sci. Rev. Res. 2012, 16, 29–33. [Google Scholar]
- Draize, J.H.; Kelley, E.A. Surface-active agents and the eye. Drug Cosmet. Ind. 1952, 71, 118–120. [Google Scholar]
- Furrer, P.; Mayer, J.M.; Gurny, R. Ocular tolerance of preservatives and alternatives. Eur. J. Pharm. Biopharm. 2002, 53, 263–280. [Google Scholar] [CrossRef]
- Carmignani, C.; Rossi, S.; Saettone, M.F.; Burgalassi, S. Ophthalmic vehicles containing polymer-solubilized tropicamide: “in vitro/in vivo” evaluation. Drug Dev. Ind. Pharm. 2002, 28, 101–105. [Google Scholar] [CrossRef]
- Rodriguez-Aller, M.; Kaufmann, B.; Guillarme, D.; Stella, C.; Furrer, P.; Rudaz, S.; El Zaoui, I.; Valamanesh, F.; Di, T.C.; Behar-Cohen, F.; et al. In vivo characterisation of a novel water-soluble cyclosporine a prodrug for the treatment of dry eye disease. Eur. J. Pharm. Biopharm. 2012, 80, 544–552. [Google Scholar] [CrossRef]
- Gonzalez-Mira, E.; Egea, M.A.; Souto, E.B.; Calpena, A.C.; García, M.L. Optimizing flurbiprofen-loaded NLC by central composite factorial design for ocular delivery. Nanotechnology 2011, 22, 045101. [Google Scholar] [CrossRef]
- Bucolo, C.; Maltese, A.; Puglisi, G.; Pignatello, R. Enhanced ocular anti-inflammatory activity of ibuprofen carried by an Eudragit RS100 nanoparticle suspension. Ophthalmic Res. 2002, 34, 319–323. [Google Scholar] [CrossRef]
- Hippalgaonkar, K.; Adelli, G.R.; Hippalgaonkar, K.; Repka, M.A.; Majumdar, S. Indomethacin-loaded solid lipid nanoparticles for ocular delivery: Development, characterization, and in vitro evaluation. J. Ocul Pharmacol Ther. 2013, 29, 216–228. [Google Scholar] [CrossRef]
- Patel, S.; Garapati, C.; Chowdhury, P.; Gupta, H.; Nesamony, J.; Nauli, S.; Boddu, S.H. Development and evaluation of dexamethasone nanomicelles with potential for treating posterior uveitis after topical application. J. Ocul. Pharmacol. Ther. 2015, 31, 215–227. [Google Scholar] [CrossRef]
- Lv, F.F.; Li, N.; Zheng, L.Q.; Tung, C.H. Studies on the stability of the chloramphenicol in the microemulsion free of alcohols. Eur. J. Pharm. Biopharm. 2006, 62, 288–294. [Google Scholar] [CrossRef]
- Zimmer, A.K.; Maincent, P.; Thouvenot, P.; Kreuter, J. Hydrocortisone delivery to healthy and inflamed eyes using a micellar polysorbate 80 solution or albumin nanoparticles. Int. J. Pharm. 1994, 110, 211–222. [Google Scholar] [CrossRef]
- Abdelkader, H.; Alani, A.W.G.; Alany, R.G. Recent advances in non-ionic surfactant vesicles (niosomes): Self-assembly, fabrication, characterization, drug delivery applications and limitations. Drug Deliv. 2014, 21, 87–100. [Google Scholar] [CrossRef]
- Ag Seleci, D.; Seleci, M.; Walter, J.G.; Stahl, F.; Scheper, T. Niosomes as nanoparticular drug carriers: Fundamentals and recent applications. J. Nanomater. 2016, 2016, 7372306. [Google Scholar] [CrossRef]
- Bartelds, R.; Nematollahi, M.H.; Pols, T.; Stuart, M.C.A.; Pardakhty, A.; Asadikaram, G.; Poolman, B. Niosomes, an alternative for liposomal delivery. PLoS ONE 2018, 13, e0194179. [Google Scholar] [CrossRef] [PubMed]
- Mazet, R.; Yaméogo, J.B.G.; Wouessidjewe, D.; Choisnard, L.; Gèze, A. Recent advances in the design of topical ophthalmic delivery systems in the treatment of ocular surface inflammation and their biopharmaceutical evaluation. Pharmaceutics 2020, 12, 570. [Google Scholar] [CrossRef] [PubMed]
- Mashal, M.; Attia, N.; Puras, G.; Martinez-Navarrete, G.; Fernandez, E.; Pedraz, J.L. Retinal gene delivery enhancement by lycopene incorporation into cationic niosomes based on DOTMA and polysorbate 60. J. Controlled Release 2017, 254, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Villate-Beitia, I.; Gallego, I.; Martinez-Navarrete, G.; Zarate, J.; Lopez-Mendez, T.; Soto-Sanchez, C.; Santos-Vizcaino, E.; Puras, G.; Fernandez, E.; Pedraz, J.L. Polysorbate 20 non-ionic surfactant enhances retinal gene delivery efficiency of cationic niosomes after intravitreal and subretinal administration. Int. J. Pharm. 2018, 550, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Shukla, T.; Upmanyu, N.; Agrawal, M.; Saraf, S.; Saraf, S.; Alexander, A. Biomedical applications of microemulsion through dermal and transdermal route. Biomed. Pharmacother. 2018, 108, 1477–1494. [Google Scholar] [CrossRef]
- Michel, C.; Purmann, T.; Mentrup, E.; Seiller, E.; Kreuter, J. Effect of liposomes on percutaneous penetration of lipophilic materials. Int. J. Pharm. 1992, 84, 93–105. [Google Scholar] [CrossRef]
- Liu, C.H.; Chang, F.Y. Development and characterization of eucalyptol microemulsions for topic delivery of curcumin. Chem. Pharm. Bull. 2011, 59, 172–178. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chang, F.-Y.; Hung, D.-K. Terpene microemulsions for transdermal curcumin delivery: Effects of terpenes and cosurfactants. Colloids Surf. B 2011, 82, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Hosmer, J.; Reed, R.; Bentley, M.V.L.B.; Nornoo, A.; Lopes, L.B. Microemulsions containing medium-chain glycerides as transdermal delivery systems for hydrophilic and hydrophobic drugs. AAPS Pharm. Sci. Tech. 2009, 10, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Hathout, R.M.; Woodman, T.J.; Mansour, S.; Mortada, N.D.; Geneidi, A.S.; Guy, R.H. Microemulsion formulations for the transdermal delivery of testosterone. Eur. J. Pharm. Sci. 2010, 40, 188–196. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, S.M.; Mo, F.K.; Zhong, D.F. Investigation of microemulsion system for transdermal delivery of meloxicam. Int. J. Pharm. 2006, 321, 117–123. [Google Scholar] [CrossRef]
- Tavano, L.; Alfano, P.; Muzzalupo, R.; de Cindio, B. Niosomes vs microemulsions: New carriers for topical delivery of Capsaicin. Colloids Surf. B 2011, 87, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.Y.; Liu, F.; Chen, Z.P.; Ping, Q.N. Water in oil microemulsions containing NaCl for transdermal delivery of fluorouracil. Yao Xue Xue Bao 2011, 46, 720–726. [Google Scholar]
- Dhamankar, A.K.; Manwar, J.V.; Kumbhar, D.D. The novel formulation design of O/W microemulsion of ketoprofen for improving transdermal absorption. Int. J. Pharm. Tech. Res. 2009, 1, 1449–1457. [Google Scholar]
- Thiebaut, F.; Tsuruo, T.; Hamada, H.; Gottesman, M.M.; Pastan, I.; Willingham, M.C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc. Natl. Acad. Sci. USA 1987, 84, 7735–7738. [Google Scholar] [CrossRef]
- Giacomini, K.M.; Huang, S.M.; Tweedie, D.J.; Benet, L.Z.; Brouwer, K.L.; Chu, X.; Dahlin, A.; Evers, R.; Fischer, V.; Hillgren, K.M.; et al. Membrane transporters in drug development. Nat. Rev. Drug Discov. 2010, 9, 215–236. [Google Scholar]
- Leslie, E.M.; Deeley, R.G.; Cole, S.P. Multidrug resistance proteins: Role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol. Appl. Pharmacol. 2005, 204, 216–237. [Google Scholar] [CrossRef]
- Cornaire, G.; Woodley, J.; Hermann, P.; Cloarec, A.; Arellano, C.; Houin, G. Impact of excipients on the absorption of P-glycoprotein substrates in vitro and in vivo. Int. J. Pharm. 2004, 278, 119–131. [Google Scholar] [CrossRef]
- Nielsen, C.U.; Abdulhussein, A.A.; Colak, D.; Holm, R. Polysorbate 20 increases oral absorption of digoxin in wild-type Sprague Dawley rats, but not in mdr1a(-/-) Sprague Dawley rats. Int. J. Pharm. 2016, 513, 78–87. [Google Scholar] [CrossRef]
- Al-Ali, A.A.A.; Quach, J.R.C.; Bundgaard, C.; Steffansen, B.; Holm, R.; Nielsen, C.U. Polysorbate 20 alters the oral bioavailability of etoposide in wild type and mdr1a deficient Sprague-Dawley rats. Int. J. Pharm. 2018, 543, 352–360. [Google Scholar] [CrossRef]
- Al-Ali, A.A.A.; Nielsen, R.B.; Steffansen, B.; Holm, R.; Nielsen, C.U. Nonionic surfactants modulate the transport activity of ATP-binding cassette (ABC) transporters and solute carriers (SLC): Relevance to oral drug absorption. Int. J. Pharm. 2019, 566, 410–433. [Google Scholar] [CrossRef]
- Serdoz, F.; Voinovich, D.; Perissutti, B.; Grabnar, I.; Hasa, D.; Ballestrazzi, R.; Coni, E.; Pellegrini, E. Development and pharmacokinetic evaluation of erythromycin lipidic formulations for oral administration in rainbow trout (Oncorhynchus mykiss). Eur. J. Pharm. Biopharm. 2011, 78, 401–407. [Google Scholar] [CrossRef]
- Fan, Y.; Li, X.; Zhou, Y.; Fan, C.; Wang, X.; Huang, Y.; Liu, Y. Improved intestinal delivery of salmon calcitonin by water-in-oil microemulsions. Int. J. Pharm. 2011, 416, 323–330. [Google Scholar] [CrossRef]
- Govindaraju, R.; Karki, R.; Chandrashekarappa, J.; Santhanam, M.; Shankar, A.K.K.; Joshi, H.K.; Divakar, G. Enhanced water dispersibility of curcumin encapsulated in alginate-polysorbate 80 nano particles and bioavailability in healthy human volunteers. Pharm. Nanotechnol. 2019, 7, 39–56. [Google Scholar] [CrossRef]
- Lu, J.L.; Wang, J.C.; Zhao, S.X.; Liu, X.Y.; Zhao, H.; Zhang, X.; Zhou, S.F.; Zhang, Q. Self-microemulsifying drug delivery system (SMEDDS) improves anticancer effect of oral 9-nitrocamptothecin on human cancer xenografts in nude mice. Eur. J. Pharm. Biopharm. 2008, 69, 899–907. [Google Scholar] [CrossRef]
- Mestry, M.; Rane, M.; Kadu, P.; More, S. Self-emulsifying drug delivery system of rosuvastatin calcium. Int. J. Pharm. 2017, 7, 130–139. [Google Scholar]
- Seo, J.K.; Sang, E.L.; Choon, L.N.; Joon, K.L.; Tae, H.K.; Cheong, W.C.; Jeong, S.P. Preformulation and in vitro physicochemical characterization of fenofibrate-loaded emulsion. J. Pharm. Investig. 2015, 45, 669–674. [Google Scholar]
- Patel, A.R.; Vavia, P.R. Preparation and in vivo evaluation of SMEDDS (self-microemulsifying drug delivery system) containing fenofibrate. AAPS J. 2007, 9, E344–E352. [Google Scholar] [CrossRef]
- Gao, M.; Mei, D.; Huo, Y.; Mao, S. Effect of polysorbate 80 on the intranasal absorption and brain distribution of tetramethylpyrazine phosphate in rats. Drug Deliv. Transl. 2019, 9, 311–318. [Google Scholar] [CrossRef]
- Hilmarsson, H.; Kristmundsdottir, T.; Gunnarsson, E.; Thormar, H. Intranasal delivery of formulations containing virucidal lipids for treatment of respiratory syncytial virus (RSV) infection in rats. J. Drug Deliv. Sci. Technol. 2013, 23, 455–457. [Google Scholar] [CrossRef]
- Ruan, Y.; Yao, L.; Zhang, B.; Zhang, S.; Guo, J. Nanoparticle-mediated delivery of neurotoxin-II to the brain with intranasal administration: An effective strategy to improve antinociceptive activity of neurotoxin. Drug Dev. Ind. Pharm. 2012, 38, 123–128. [Google Scholar] [CrossRef]
- Deshpande, N.U.; Jayakannan, M. Cisplatin-stitched polysaccharide vesicles for synergistic cancer therapy of triple antagonistic drugs. Biomacromolecules 2017, 18, 113–126. [Google Scholar] [CrossRef]
- Jensen, A.I.; Binderup, T.; Kumar, E.P.; Kjær, A.; Rasmussen, P.H.; Andresen, T.L. Positron emission tomography based analysis of long-circulating cross-linked triblock polymeric micelles in a U87MG mouse xenograft model and comparison of DOTA and CB-TE2A as chelators of copper-64. Biomacromolecules 2014, 15, 1625–1633. [Google Scholar] [CrossRef]
- Kumar, S.; Alnasif, N.; Fleige, E.; Kurniasih, I.; Kral, V.; Haase, A.; Luch, A.; Weindl, G.; Haag, R.; Schäfer-Korting, M.; et al. Impact of structural differences in hyperbranched polyglycerol–polyethylene glycol nanoparticles on dermal drug delivery and biocompatibility. Eur. J. Pharm. Biopharm. 2014, 88, 625–634. [Google Scholar] [CrossRef]
- Sousa-Herves, A.; Würfel, P.; Wegner, N.; Khandare, J.; Licha, K.; Haag, R.; Welker, P.; Calderón, M. Dendritic polyglycerol sulfate as a novel platform for paclitaxel delivery: Pitfalls of ester linkage. Nanoscale 2015, 7, 3923–3932. [Google Scholar] [CrossRef]
- Ray, P.; Ferraro, M.; Haag, R.; Quadir, M. Dendritic polyglycerol-derived nano-architectures as delivery platforms of gemcitabine for pancreatic cancer. Macromol. Biosci. 2019, 19, e1900073. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Kim, J.C. Photo responsive monoolein cubic phase containing coumarin-Tween 20 conjugates. Drug Dev. Ind. Pharm. 2013, 39, 1457–1463. [Google Scholar] [CrossRef]
- Seo, H.J.; Dai, J.; Kim, J.C. The effect of UV irradiation on air/water interfacial activity of Tween 20-coumarin conjugates. Colloid Polym. Sci. 2013, 291, 2311–2318. [Google Scholar] [CrossRef]
- Wang, M.; Kim, J.C. Tween 20-cinnamic acid conjugate as a UV-absorbing emulsifier. Colloids Surf. A Physicochem. Eng. Asp. 2014, 453, 62–67. [Google Scholar] [CrossRef]
- Kim, J.C. Characterization of cinnamic acid-attached nonionic amphiphiles in UV extinction, emulsification, and in vitro toxicity. J. Dispers. Sci. Technol. 2016, 37, 104–112. [Google Scholar] [CrossRef]
- Vasanthan, R.; Venkitasamy, K.; Sandrine, C.; Philippe, M.L.; Jayakrishnan, A. Polysorbate surfactants as drug carriers: Tween 20-Amphotericin B conjugates as anti-fungal and anti-leishmanial agents. Curr. Drug Deliv. 2018, 15, 1028–1037. [Google Scholar]
- Borges, A.C.; Jayakrishnan, A.; Bourban, P.E.; Plummer, C.J.G.; Pioletti, D.P.; Manson, J.-A.E. Synthesis and photopolymerization of tween 20 methacrylate/n-vinyl-2-pyrrolidone blends. Mater. Sci. Eng. C 2012, 32, 2235–2241. [Google Scholar] [CrossRef][Green Version]
- Lai, W.F.; Shum, H.C. A stimuli-responsive nanoparticulate system using poly(ethylenimine)-graft-polysorbate for controlled protein release. Nanoscale 2016, 8, 517–528. [Google Scholar] [CrossRef]
- Kreuter, J.; Alyautdin, R.N.; Kharkevich, D.A.; Ivanov, A.A. Passage of peptides through the blood-brain barrier with colloidal polymer particles (nanoparticles). Brain Res. 1995, 674, 171–174. [Google Scholar] [CrossRef]
- Kreuter, J. Nanoparticulate systems for brain delivery of drugs. Adv. Drug Deliv. Rev. 2012, 64, 213–222. [Google Scholar] [CrossRef]
- Kreuter, J.; Shamenkov, D.; Petrov, V.; Ramge, P.; Cychutek, K.; Koch-Brandt, C.; Alyautdin, R. Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood-brain barrier. J. Drug Target. 2002, 10, 317–325. [Google Scholar] [CrossRef]
- Hartl, N.; Adams, F.; Merkel, O.M. From adsorption to covalent bonding: Apolipoprotein E functionalization of polymeric nanoparticles for drug delivery across the blood–brain barrier. Adv. Ther. 2021, 4, 2000092. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Anju, S.S.; Cinu, T.A.; Aleykutty, N.A.; Thomas, S.; Souto, E.B. In vivo pharmacokinetics and biodistribution of resveratrol-loaded solid lipid nanoparticles for brain delivery. Int. J. Pharm. 2014, 474, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Samanta, M.K.; Santhi, K.; Kumar, K.P.S.; Paramakrishnan, N.; Suresh, B. Targeted delivery of tacrine into the brain with polysorbate 80-coated poly(n-butylcyanoacrylate) nanoparticles. Eur. J. Pharm. Biopharm. 2008, 70, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Gulyaev, A.E.; Gelperina, S.E.; Skidan, I.N.; Antropov, A.S.; Kivman, G.Y.; Kreuter, J. Significant transport of doxorubicin into the brain with polysorbate 80-coated nanoparticles. Pharm. Res. 1999, 16, 1564–1569. [Google Scholar] [CrossRef]
- Alyautdin, R.; Gothier, D.; Ve, P.; Kharkevich, D.A.; Kreuter, J. Analgesic activity of the hexapeptide dalargin adsorbed on the surface of polysorbate 80-coated poly(butyl cyanoacrylate) nanoparticles. Eur. J. Pharm. Biopharm. 1995, 41, 44–48. [Google Scholar]
- Alyautdin, R.N.; Petrov, V.E.; Langer, K.; Berthold, A.; Kharkevich, D.A.; Kreuter, J. Delivery of loperamide across the blood-brain barrier with polysorbate 80-coated polybutylcyanoacrylate nanoparticles. Pharm. Res. 1997, 14, 325–328. [Google Scholar] [CrossRef]
- Alyautdin, R.N.; Tezikov, E.B.; Ramge, P.; Kharkevich, D.A.; Begley, D.J.; Kreuter, J. Significant entry of tubocurarine into the brain of rats by adsorption to polysorbate 80-coated polybutylcyanoacrylate nanoparticles: An in situ brain perfusion study. J. Microencapsul. 1998, 15, 67–74. [Google Scholar] [CrossRef]
- Prabhakar, K.; Afzal, S.M.; Surender, G.; Kishan, V. Tween 80 containing lipid nanoemulsions for delivery of indinavir to brain. Acta Pharm. Sin. B. 2013, 3, 345–353. [Google Scholar] [CrossRef]
- Schröder, U.; Sabel, B.A. Nanoparticles, a drug carrier system to pass the blood-brain barrier, permit central analgesic effects of i.v. dalargin injections. Brain Res. 1996, 710, 121–124. [Google Scholar] [CrossRef]
- Kreuter, J.; Petrov, V.E.; Kharkevich, D.A.; Alyautdin, R.N. Influence of the type of surfactant on the analgesic effects induced by the peptide dalargin after its delivery across the blood–brain barrier using surfactant-coated nanoparticles. J. Control. Release 1997, 49, 81–87. [Google Scholar] [CrossRef]
- Wilson, B.; Samanta, M.K.; Santhi, K.; Kumar, K.P.S.; Paramakrishnan, N.; Suresh, B. Poly(n-butyl cyanoacrylate) nanoparticles coated with Polysorbate 80 for the targeted delivery of rivastigmine into the brain to treat Alzheimer’s disease. Brain Res. 2008, 1200, 159–168. [Google Scholar] [CrossRef]
- Chintamaneni, P.K.; Krishnamurthy, P.T.; Pindiprolu, S.K.S.S. Polysorbate-80 surface modified nano-stearylamine BQCA conjugate for the management of Alzheimer’s disease. RSC Adv. 2021, 11, 5325–5334. [Google Scholar] [CrossRef]
- Kreuter, J. Influence of the surface properties on nanoparticle-mediated transport of drugs to the brain. J. Nanosci. Nanotechnol. 2004, 4, 484–488. [Google Scholar] [CrossRef]
- Wilson, B. Brain targeting PBCA nanoparticles and the blood-brain barrier. Nanomedicine 2009, 4, 499–502. [Google Scholar] [CrossRef]
- Kreuter, J. Application of nanoparticles for the delivery of drugs to the brain. Int. Congr. Ser. 2005, 1277, 85–94. [Google Scholar] [CrossRef]
- Zhang, W.; Mehta, A.; Tong, Z.; Esser, L.; Voelcker, N.H. Development of polymeric nanoparticles for blood–brain barrier transfer—strategies and challenges. Adv. Sci. 2021, 8, 2003937. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Xie, C.; Wang, H.; Hu, Y. Specific role of polysorbate 80 coating on the targeting of nanoparticles to the brain. Biomaterials 2004, 25, 3065–3071. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Xue, A.; Zhang, B.; Lou, H.; Shi, H.; Zhang, X. Polysorbate 80-coated PLGA nanoparticles improve the permeability of acetylpuerarin and enhance its brain-protective effects in rats. J. Pharm. Pharmacol. 2015, 67, 1650–1662. [Google Scholar] [CrossRef]
- Wang, C.X.; Huang, L.S.; Hou, L.B.; Jiang, L.; Yan, Z.T.; Wang, Y.L.; Chen, Z.L. Antitumor effects of polysorbate-80 coated gemcitabine polybutylcyanoacrylate nanoparticles in vitro and its pharmacodynamics in vivo on C6 glioma cells of a brain tumor model. Brain Res. 2009, 1261, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Gelperina, S.E.; Khalansky, A.S.; Skidan, I.N.; Smirnova, Z.S.; Bobruskin, A.I.; Severin, S.E.; Turowski, B.; Zanella, F.E.; Kreuter, J. Toxicological studies of doxorubicin bound to polysorbate 80-coated poly(butyl cyanoacrylate) nanoparticles in healthy rats and rats with intracranial glioblastoma. Toxicol. Lett. 2002, 126, 131–141. [Google Scholar] [CrossRef]
- Ren, T.; Xu, N.; Cao, C.; Yuan, W.; Yu, X.; Chen, J.; Ren, J. Preparation and therapeutic efficacy of polysorbate-80-coated amphotericin B/PLA-b-PEG nanoparticles. J. Biomater. Sci. Polym. Ed. 2009, 20, 1369–1380. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, B.; Xie, S.; Yang, B.; Xu, Q.; Tan, J. Superparamagnetic iron oxide nanoparticles modified with tween 80 pass through the intact blood-brain barrier in rats under magnetic field. ACS Appl. Mater. Interfaces 2016, 8, 11336–11341. [Google Scholar] [CrossRef]
- Tian, C.; Asghar, S.; Xu, Y.; Chen, Z.; Zhang, J.; Ping, Q.; Xiao, Y. Tween 80-modified hyaluronic acid-ss-curcumin micelles for targeting glioma: Synthesis, characterization and their in vitro evaluation. Int. J. Biol. Macromol. 2018, 120, 2579–2588. [Google Scholar] [CrossRef] [PubMed]
- Koffie, R.M.; Farrar, C.T.; Saidi, L.J.; William, C.M.; Hyman, B.T.; Spires-Jones, T.L. Nanoparticles enhance brain delivery of blood-brain barrier-impermeable probes for in vivo optical and magnetic resonance imaging. Proc. Natl. Acad. Sci. USA 2011, 108, 18837–18842. [Google Scholar] [CrossRef]
- Parmar, A.; Jain, A.; Uppal, S.; Mehta, S.K.; Kaur, K.; Singh, B.; Sandhir, R.; Sharma, S. Anti-proliferate and apoptosis triggering potential of methotrexate-transferrin conjugate encapsulated PLGA nanoparticles with enhanced cellular uptake by high-affinity folate receptors. Artif. Cells Nanomed. Biotechnol. 2018, 46, 704–719. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Khan, M.; Alrobaian, M.M.; Alghamdi, S.A.; Warsi, M.H.; Sultana, S.; Khan, R.A. Brain targeted Polysorbate-80 coated PLGA thymoquinone nanoparticles for the treatment of Alzheimer’s disease, with biomechanistic insights. J. Drug. Deliv. Sci. Technol. 2021, 61, 102214. [Google Scholar] [CrossRef]
- Li, W.; Qiu, J.; Li, X.L.; Aday, S.; Zhang, J.; Conley, G.; Xu, J.; Joseph, J.; Lan, H.; Langer, R.; et al. BBB pathophysiology-independent delivery of siRNA in traumatic brain injury. Sci. Adv. 2021, 7, eabd6889. [Google Scholar]
- Hwang, T.L.; Fang, C.L.; Chen, C.H.; Fang, J.Y. Permeation enhancer-containing water-in-oil nanoemulsions as carriers for intravesical cisplatin delivery. Pharm. Res. 2009, 26, 2314–2323. [Google Scholar] [CrossRef] [PubMed]
- Pectasides, D.; Pectasides, M.; Economopoulos, T. Systemic chemotherapy in locally advanced and/or metastatic bladder cancer. Cancer Treat. Rev. 2006, 32, 456–470. [Google Scholar] [CrossRef]
- Wahgiman, N.A.; Salim, N.; Abdul Rahman, M.B.; Ashari, S.E. Optimization of nanoemulsion containing gemcitabine and evaluation of its cytotoxicity towards human fetal lung fibroblast (MRC5) and human lung carcinoma (A549) cells. Int. J. Nanomed. 2019, 14, 7323–7338. [Google Scholar] [CrossRef]
- Clarke, S.J.; Rivory, L.P. Clinical pharmacokinetics of docetaxel. Clin. Pharmacokinet. 1999, 36, 99–114. [Google Scholar] [CrossRef]
- Song, H.; Geng, H.; Ruan, J.; Wang, K.; Bao, C.; Wang, J.; Peng, X.; Zhang, X.; Cui, D. Development of polysorbate 80/Phospholipid mixed micellar formation for docetaxel and assessment of its in vivo distribution in animal models. Nanoscale Res. Lett. 2011, 6, 354. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Kim, J.K.; Lim, J.S.; Lim, S.J. Enhancement of indocyanine green stability and cellular uptake by incorporating cationic lipid into indocyanine green-loaded nanoemulsions. Colloids Surf. B 2015, 136, 305–313. [Google Scholar] [CrossRef]
- Boni, L.; David, G.; Mangano, A.; Dionigi, G.; Rausei, S.; Spampatti, S.; Cassinotti, E.; Fingerhut, A. Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery. Surg. Endosc. 2015, 29, 2046–2055. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, V.; Nguyen Cao, T.G.; Choi, D.G.; Kang, H.C.; Shim, M.S. Non-ionic polysorbate-based nanoparticles for efficient combination chemo/photothermal/photodynamic therapy. J. Ind. Eng. Chem. 2020, 88, 260–267. [Google Scholar] [CrossRef]
- Martos, A.; Koch, W.; Jiskoot, W.; Wuchner, K.; Winter, G.; Friess, W.; Hawe, A. Trends on analytical characterization of polysorbates and their degradation products in biopharmaceutical formulations. J. Pharm. Sci. 2017, 106, 1722–1735. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.R.; Zhang, J.; O’Dell, C.; Hsieh, M.C.; Goldstein, J.; Liu, J.; Srivastava, A. Effect of polysorbate 80 quality on photostability of a monoclonal antibody. AAPS Pharm. Sci. Tech. 2012, 13, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Hampl, V.; Guo, X.; Ehrenstrasser, C.; Viertler, M.; Rayner, L.; Campanelli, G.; Schipflinger, R.; Thewes, K.; Cerreti, A.; Boehm, S.; et al. A newly identified impurity in polysorbate 80, the long-chain ketone 12-tricosanone, forms visible particles in a biopharmaceutical drug product. J. Pharm. Sci. 2018, 107, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Hewarathna, A.; Geerlof-Vidavsky, I.; Rao, V.A.; Gryniewicz-Ruzicka, C.; Keire, D. Screening of polysorbate-80 composition by high resolution mass spectrometry with rapid H/D exchange. Anal. Chem. 2019, 91, 14649–14656. [Google Scholar] [CrossRef]
- Tomlinson, A.; Zarraga, I.E.; Demeule, B. Characterization of polysorbate ester fractions and implications in protein drug product stability. Mol. Pharm. 2020, 17, 2345–2353. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravichandran, V.; Lee, M.; Nguyen Cao, T.G.; Shim, M.S. Polysorbate-Based Drug Formulations for Brain-Targeted Drug Delivery and Anticancer Therapy. Appl. Sci. 2021, 11, 9336. https://doi.org/10.3390/app11199336
Ravichandran V, Lee M, Nguyen Cao TG, Shim MS. Polysorbate-Based Drug Formulations for Brain-Targeted Drug Delivery and Anticancer Therapy. Applied Sciences. 2021; 11(19):9336. https://doi.org/10.3390/app11199336
Chicago/Turabian StyleRavichandran, Vasanthan, Minjong Lee, Thuy Giang Nguyen Cao, and Min Suk Shim. 2021. "Polysorbate-Based Drug Formulations for Brain-Targeted Drug Delivery and Anticancer Therapy" Applied Sciences 11, no. 19: 9336. https://doi.org/10.3390/app11199336
APA StyleRavichandran, V., Lee, M., Nguyen Cao, T. G., & Shim, M. S. (2021). Polysorbate-Based Drug Formulations for Brain-Targeted Drug Delivery and Anticancer Therapy. Applied Sciences, 11(19), 9336. https://doi.org/10.3390/app11199336






