The Start-Up of Continuous Biohydrogen Production from Cheese Whey: Comparison of Inoculum Pretreatment Methods and Reactors with Moving and Fixed Polyurethane Carriers
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrates and Inoculates
- (1)
- Thermophilic anaerobic sludge from CSTR for co-fermentation of food waste and sewage sludge (TS = 3.57%, VS = 68.03% of TS) (inoculum 1);
- (2)
- Mesophilic anaerobic sludge from the industrial UASB, which treated brewery wastewater (TS = 10.96%, VS = 88.3% of TS) (inoculum 2).
2.2. Experiment on Inactivation of Methanogens in Inoculums
2.3. Experiment on DF of CW in Continuous Mode
2.4. Analytical Methods
2.5. Microbial Community Analysis
3. Results
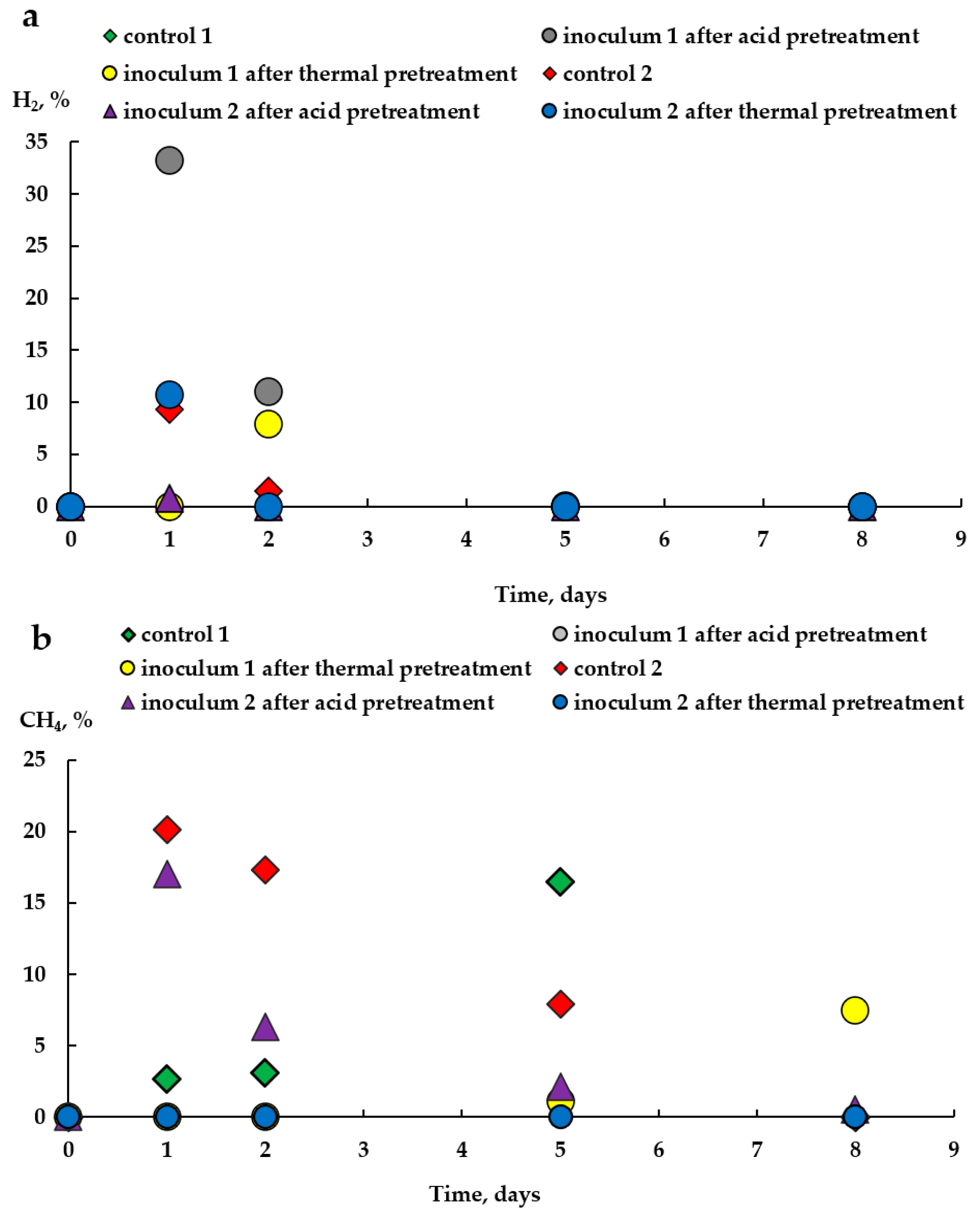
3.1. Experiment on Inactivation of Methanogens in the Inoculum
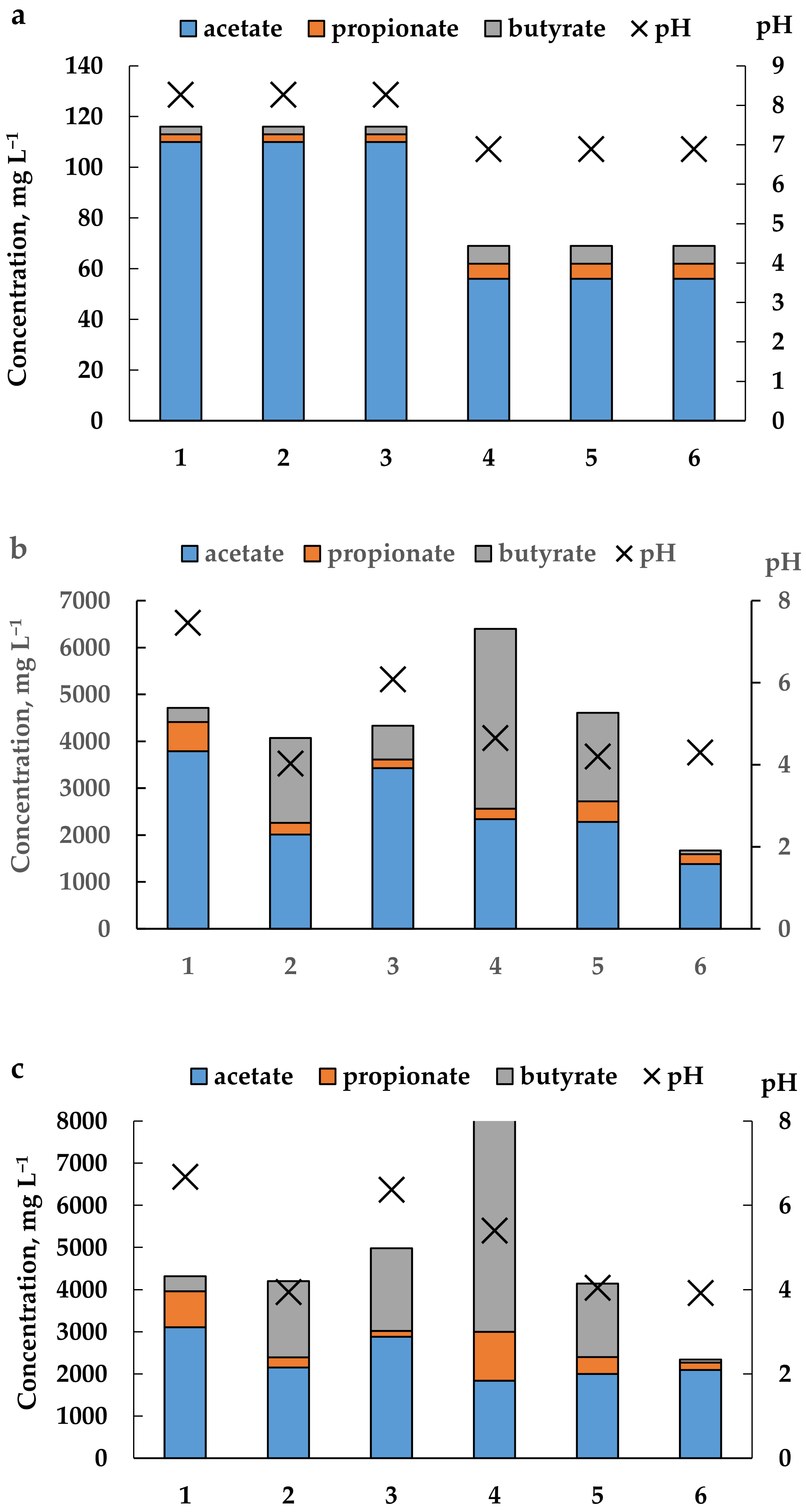
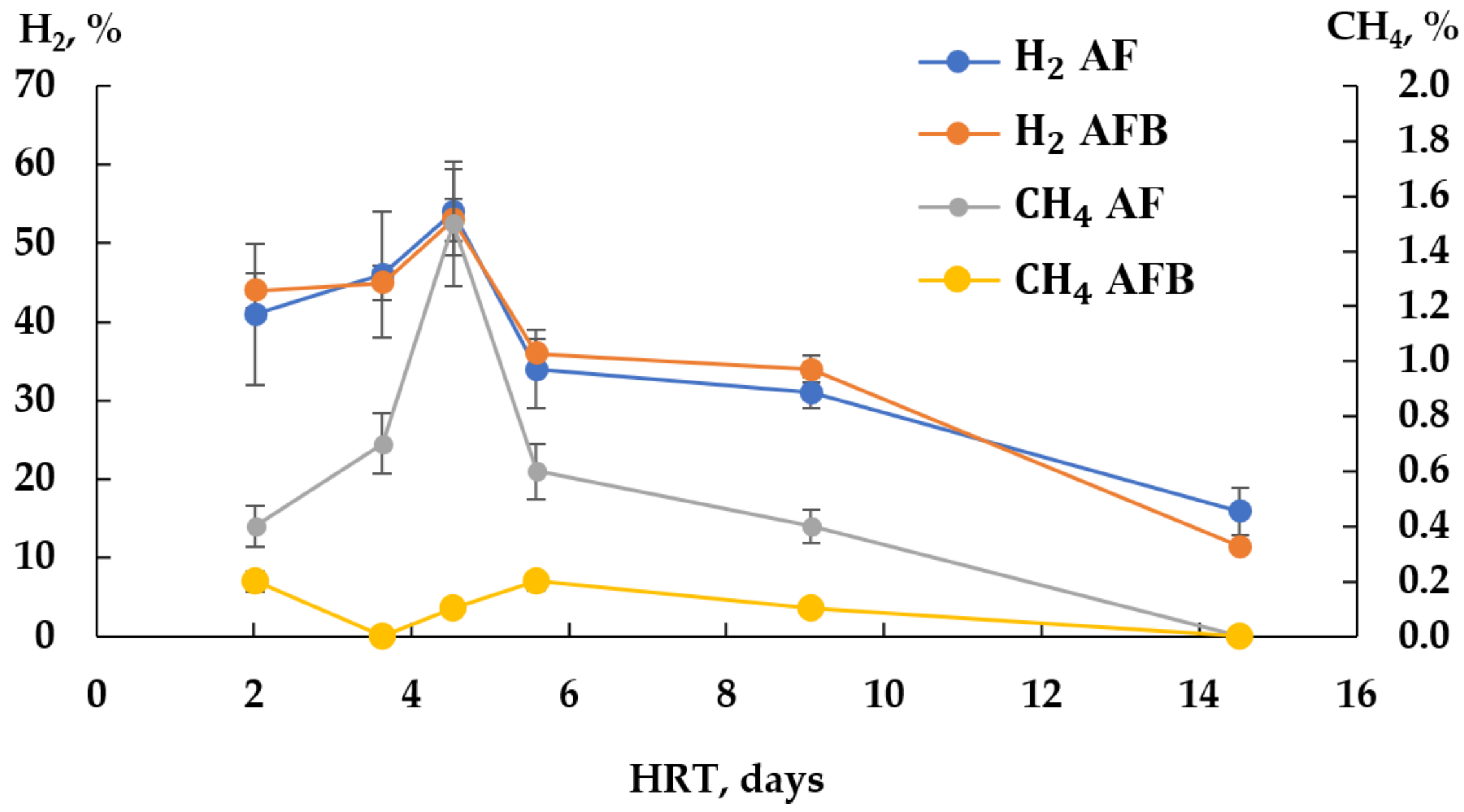
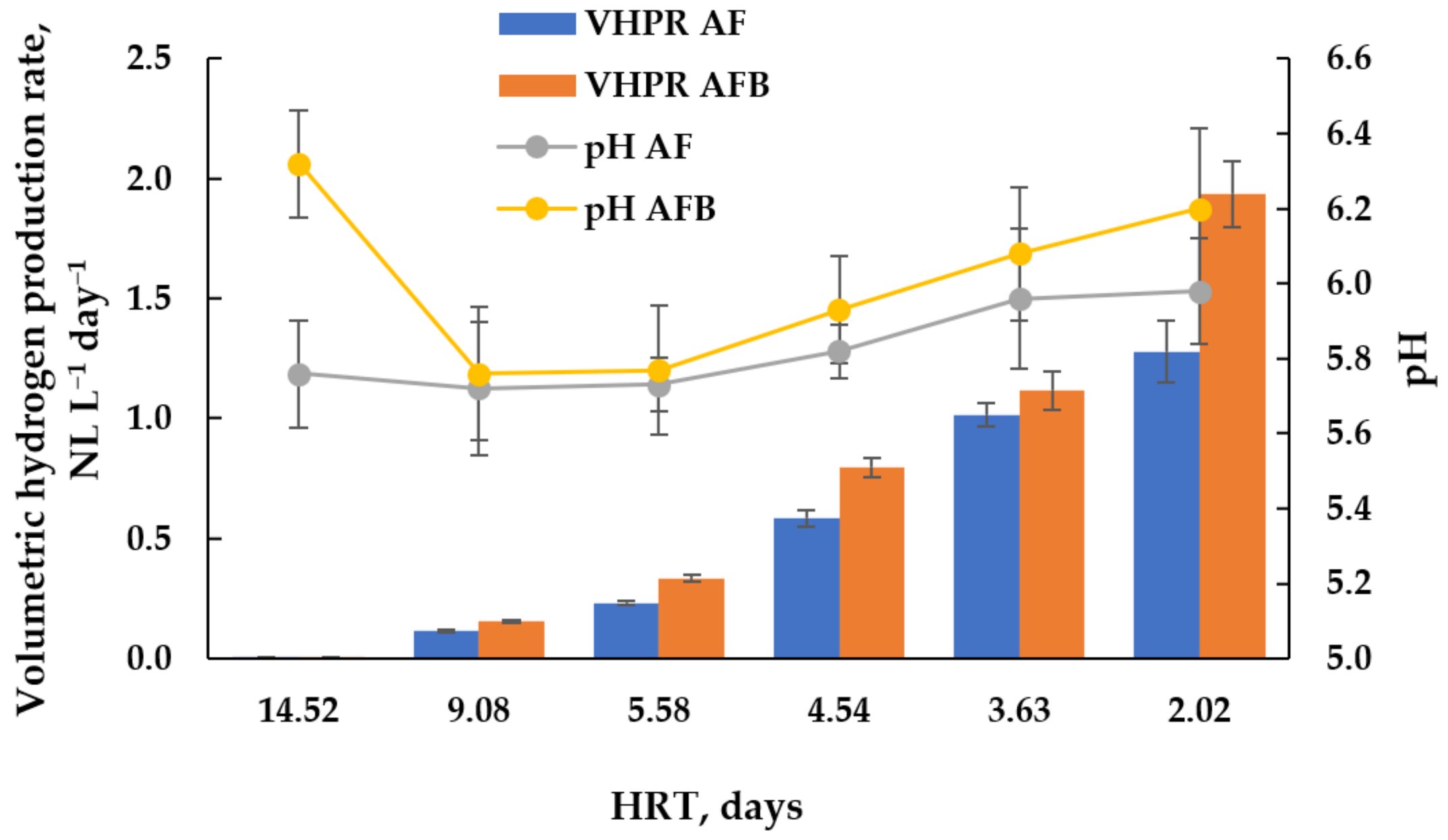
3.2. Experiment on DF of CW in Continuous Mode in Two Types of Reactors
- -
- Thermal inactivation was chosen primarily because of its relative simplicity both in laboratory conditions and in the full-scale implementation of the technology [7];
- -
- Mesophilic sludge was chosen since it contains a microbial community naturally adapted to mesophilic conditions. In turn, the mesophilic mode of operation of AF and AFB was chosen because is considered more stable than thermophilic.
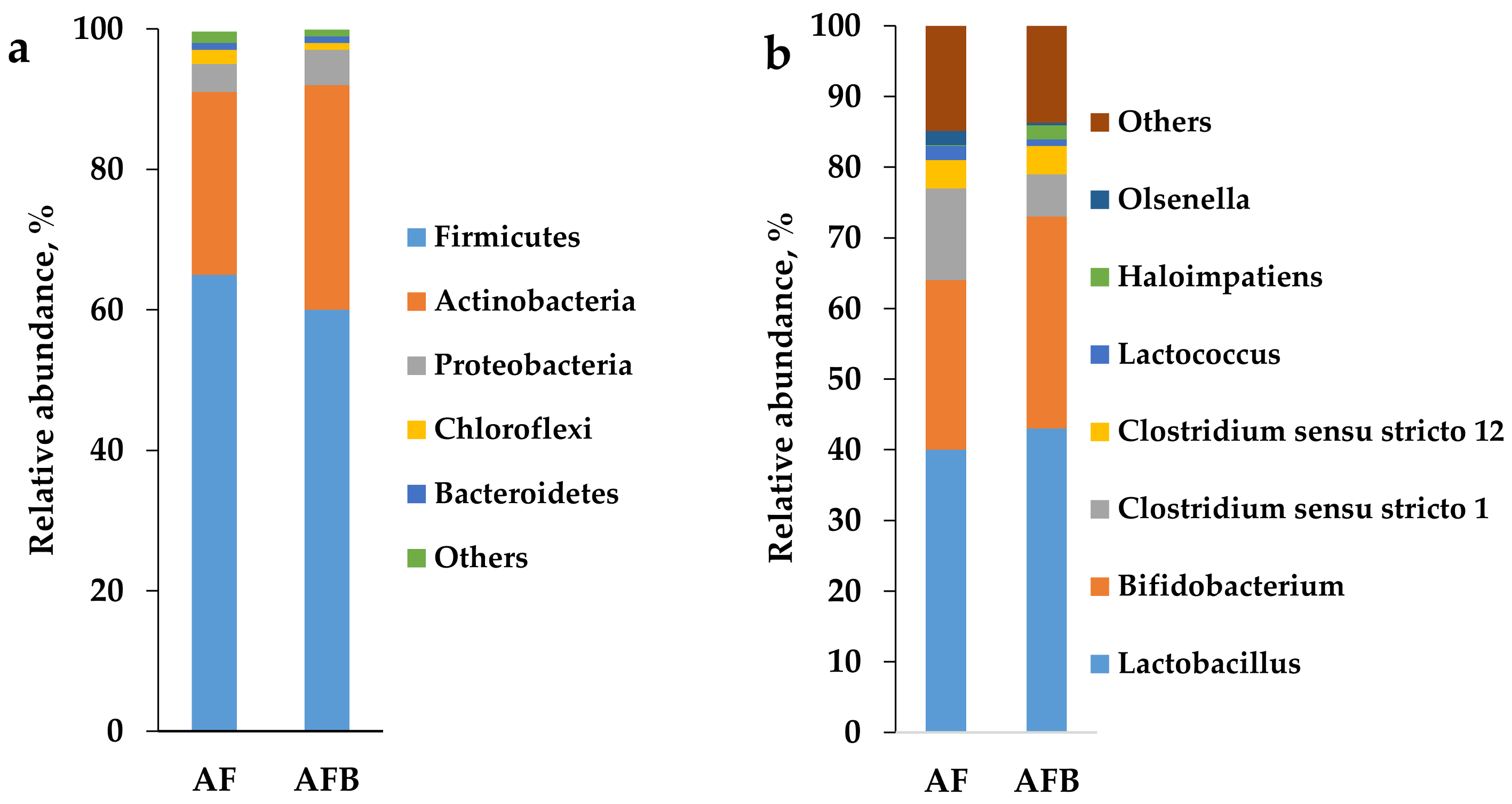
3.3. AF and AFB Microbial Community at the End of the Start-Up
4. Discussion
4.1. Inactivation of Methanogens in the Inoculum
4.2. DF of CW in Continuous Mode in Two Types of Reactors
4.3. AF and AFB Microbial Community at the End of the Start-Up
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carvalho, F.; Prazeres, A.R.; Rivas, J. Cheese whey wastewater: Characterization and treatment. Sci. Total Environ. 2013, 445–446, 385–396. [Google Scholar] [CrossRef]
- De Gioannis, G.; Friargiu, M.; Massi, E.; Muntoni, A.; Polettini, A.; Pomi, R.; Spiga, D. Biohydrogen production from dark fermentation of cheese whey: Influence of pH. Int. J. Hydrog. Energy 2014, 39, 20930–20941. [Google Scholar] [CrossRef]
- Çalli, B. Dark fermentative H2H2 production from xylose and lactose—Effects of on-line pH control. Int. J. Hydrog. Energy 2008, 33, 522–530. [Google Scholar] [CrossRef]
- Grossiord, B.; Vaughan, E.E.; Luesink, E.; De Vos, W.M. Genetics of galactose utilisation via the Leloir pathway in lactic acid bacteria. Le Lait 1998, 78, 77–84. [Google Scholar] [CrossRef][Green Version]
- Kovalev, A.; Litti, Y.; Katraeva, I. Biohydrogen production in the two-stage process of anaerobic bioconversion of organic matter of liquid organic waste with recirculation of digister effluent. Int. J. Hydrog. Energy 2020, 45, 26831–26839. [Google Scholar] [CrossRef]
- Kothari, R. A critical review on factors influencing fermentative hydrogen production. Front. Biosci. 2017, 22, 1195–1220. [Google Scholar] [CrossRef] [PubMed]
- Davila-Vazquez, G.; Alatriste-Mondragón, F.; De Leon-Rodriguez, A.; Razo-Flores, E. Fermentative hydrogen production in batch experiments using lactose, cheese whey and glucose: Influence of initial substrate concentration and pH. Int. J. Hydrog. Energy 2008, 33, 4989–4997. [Google Scholar] [CrossRef]
- Akinbomi, J.; Wikandari, R.; Taherzadeh, M.J. Enhanced Fermentative Hydrogen and Methane Production from an Inhibitory Fruit-Flavored Medium with Membrane-Encapsulated Cells. Membranes 2015, 5, 616–631. [Google Scholar] [CrossRef]
- Andreani, C.L.; Torres, D.G.B.; Schultz, L.; De Carvalho, K.Q.; Gomes, S.D. Hydrogen production from cassava processing wastewater in an anaerobic fixed bed reactor with bamboo as a support material. Eng. Agríc. 2015, 35, 578–587. [Google Scholar] [CrossRef]
- Patra, A.; Park, T.; Kim, M.; Yu, Z. Rumen methanogens and mitigation of methane emission by anti-methanogenic compounds and substances. J. Anim. Sci. Biotechnol. 2017, 8, 1–18. [Google Scholar] [CrossRef]
- Kongjan, P.; O-Thong, S.; Angelidaki, I. Biohydrogen production from desugared molasses (DM) using thermophilic mixed cultures immobilized on heat treated anaerobic sludge granules. Int. J. Hydrog. Energy 2011, 36, 14261–14269. [Google Scholar] [CrossRef]
- Azbar, N.; Dokgöz, F.T.; Keskin, T.; Eltem, R.; Korkmaz, K.S.; Gezgin, Y.; Akbal, Z.; Öncel, S.; Dalay, M.C.; Gönen, Ç.; et al. Comparative Evaluation of Bio-Hydrogen Production from Cheese Whey Wastewater Under Thermophilic and Mesophilic Anaerobic Conditions. Int. J. Green Energy 2009, 6, 192–200. [Google Scholar] [CrossRef]
- Kargi, F.; Eren, N.S.; Ozmihci, S. Hydrogen gas production from cheese whey powder (CWP) solution by thermophilic dark fermentation. Int. J. Hydrog. Energy 2012, 37, 2260–2266. [Google Scholar] [CrossRef]
- Dębowski, M.; Korzeniewska, E.; Filipkowska, Z.; Zieliński, M.; Kwiatkowski, R. Possibility of hydrogen production during cheese whey fermentation process by different strains of psychrophilic bacteria. Int. J. Hydrog. Energy 2014, 39, 1972–1978. [Google Scholar] [CrossRef]
- Badiei, M.; Jahim, J.M.; Anuar, N.; Abdullah, S.R.S. Effect of hydraulic retention time on biohydrogen production from palm oil mill effluent in anaerobic sequencing batch reactor. Int. J. Hydrog. Energy 2011, 36, 5912–5919. [Google Scholar] [CrossRef]
- Oh, S.-E.; Iyer, P.; Bruns, M.A.; Logan, B.E. Biological hydrogen production using a membrane bioreactor. Biotechnol. Bioeng. 2004, 87, 119–127. [Google Scholar] [CrossRef]
- Azbar, N.; Dokgöz, F.T.Ç.; Keskin-Gundogdu, T.; Korkmaz, K.S.; Syed, H.M. Continuous fermentative hydrogen production from cheese whey wastewater under thermophilic anaerobic conditions. Int. J. Hydrog. Energy 2009, 34, 7441–7447. [Google Scholar] [CrossRef]
- Castelló, E.; Santos, C.G.Y.; Iglesias, T.; Paolino, G.; Wenzel, J.; Borzacconi, L.; Etchebehere, C. Feasibility of biohydrogen production from cheese whey using a UASB reactor: Links between microbial community and reactor performance. Int. J. Hydrog. Energy 2009, 34, 5674–5682. [Google Scholar] [CrossRef]
- Perna, V.; Castelló, E.; Wenzel, J.J.; Zampol, C.; Lima, D.F.; Borzacconi, L.; Varesche, M.; Zaiat, M.; Etchebehere, C. Hydrogen production in an upflow anaerobic packed bed reactor used to treat cheese whey. Int. J. Hydrog. Energy 2013, 38, 54–62. [Google Scholar] [CrossRef]
- Rosa, P.R.F.; Santos, S.C.; Sakamoto, I.K.; Varesche, M.B.A.; Silva, E.L. Hydrogen production from cheese whey with ethanol-type fermentation: Effect of hydraulic retention time on the microbial community composition. Bioresour. Technol. 2014, 161, 10–19. [Google Scholar] [CrossRef]
- Ottaviano, L.M.; Ramos, L.R.; Botta, L.S.; Varesche, M.B.A.; Silva, E.L. Continuous thermophilic hydrogen production from cheese whey powder solution in an anaerobic fluidized bed reactor: Effect of hydraulic retention time and initial substrate concentration. Int. J. Hydrog. Energy 2017, 42, 4848–4860. [Google Scholar] [CrossRef]
- Bakonyi, P.; Nemestóthy, N.; Simon, V.; Bélafi-Bakó, K. Review on the start-up experiences of continuous fermentative hydrogen producing bioreactors. Renew. Sustain. Energy Rev. 2014, 40, 806–813. [Google Scholar] [CrossRef]
- Lima, D.M.F.; Inoue, R.K.; Rodrigues, J.A.D.; Ratusznei, S.M.; Zaiat, M. Biohydrogen from Cheese whey Treatment in an AnSBBR: Achieving Process Stability. Braz. J. Chem. Eng. 2015, 32, 397–408. [Google Scholar] [CrossRef][Green Version]
- Pandey, A.; Srivastava, S.; Rai, P.; Duke, M. Cheese whey to biohydrogen and useful organic acids: A non-pathogenic microbial treatment by L. acidophilus. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mikheeva, E.R.; Katraeva, I.V.; Vorozhtsov, D.L.; Litti, Y.V.; Nozhevnikova, A.N. Efficiency of Two-Phase Anaerobic Fermentation and the Physicochemical Properties of the Organic Fraction of Municipal Solid Waste Processed in a Vortex-Layer Apparatus. Appl. Biochem. Microbiol. 2020, 56, 736–742. [Google Scholar] [CrossRef]
- Lever, M.A.; Etorti, A.; Eeickenbusch, P.; Michaud, A.B.; Antl-Temkiv, T.; Jørgensen, B.B. A modular method for the extraction of DNA and RNA, and the separation of DNA pools from diverse environmental sample types. Front. Microbiol. 2015, 6, 476. [Google Scholar] [CrossRef] [PubMed]
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Brotman, R.M.; Ravel, J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014, 2, 6. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Ren, N.-Q.; Guo, W.-Q.; Wang, X.-J.; Xiang, W.-S.; Liu, B.-F.; Ding, J.; Chen, Z.-B. Effects of different pretreatment methods on fermentation types and dominant bacteria for hydrogen production. Int. J. Hydrog. Energy 2008, 33, 4318–4324. [Google Scholar] [CrossRef]
- Wang, J.; Wan, W. Comparison of different pretreatment methods for enriching hydrogen-producing bacteria from digested sludge. Int. J. Hydrog. Energy 2008, 33, 2934–2941. [Google Scholar] [CrossRef]
- Liu, D. Bio-Hydrogen Production by Dark Fermentation from Organic Wastes and Residues. Ph.D. Thesis, Technical University of Denmark, Kongens Lyngby, Denmark, 2008. [Google Scholar]
- Ruggeri, B.; Tommasi, T.; Sanfilippo, S. BioH2 & BioCH4 through Anaerobic Digestion, 1st ed.; Springer: London, UK, 2015; p. 218. [Google Scholar]
- Bundhoo, M.Z.; Mohee, R. Inhibition of dark fermentative bio-hydrogen production: A review. Int. J. Hydrog. Energy 2016, 41, 6713–6733. [Google Scholar] [CrossRef]
- Wang, S.; Ma, F.; Ma, W.; Wang, P.; Zhao, G.; Lu, X. Influence of Temperature on Biogas Production Efficiency and Microbial Community in a Two-Phase Anaerobic Digestion System. Water 2019, 11, 133. [Google Scholar] [CrossRef]
- Xiao, L.; Deng, Z.; Fung, K.Y.; Ng, K.M. Biohydrogen generation from anaerobic digestion of food waste. Int. J. Hydrog. Energy 2013, 38, 13907–13913. [Google Scholar] [CrossRef]
- Noike, T. Inhibition of hydrogen fermentation of organic wastes by lactic acid bacteria. Int. J. Hydrog. Energy 2002, 27, 1367–1371. [Google Scholar] [CrossRef]
- Fernández, C.; Carracedo, B.; Martínez, E.J.; Gómez, X.; Morán, A. Application of a packed bed reactor for the production of hydrogen from cheese whey permeate: Effect of organic loading rate. J. Environ. Sci. Health Part A 2013, 49, 210–217. [Google Scholar] [CrossRef]
- Blanco, V.; Oliveira, G.H.D.; Zaiat, M. Dark fermentative biohydrogen production from synthetic cheese whey in an anaerobic structured-bed reactor: Performance evaluation and kinetic modeling. Renew. Energy 2019, 139, 1310–1319. [Google Scholar] [CrossRef]
- Davila-Vazquez, G.; Cota-Navarro, C.B.; Rosales-Colunga, L.M.; De Leon-Rodriguez, A.; Razo-Flores, E. Continuous biohydrogen production using cheese whey: Improving the hydrogen production rate. Int. J. Hydrog. Energy 2009, 34, 4296–4304. [Google Scholar] [CrossRef]
- Fernandes, B.S.; Saavedra, N.K.; Maintinguer, S.I.; Sette, L.D.; Oliveira, V.M.; Varesche, M.B.A.; Zaiat, M. The Effect of Biomass Immobilization Support Material and Bed Porosity on Hydrogen Production in an Upflow Anaerobic Packed-Bed Bioreactor. Appl. Biochem. Biotechnol. 2013, 170, 1348–1366. [Google Scholar] [CrossRef]
- Lee, K.-S.; Lo, Y.-S.; Lo, Y.-C.; Lin, P.-J.; Chang, J.-S. H2 production with anaerobic sludge using activated-carbon supported packed-bed bioreactors. Biotechnol. Lett. 2003, 25, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Cho, K.-S. Effect of hydraulic retention time on suppression of methanogens during a continuous biohydrogen production process using molasses wastewater. J. Environ. Sci. Health Part A 2016, 52, 1–8. [Google Scholar] [CrossRef]
- Zhang, B.; Cai, W.-M.; He, P.-J. Influence of lactic acid on the two-phase anaerobic digestion of kitchen wastes. J. Environ. Sci. 2007, 19, 244–249. [Google Scholar] [CrossRef]
- Doyle, N.; Mbandlwa, P.; Kelly, W.J.; Attwood, G.; Li, Y.; Ross, R.P.; Stanton, C.; Leahy, S.C. Use of Lactic Acid Bacteria to Reduce Methane Production in Ruminants, a Critical Review. Front. Microbiol. 2019, 10, 2207. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-Pérez, C.; Carrillo-Reyes, J.; Celis, L.B.; Alatriste-Mondragón, F.; Etchebehere, C.; Razo-Flores, E. Inoculum pretreatment promotes differences in hydrogen production performance in EGSB reactors. Int. J. Hydrog. Energy 2015, 40, 6329–6339. [Google Scholar] [CrossRef]
- Sivarajan, A.; Shanmugasundaram, T.; Thirumalairaj, J.; Balagurunathan, R. Production and optimization of biohydrogen from saccharolytic actinobacterium, Streptomyces rubiginosus (SM16), using sugarcane molasses. Biofuels 2016, 8, 717–723. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, R.; McGarvey, J.; Benemann, J. Biohydrogen production from cheese processing wastewater by anaerobic fermentation using mixed microbial communities. Int. J. Hydrog. Energy 2007, 32, 4761–4771. [Google Scholar] [CrossRef]
- Gomez-Romero, J.; Gonzalez-Garcia, A.; Chairez, I.; Torres, L.G.; García-Peña, E. Selective adaptation of an anaerobic microbial community: Biohydrogen production by co-digestion of cheese whey and vegetables fruit waste. Int. J. Hydrog. Energy 2014, 39, 12541–12550. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Hung, C.-H.; Lee, K.-S.; Liau, P.-Y.; Liang, C.-M.; Yang, L.-H.; Lin, P.-J.; Lin, C.-Y. Microbial community structure of a starch-feeding fermentative hydrogen production reactor operated under different incubation conditions. Int. J. Hydrog. Energy 2008, 33, 5242–5249. [Google Scholar] [CrossRef]


| Parameter | Units | Value | |||||
|---|---|---|---|---|---|---|---|
| Stage | - | S-I | S-II | S-III | S-IV | S-V | S-VI |
| Time | D | 1–30 | 31–50 | 51–66 | 67–80 | 81–90 | 91–100 |
| HRT | D | 14.5 | 9.1 | 5.6 | 4.5 | 3.6 | 2 |
| OLR | kg COD/(m3 d) | 2.07 | 3.31 | 5.37 | 6.61 | 8.26 | 14.88 |
| Inoculum | Final pH | H2 in Biogas Max, % | CH4 in Biogas Max, % | COD Removal, % |
|---|---|---|---|---|
| Inoculum 1 untreated (Control 1) | 6.67 | 0 | 16.5 (5) * | 71.4 |
| Inoculum 1 after acid pretreatment | 3.94 | 33.3 (1) * | 0 | 65.2 |
| Inoculum 1 after thermal pretreatment | 6.37 | 8.0 (2) * | 7.5 (8) * | 46.2 |
| Inoculum 2 untreated (Control 2) | 5.4 | 9.3 (1) * | 20.1(1) * | 66.7 |
| Inoculum 2 after acid pretreatment | 4.04 | 0.8 (1) * | 17.0 (1) * | 63.8 |
| Inoculum 2 after thermal pretreatment | 3.92 | 10.7 (1) * | 0 | 43.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikheeva, E.R.; Katraeva, I.V.; Kovalev, A.A.; Kovalev, D.A.; Nozhevnikova, A.N.; Panchenko, V.; Fiore, U.; Litti, Y.V. The Start-Up of Continuous Biohydrogen Production from Cheese Whey: Comparison of Inoculum Pretreatment Methods and Reactors with Moving and Fixed Polyurethane Carriers. Appl. Sci. 2021, 11, 510. https://doi.org/10.3390/app11020510
Mikheeva ER, Katraeva IV, Kovalev AA, Kovalev DA, Nozhevnikova AN, Panchenko V, Fiore U, Litti YV. The Start-Up of Continuous Biohydrogen Production from Cheese Whey: Comparison of Inoculum Pretreatment Methods and Reactors with Moving and Fixed Polyurethane Carriers. Applied Sciences. 2021; 11(2):510. https://doi.org/10.3390/app11020510
Chicago/Turabian StyleMikheeva, Elza R., Inna V. Katraeva, Andrey A. Kovalev, Dmitriy A. Kovalev, Alla N. Nozhevnikova, Vladimir Panchenko, Ugo Fiore, and Yuri V. Litti. 2021. "The Start-Up of Continuous Biohydrogen Production from Cheese Whey: Comparison of Inoculum Pretreatment Methods and Reactors with Moving and Fixed Polyurethane Carriers" Applied Sciences 11, no. 2: 510. https://doi.org/10.3390/app11020510
APA StyleMikheeva, E. R., Katraeva, I. V., Kovalev, A. A., Kovalev, D. A., Nozhevnikova, A. N., Panchenko, V., Fiore, U., & Litti, Y. V. (2021). The Start-Up of Continuous Biohydrogen Production from Cheese Whey: Comparison of Inoculum Pretreatment Methods and Reactors with Moving and Fixed Polyurethane Carriers. Applied Sciences, 11(2), 510. https://doi.org/10.3390/app11020510










