Size Effect of Mesoporous Silica Nanoparticles on Pesticide Loading, Release, and Delivery in Cucumber Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Carbon Quantum Dots-MSN with Different Sizes
2.3. Construction of Pyr@M15, Pyr@M100, and Pyr@M200
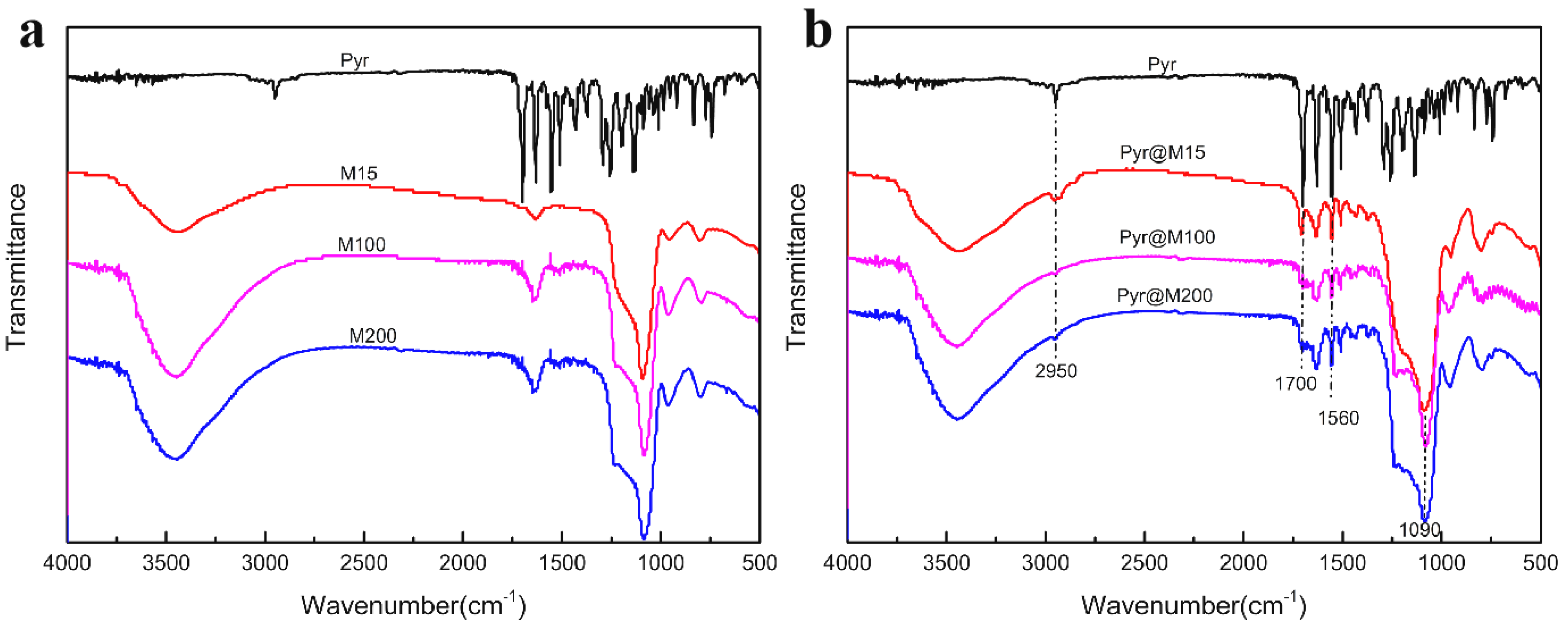
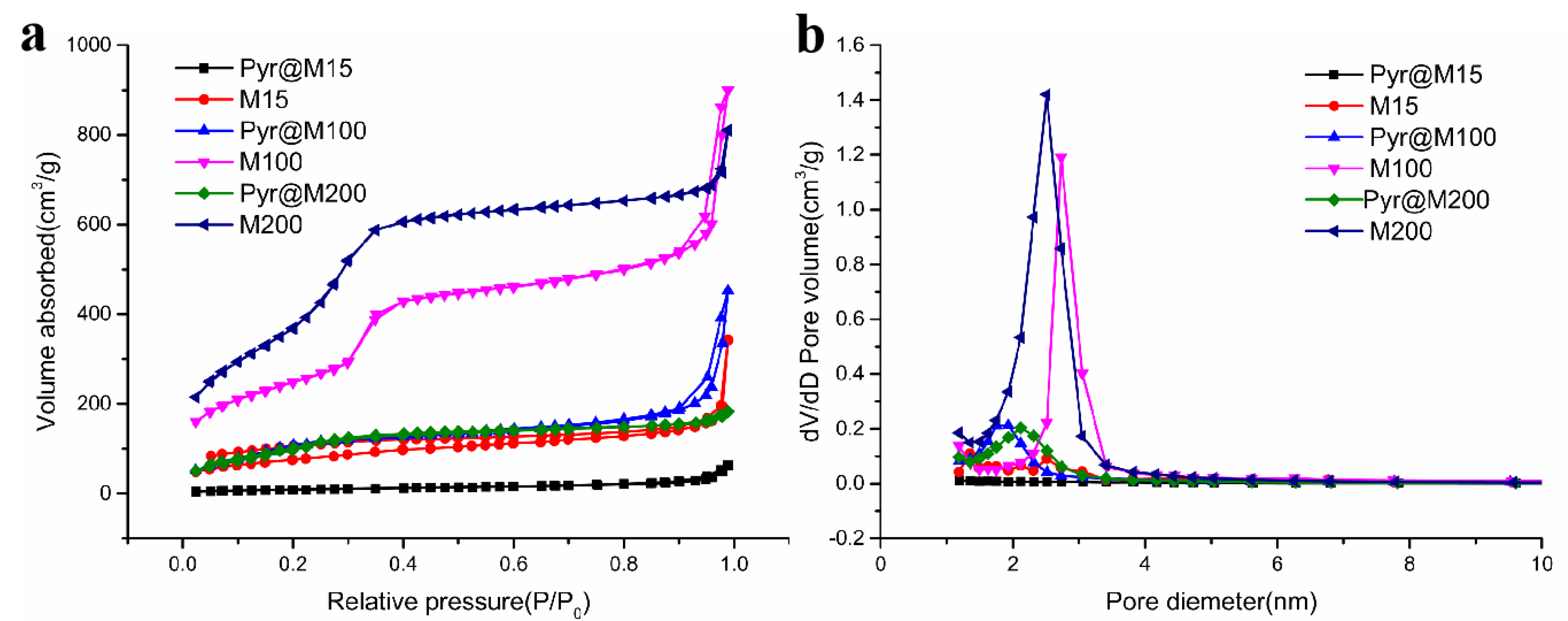
2.4. Sample Characterization
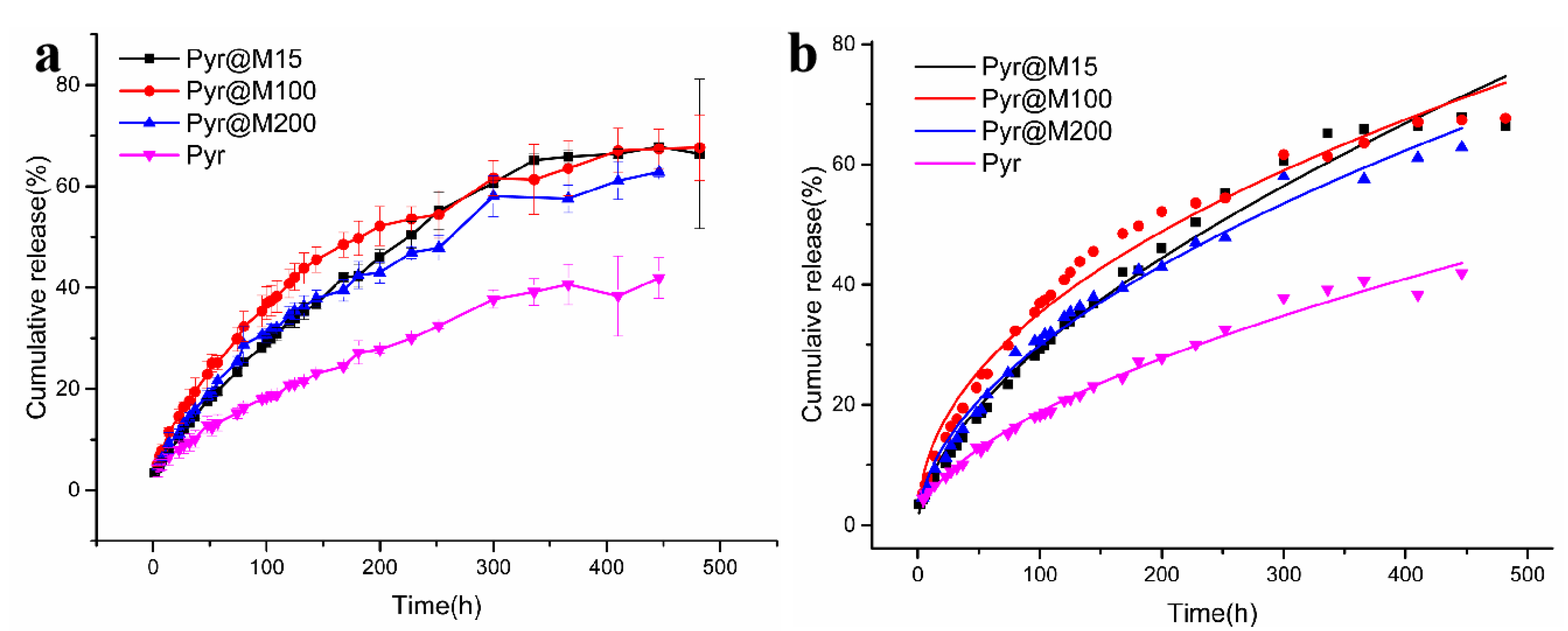
2.5. In Vitro Release of Pyr from Pyr@M15, Pyr@M100, and Pyr@M200
- Er: the accumulative release percentage of Pyr, %;
- Ve: the volume of the release medium taken out at each sampling, Ve = 1 mL;
- V0: the total volume of release solution, V0 = 200 mL;
- Cn: the concentration of Pyr in release medium at sampling time n, mg/L;
- mpesticide: the total mass of Pry in Pry@M, mg.
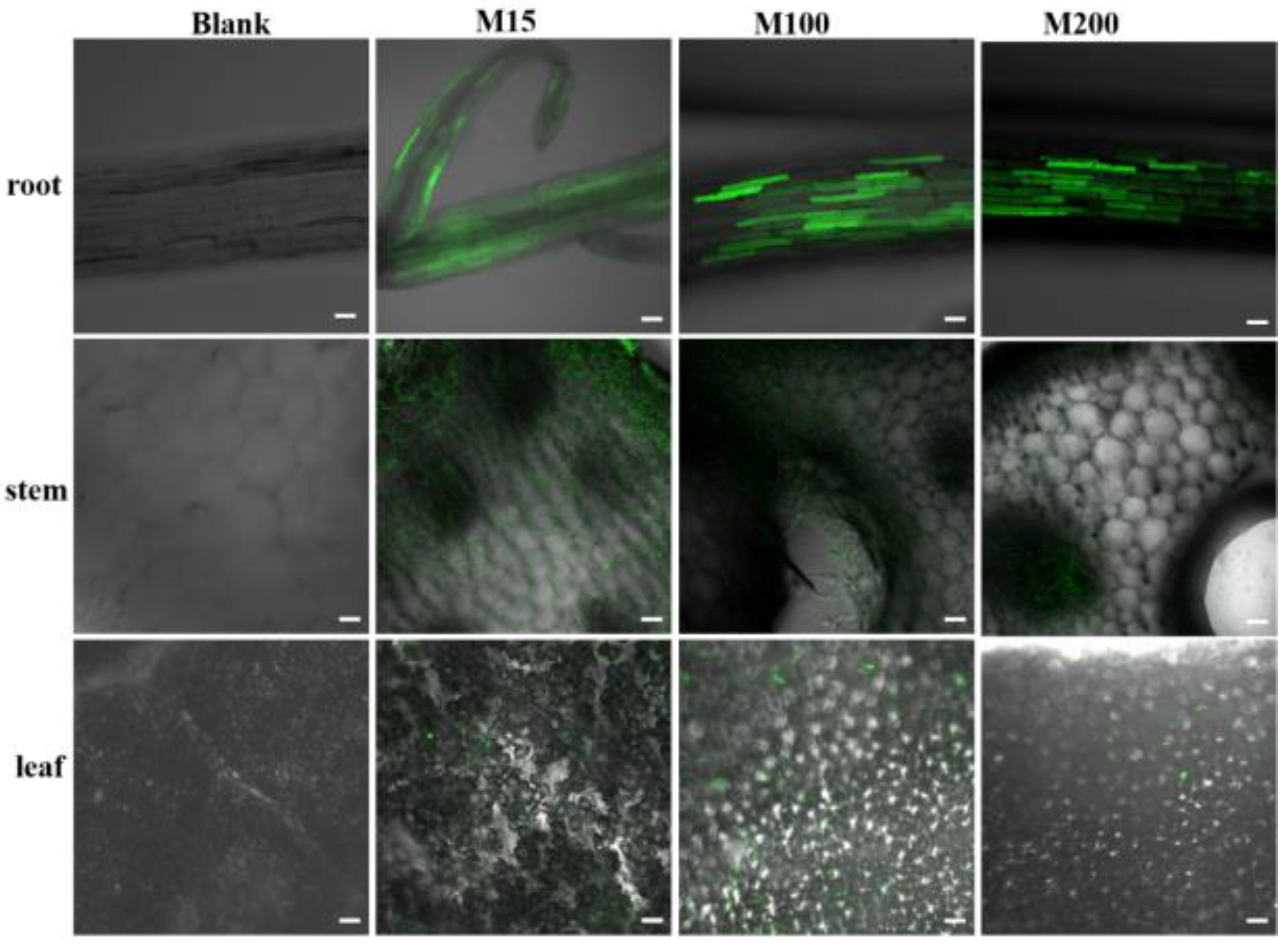
2.6. Translocation of Carbon Quantum Dots-MSN with Different Sizes in Cucumber Plants
2.7. Uptake and Dose Distribution of Pyr in Cucumber Plants
2.8. Analysis Method of Pyr in Cucumber
2.9. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of Carbon Quantum dots-MSN, Pyr@M15, Pyr@M100, and Pyr@M200
3.2. Opitimization of Pyr Loading Content
3.3. Controlled Release
3.4. Transportation of MSN in Cucumber Plants
3.5. Analytical Method Validation
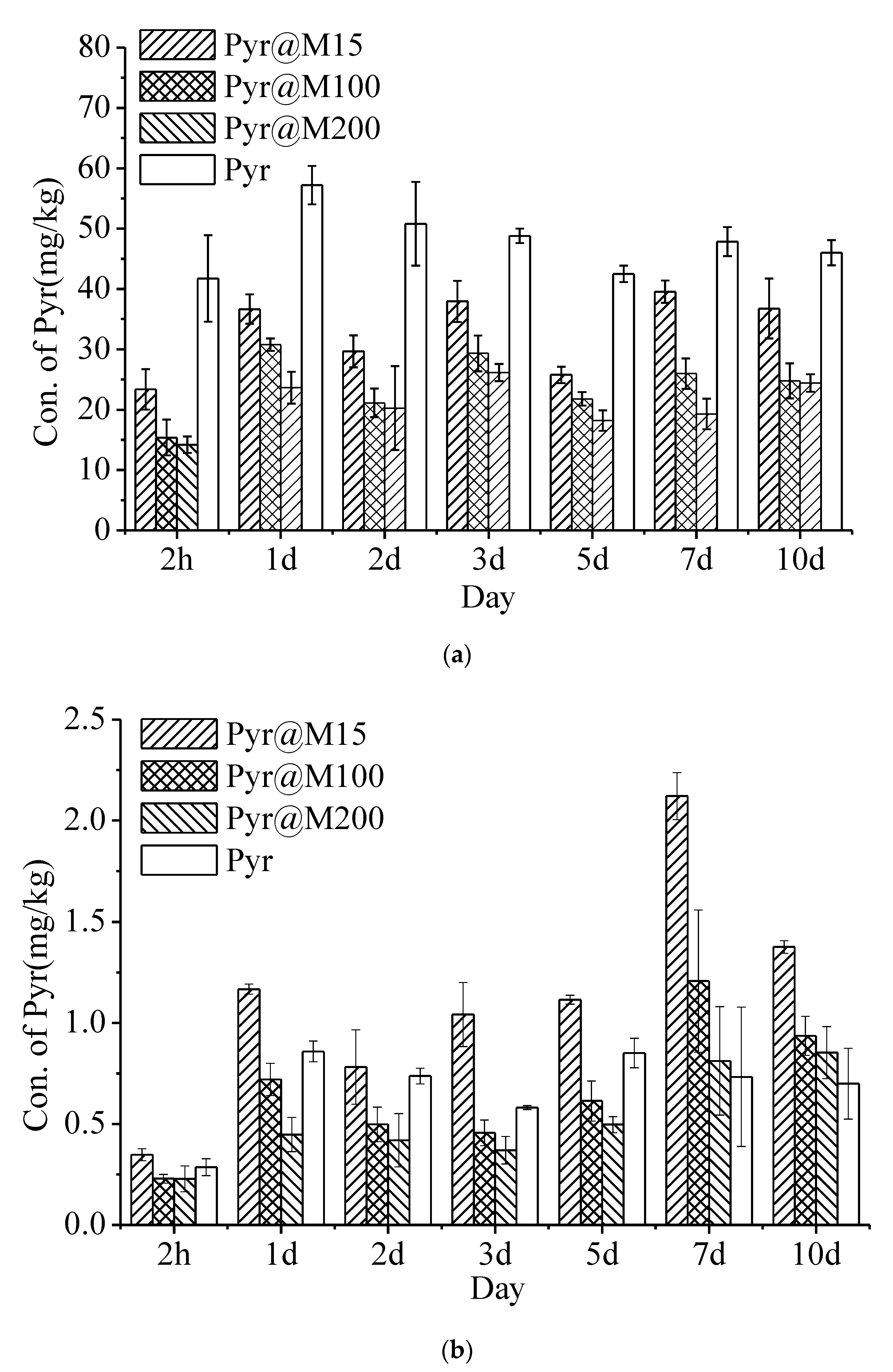
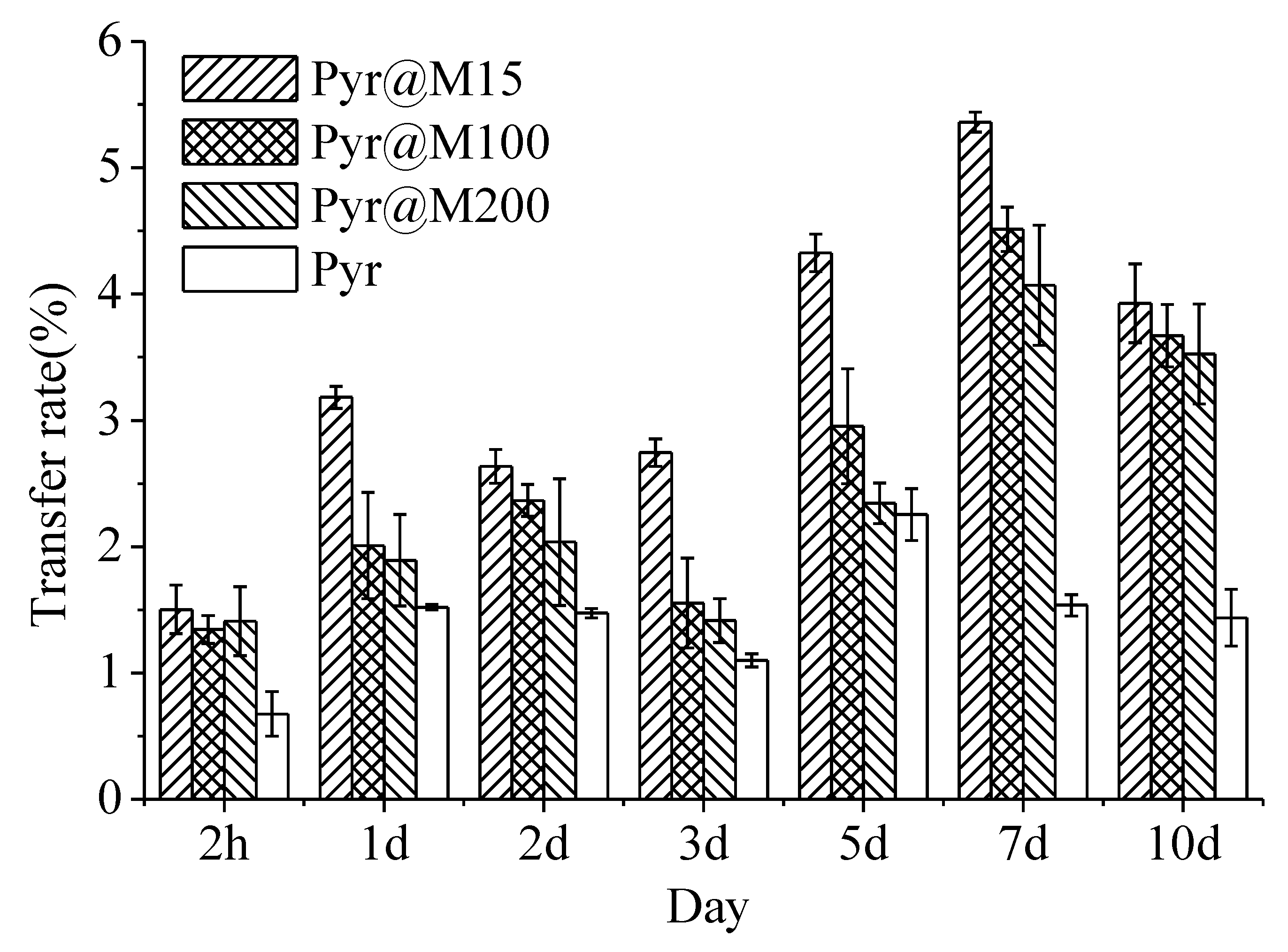
3.6. Uptake and Distribution of Pyr@MSN in Cucumber Plants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Guan, W.; Tang, L.; Zhang, W.; Cui, H. Fabrication of novel avermectin nanoemulsion using a polyurethane emulsifier with cleavable disulfide bonds. J. Agric. Food Chem. 2018, 66, 6569–6577. [Google Scholar]
- Zhu, F.; Liu, X.; Cao, L.; Cao, C.; Li, F.; Chen, C.; Xu, C.; Huang, Q.; Du, F. Uptake and distribution of fenoxanil-Loaded mesoporous silica nanoparticles in rice plants. Int. J. Mol. Sci. 2018, 19, 2854. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xi, W.; Su, Q.; Yan, G.; Liu, Y.; Wang, H.; Cao, A. Unexpected size effect: The interplay between different sized nanoparticles in their cellular uptake. Small 2019, 15, 1901687. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Marhadour, S.; Lei, Z.; Yang, W.; Marivingt-Mounir, C.; Bonnemain, J.; Chollet, J. Vectorization of agrochemicals: Amino acid carriers are more efficient than sugar carriers to translocate phenylpyrrole conjugates in the Ricinus system. Environ. Sci. Pollut. R. 2018, 25, 14336–14349. [Google Scholar] [CrossRef]
- Mao, G.L.; Yan, Y.; Chen, Y.; Wang, B.F.; Xu, F.F.; Zhang, Z.X.; Lin, F.; Xu, H.H. Family of Ricinus communis Monosaccharide Transporters and RcSTP1 in Promoting the Uptake of a Glucose-Fipronil Conjugate. J. Agric. Food Chem. 2017, 65, 6169–6178. [Google Scholar] [CrossRef]
- Wu, H.; Xu, H.; Marivingt Mounir, C.; Bonnemain, J.L.; Chollet, J.F. Vectorizing agrochemicals: Enhancing bioavailability via carrier-mediated transport. Pest Manag. Sci. 2019, 75, 1507–1516. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, C.; Qian, J.; Wang, J.; Zhang, Y. Size-mediated cytotoxicity and apoptosis of hydroxyapatite nanoparticles in human hepatoma HepG2 cells. Biomaterials 2010, 31, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Popat, A.; Liu, J.; Hu, Q.; Kennedy, M.; Peters, B.; Lu, G.Q.M.; Qiao, S.Z. Adsorption and release of biocides with mesoporous silica nanoparticles. Nanoscale 2012, 4, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Fan, W.; Jiang, S.; Ma, Y.; Lu, Y.; Qi, J.; Ahmad, E.; Dong, X.; Zhao, W.; Wu, W. Size-dependent translocation of nanoemulsions via oral delivery. ACS Appl. Mater. Inter. 2017, 9, 21660–21672. [Google Scholar] [CrossRef]
- Lu, F.; Wu, S.; Hung, Y.; Mou, C. Size effect on cell uptake in well-suspended, uniform mesoporous silica nanoparticles. Small 2009, 5, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Cruje, C.; Chithrani, B.D. Integration of peptides for enhanced uptake of PEG ylayed gold nanoparticles. J. Nanosci. Nanotechnol. 2015, 15, 2125–2131. [Google Scholar] [CrossRef]
- Khot, L.R.; Sankaran, S.; Maja, J.M.; Ehsani, R.; Schuster, E.W. Applications of nanomaterials in agricultural production and crop protection: A review. Crop Prot. 2012, 35, 64–70. [Google Scholar] [CrossRef]
- Xiang, Y.; Han, J.; Zhang, G.; Zhan, F.; Cai, D.; Wu, Z. Efficient synthesis of starch-regulated porous calcium carbonate microspheres as a Carrier for slow-release herbicide. ACS Sustain Chem. Eng. 2018, 6, 3649–3658. [Google Scholar] [CrossRef]
- Wang, L.; Lei, L.; Kong, T.; Zhu, P.; Tian, X.; Kang, Z. Self-Assembly of TiO2 nanofiber-based microcapsules by spontaneously evolved multiple emulsions. Langmuir 2018, 34, 8785–8791. [Google Scholar]
- Li, Z.; Xu, S.; Wen, L.; Liu, F.; Liu, A.; Wang, Q.; Sun, H.; Yu, W.; Chen, J. Controlled release of avermectin from porous hollow silica nanoparticles: Influence of shell thickness on loading efficiency, UV-shielding property and release. J. Control Release 2006, 111, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Trofimov, A.D.; Ivanova, A.A.; Zyuzin, M.V.; Timin, A.S. Porous inorganic carriers based on silica, calcium carbonate and calcium phosphate for controlled/modulated drug delivery: Fresh outlook and future perspectives. Pharmaceutics 2018, 10, 167. [Google Scholar] [CrossRef]
- Assadi, Z.; Emtiazi, G.; Zarrabi, A. Hyperbranched polyglycerol coated on copper oxide nanoparticles as a novel core-shell nano-carrier hydrophilic drug delivery model. J. Mol. Liq. 2018, 250, 375–380. [Google Scholar] [CrossRef]
- Ziaee, M.; Moharramipour, S.; Mohsenifar, A. MA-chitosan nanogel loaded with Cuminum cyminum essential oil for efficient management of two stored product beetle pests. J. Pest Sci. 2014, 87, 691–699. [Google Scholar] [CrossRef]
- Sun, H.; Liu, S.; Zeng, X.; Meng, X.; Zhao, L.; Wan, Y.; Zuo, G. Morphology effect of nano-hydroxyapatite as a drug carrier of methotrexate. J. Mater. Sci.: Mater. Med. 2017, 28, 158. [Google Scholar] [CrossRef]
- Shan, Y.; Cao, L.; Xu, C.; Zhao, P.; Cao, C.; Li, F.; Xu, B.; Huang, Q. Sulfonate-functionalized mesoporous silica nanoparticles as carriers for controlled herbicide diquat dibromide release through electrostatic interaction. Int. J. Mol. Sci. 2019, 20, 1330. [Google Scholar] [CrossRef]
- Gan, Q.; Dai, D.; Yuan, Y.; Qian, J.; Sha, S.; Shi, J.; Liu, C. Effect of size on the cellular endocytosis and controlled release of mesoporous silica nanoparticles for intracellular delivery. Biomed. Microdevices 2012, 14, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Rascol, E.; Pisani, C.; Dorandeu, C.; Nyalosaso, J.; Charnay, C.; Daurat, M.; Silva, A.; Devoisselle, J.; Gaillard, J.; Armengaud, J.; et al. Biosafety of Mesoporous Silica Nanoparticles. Biomimetics 2018, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Derakhshankhah, H.; Alaei, L.; Fattahi, A.; Varnamkhasti, B.S.; Saboury, A.A. Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomed. Pharmacother. 2019, 109, 1100–1111. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Liang, Y.; Erichsen, E.S.; Anwander, R. Hierarchical mesoporous organosilica–silica core–shell nanoparticles capable of controlled fungicide release. Chemistry 2018, 24, 7200–7209. [Google Scholar] [CrossRef]
- Liang, Y.; Gao, Y.; Wang, W.; Dong, H.; Tang, R.; Yang, J.; Niu, J.; Zhou, Z.; Jiang, N.; Cao, Y.J. Fabrication of smart stimuli-responsive mesoporous organosilica nano-vehicles for targeted pesticide delivery. Hazard. Mater. 2020, 389, 122075. [Google Scholar] [CrossRef]
- Chang, F.; Kuang, L.; Huang, C.; Jane, W.; Hung, Y.; Hsing, Y.C.; Mou, C. A simple plant gene delivery system using mesoporous silica nanoparticles as carriers. J. Mater. Chem. B 2013, 1, 5279. [Google Scholar] [CrossRef]
- Slomberg, D.L.; Schoenfisch, M.H. Silica nanoparticle phytotoxicity to arabidopsis thaliana. Environ. Sci. Technol. 2012, 46, 10247–10254. [Google Scholar]
- Sun, D.; Hussain, H.I.; Yi, Z.; Siegele, R.; Cresswell, T.; Kong, L.; Cahill, D.M. Uptake and cellular distribution, in four plant species, of fluorescently labeled mesoporous silica nanoparticles. Plant Cell Rep. 2014, 33, 1389–1402. [Google Scholar] [CrossRef]
- Zhao, P.; Yuan, W.; Xu, C.; Li, F.; Cao, L.; Huang, Q. Enhancement of spirotetramat transfer in cucumber plant using mesoporous silica nanoparticles as carriers. J. Agric. Food Chem. 2018, 66, 11592–11600. [Google Scholar] [CrossRef]
- Dietz, K.; Herth, S. Plant nanotoxicology. Trends Plant Sci. 2011, 16, 582–589. [Google Scholar] [CrossRef]
- Zhao, X.; Cui, H.; Wang, Y.; Sun, C.; Cui, B.; Zeng, Z. Development Strategies and Prospects of nano-based Smart Pesticide Formulation. J. Agric. Food Chem. 2018, 66, 6504–6512. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Yu, W.; Pang, Y.; Sun, C.; Meng, Q.; Li, X.; Guo, X. Validation of an LC-MS/MS method for the quantitative determination of a novel fungicide: Pyraoxystrobin in rat plasma and its application to a toxicokinetics study. Anal. Bioanal. Chem. 2014, 406, 5521–5526. [Google Scholar] [CrossRef]
- Yang, X.; Liu, H.; Jia, M.; Wang, J.; Wu, J.; Song, J.; Liu, Y. Evaluation of pyraoxystrobin bioconcentration in zebrafish (Danio rerio) using modified QuEChERS extraction. Journal of environmental science and health. J. Environ. Sci. Heath Part B Pesticides Food Contam. Agric. Wastes 2020, 55, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ye, Q.; Kan, D.; Zhang, Z.; Ding, X. Synthesis of carbon-14 labeled pyraoxystrobin, a novel fungicide. J. Label. Compd. Rad. 2011, 54, 780–782. [Google Scholar] [CrossRef]
- Yang, T.; Xu, C.; Liu, X.; Chen, X.; Zhang, J.; Ding, X. Fate of a novel strobilurin fungicide pyraoxystrobin in flooded soil. Environ. Sci. Proc. Imp. 2014, 16, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, H.; Hu, T.; Chen, X.; Ding, X. Adsorption and leaching of novel fungicide pyraoxystrobin on soils by 14C tracing method. Environ. Monit. Assess. 2018, 190, 86. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Ding, X.; Wu, H. Uptake and distribution characteristics of the novel fungicide pyraoxystrobin in cucumber plants. RSC Adv. 2018, 8, 27152–27156. [Google Scholar] [CrossRef]
- Zhao, P.; Cao, L.; Ma, D.; Zhou, Z.; Huang, Q.; Pan, C. Translocation, distribution and degradation of prochloraz-loaded mesoporous silica nanoparticles in cucumber plants. Nanoscale 2018, 10, 1798–1806. [Google Scholar] [CrossRef]
- Zhao, P.; Cao, L.; Ma, D.; Zhou, Z.; Huang, Q.; Pan, C. Synthesis of Pyrimethanil-Loaded Mesoporous Silica Nanoparticles and Its Distribution and Dissipation in Cucumber Plants. Molecules 2017, 22, 817. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Lisuzzo, L.; Milioto, S.; Parisi, F. Selective adsorption of oppositely charged PNIPAAM on halloysite surfaces: A route to thermo-responsive nanocarriers. Nanotechnology 2018, 29, 325702. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, M.; Rocher, F.; Legros, S.; Bonnemain, J.; Chollet, J. A non-destructive method for testing two components of the behaviour of soil-applied agricultural chemicals over a long period. Pest Manag. Sci. 2012, 68, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; He, X.; Zhang, H.; Ma, Y.; Zhang, P.; Ding, Y.; Zhao, Y. Uptake and distribution of ceria nanoparticles in cucumber plants. Metallomics 2011, 3, 816–822. [Google Scholar] [CrossRef]
- Yazdimamaghani, M.; Barber, Z.B.; Hadipour Moghaddam, S.P.; Ghandehari, H. Influence of silica nanoparticle density and flow conditions on sedimentation, cell uptake, and cytotoxicity. Mol. Pharmaceut. 2018, 15, 2372–2383. [Google Scholar] [CrossRef]
- Chou, C.; Chen, W.; Hung, Y.; Mou, C. Molecular elucidation of biological response to mesoporous silica nanoparticles in vitro and in vivo. ACS Appl. Mater. Inter. 2017, 9, 22235–22251. [Google Scholar] [CrossRef] [PubMed]


| Sample | CTAB (g) | NaOH (mL) | Water (mL) | TSD (mL) | Temperature (°C) | Particle Size (nm) |
|---|---|---|---|---|---|---|
| M15 | 1.2 | 1 | 576 | 0.84 | 80 | 15 |
| M100 | 1.2 | 4.2 | 576 | 0.84 | 80 | 100 |
| M200 | 1.2 | 4.2 | 288 | 0.84 | 80 | 200 |
| Nanoparticle | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| M15 | 342.09 | 0.091 | 1.487 |
| M100 | 900.01 | 0.222 | 2.743 |
| M200 | 810.47 | 1.42 | 2.524 |
| Pyr@M15 | 63.12 | 0.00636 | 1.35 |
| Pyr@M100 | 452.05 | 0.0415 | 1.879 |
| Pyr@M200 | 182.90 | 0.120 | 2.117 |
| Carrier Material | Solvent | Mass Ratio a | Loading Content (%) b |
|---|---|---|---|
| M100 | Acetonitrile | 2:1 | 4.25 ± 0.68 |
| M100 | Methanol | 2:1 | 5.86 ± 1.66 |
| M100 | Dichloromethane | 2:1 | 18.86 ± 0.61 |
| M15 | Dichloromethane | 2:1 | 8.8 ± 0.07 |
| M100 | Dichloromethane | 2:1 | 18.86 ± 0.61 |
| M200 | Dichloromethane | 2:1 | 23.06 ± 0.43 |
| M15 | Dichloromethane | 5:1 | 23.43 ± 0.42 |
| M100 | Dichloromethane | 3:1 | 22.63 ± 0.23 |
| M200 | Dichloromethane | 2:1 | 23.06 ± 0.43 |
| Sample | k/min−n | n | R2 |
|---|---|---|---|
| Pyr | 1.40235 ± 0.099 | 0.56322 ± 0.013 | 0.98953 |
| Pyr@M15 | 1.94883 ± 0.174 | 0.59100 ± 0.016 | 0.98659 |
| Pyr@M100 | 4.11986 ± 0.331 | 0.46659 ± 0.015 | 0.98049 |
| Pyr@M200 | 2.60443 ± 0.181 | 0.52989 ± 0.013 | 0.98871 |
| Sample | Fortified Level (mg·kg−1) | Average Recoveries (%) | RSD (%) | LOD (μg·kg−1) | Linear Equation | R2 |
|---|---|---|---|---|---|---|
| Leaf | 0.01 | 74 | 0.54 | 0.1 | Y = 153,971,544 x + 1,292,542 | 0.9997 |
| 0.1 | 75 | 4.16 | ||||
| 1 | 81 | 7.36 | ||||
| root | 0.01 | 97 | 5.46 | 0.1 | Y = 155,065,913 x + 16,332,063 | 0.9996 |
| 0.1 | 90 | 5.62 | ||||
| 1 | 87 | 2.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Xu, C.; Huang, Q.; Cao, L.; Teng, F.; Zhao, P.; Jia, M. Size Effect of Mesoporous Silica Nanoparticles on Pesticide Loading, Release, and Delivery in Cucumber Plants. Appl. Sci. 2021, 11, 575. https://doi.org/10.3390/app11020575
Xu Y, Xu C, Huang Q, Cao L, Teng F, Zhao P, Jia M. Size Effect of Mesoporous Silica Nanoparticles on Pesticide Loading, Release, and Delivery in Cucumber Plants. Applied Sciences. 2021; 11(2):575. https://doi.org/10.3390/app11020575
Chicago/Turabian StyleXu, Yongbing, Chunli Xu, Qiliang Huang, Lidong Cao, Feifei Teng, Pengyue Zhao, and Minghong Jia. 2021. "Size Effect of Mesoporous Silica Nanoparticles on Pesticide Loading, Release, and Delivery in Cucumber Plants" Applied Sciences 11, no. 2: 575. https://doi.org/10.3390/app11020575
APA StyleXu, Y., Xu, C., Huang, Q., Cao, L., Teng, F., Zhao, P., & Jia, M. (2021). Size Effect of Mesoporous Silica Nanoparticles on Pesticide Loading, Release, and Delivery in Cucumber Plants. Applied Sciences, 11(2), 575. https://doi.org/10.3390/app11020575





