Fructans with Varying Degree of Polymerization Enhance the Selective Growth of Bifidobacterium animalis subsp. lactis BB-12 in the Human Gut Microbiome In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Test Product
2.2. Strain Preservation and Quality Control
2.3. Fermentation of Fructans by BB-12 in Monoculture during Short-Term Colonic Incubations (Experiment 1)
2.4. Donor Screening (Experiment 2)
2.5. Fermentation of Fructans by BB-12 and Complex Microbiota during Short-Term Colonic Incubations (Experiment 3)
2.6. Analysis of Microbial Metabolic Activity
2.7. Analysis of Microbial Community Composition
2.8. Data and Statistical Analysis
3. Results
3.1. Fermentation of Fructan-Type Carbohydrates by BB-12 in Monoculture (Experiment 1)
3.2. Donor Screening (Experiment 2)
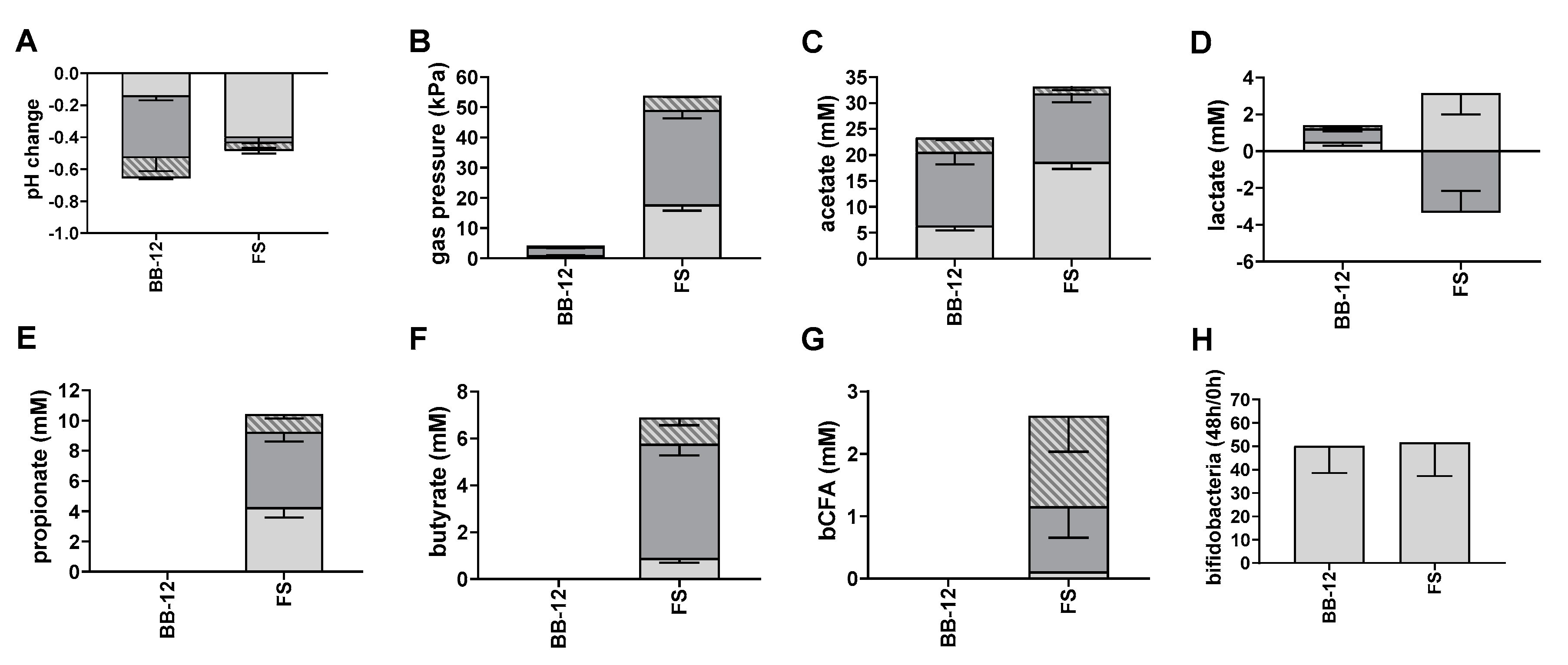
3.3. Fermentation of Fructan-Type Carbohydrates by BB-12 and Complex Microbiota (Experiment 3)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Smid, A.; Strniša, L.; Bajc, K.; Vujić-Podlipec, D.; Matijasic, B.; Rogelj, I. Randomized clinical trial: The effect of fermented milk with the probiotic cultures Lactobacillus acidophilus La-5® and Bifidobacterium BB-12® and Beneo dietary fibres on health-related quality of life and the symptoms of irritable bowel syndrome in adults. J. Funct. Foods 2016, 24, 549–557. [Google Scholar] [CrossRef]
- Fujimori, S.; Gudis, K.; Mitsui, K.; Seo, T.; Yonezawa, M.; Tanaka, S.; Tatsuguchi, A.; Sakamoto, C. A randomized controlled trial on the efficacy of synbiotic versus probiotic or prebiotic treatment to improve the quality of life in patients with ulcerative colitis. Nutrition 2009, 25, 520–525. [Google Scholar] [CrossRef]
- Chang, Y.S.; Trivedi, M.K.; Jha, A.; Lin, Y.F.; Dimaano, L.; García-Romero, M.T. Synbiotics for Prevention and Treatment of Atopic Dermatitis: A Meta-analysis of Randomized Clinical Trials. JAMA Pediatr. 2016, 170, 236–242. [Google Scholar] [CrossRef]
- Rafter, J.; Bennett, M.; Caderni, G.; Clune, Y.; Hughes, R.; Karlsson, P.C.; Klinder, A.; O’Riordan, M.; O’Sullivan, G.C.; Pool-Zobel, B.; et al. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am. J. Clin. Nutr. 2007, 85, 488–496. [Google Scholar] [CrossRef]
- Ipar, N.; Aydogdu, S.D.; Yildirim, G.K.; Inal, M.; Gies, I.; Vandenplas, Y.; Dinleyici, E.C. Effects of synbiotic on anthropometry, lipid profile and oxidative stress in obese children. Benef. Microbes 2015, 6, 775–782. [Google Scholar] [CrossRef]
- Safavi, M.; Farajian, S.; Kelishadi, R.; Mirlohi, M.; Hashemipour, M. The effects of synbiotic supplementation on some cardio-metabolic risk factors in overweight and obese children: A randomized triple-masked controlled trial. Int. J. Food Sci. Nutr. 2013, 64, 687–693. [Google Scholar] [CrossRef]
- Kolida, S.; Gibson, G.R. Synbiotics in health and disease. Annu. Rev. Food Sci. Technol. 2011, 2, 373–393. [Google Scholar] [CrossRef] [Green Version]
- Marsaux, B.; Van den Abbeele, P.; Ghyselinck, J.; Prioult, G.; Marzorati, M.; Bogićević, B. Synbiotic Effect of Bifidobacterium lactis CNCM I-3446 and Bovine Milk-Derived Oligosaccharides on Infant Gut Microbiota. Nutrients 2020, 12, 2268. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics—A review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef] [PubMed]
- Saeed, F.; Pasha, I.; Arshad, M.U.; Muhammad Anjum, F.; Hussain, S.; Rasheed, R.; Nasir, M.A.; Shafique, B. Physiological and Nutraceutical Perspectives of Fructan. Int. J. Food Prop. 2015, 18, 1895–1904. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Alhaj, O.A.; Kanekanian, A.D.; Peters, A.C.; Tatham, A.S. Hypocholesterolaemic effect of Bifidobacterium animalis subsp. lactis (Bb12) and trypsin casein hydrolysate. Food Chem. 2010, 123, 430–435. [Google Scholar] [CrossRef]
- Uchida, K.; Akashi, K.; Kusunoki, I.; Ikeda, T.; Katano, N.; Motoshima, H.; Benno, Y. Effect of fermented milk containing Bifidobacterium lactis BB-12® on stool frequency, defecation, fecal microbiota and safety of excessive ingestion in healthy female students. J. Nutr. Food 2005, 8, 39–51. [Google Scholar]
- Chouraqui, J.P.; Van Egroo, L.D.; Fichot, M.C. Acidified milk formula supplemented with bifidobacterium lactis: Impact on infant diarrhea in residential care settings. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 288–292. [Google Scholar] [CrossRef]
- Rizzardini, G.; Eskesen, D.; Calder, P.C.; Capetti, A.; Jespersen, L.; Clerici, M. Evaluation of the immune benefits of two probiotic strains Bifidobacterium animalis ssp. lactis, BB-12® and Lactobacillus paracasei ssp. paracasei, L. casei 431® in an influenza vaccination model: A randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2012, 107, 876–884. [Google Scholar] [CrossRef] [Green Version]
- Gilad, O. Discovery of Proteins Involved in the Interaction between Prebiotics Carbohydrates and Probiotics & Whole Proteome Analysis of the Probiotic Strain Bifidobacterium Animalis susp. Lactis BB-12. Ph.D. Thesis, Technical University of Denmark, Kgs. Lyngby, Denmark, 2010. [Google Scholar]
- Mueller, M.; Reiner, J.; Fleischhacker, L.; Viernstein, H.; Loeppert, R.; Praznik, W. Growth of selected probiotic strains with fructans from different sources relating to degree of polymerization and structure. J. Funct. Foods 2016, 24, 264–275. [Google Scholar] [CrossRef]
- Koropatkin, N.M.; Cameron, E.A.; Martens, E.C. How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol. 2012, 10, 323–335. [Google Scholar] [CrossRef] [Green Version]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, C.M.; Martinez, K.; Leone, V.; Hubert, N.; Chang, E.B.; Liska, D.J. Prebiotic Dietary Fiber Consumption Improves Glucose Tolerance and Modulates Gastrointestinal Microbiota Composition in Overweight/Obese Adults with Impaired Fasting Glucose. FASEB J. 2016, 30, 1166.3. [Google Scholar] [CrossRef]
- Korpela, K.; Flint, H.J.; Johnstone, A.M.; Lappi, J.; Poutanen, K.; Dewulf, E.; Delzenne, N.; de Vos, W.M.; Salonen, A. Gut microbiota signatures predict host and microbiota responses to dietary interventions in obese individuals. PLoS ONE 2014, 9, e90702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salonen, A.; Lahti, L.; Salojärvi, J.; Holtrop, G.; Korpela, K.; Duncan, S.H.; Date, P.; Farquharson, F.; Johnstone, A.M.; Lobley, G.E.; et al. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014, 8, 2218–2230. [Google Scholar] [CrossRef] [PubMed]
- Mättö, J.; Fondén, R.; Tolvanen, T.; von Wright, A.; Vilpponen-Salmela, T.; Satokari, R.; Saarela, M. Intestinal survival and persistence of probiotic Lactobacillus and Bifidobacterium strains administered in triple-strain yoghurt. Int. Dairy J. 2006, 16, 1174–1180. [Google Scholar] [CrossRef]
- Vandeputte, D.; Kathagen, G.; D’Hoe, K.; Vieira-Silva, S.; Valles-Colomer, M.; Sabino, J.; Wang, J.; Tito, R.Y.; De Commer, L.; Darzi, Y.; et al. Quantitative microbiome profiling links gut community variation to microbial load. Nature 2017, 551, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Boon, N.; Top, E.M.; Verstraete, W.; Siciliano, S.D. Bioaugmentation as a Tool To Protect the Structure and Function of an Activated-Sludge Microbial Community against a 3-Chloroaniline Shock Load. Appl. Environ. Microbiol. 2003, 69, 1511–1520. [Google Scholar] [CrossRef] [Green Version]
- Duysburgh, C.; Van den Abbeele, P.; Krishnan, K.; Bayne, T.F.; Marzorati, M. A synbiotic concept containing spore-forming Bacillus strains and a prebiotic fiber blend consistently enhanced metabolic activity by modulation of the gut microbiome in vitro. Int. J. Pharm. 2019, 1, 100021. [Google Scholar] [CrossRef]
- Kok, R.G.; de Waal, A.; Schut, F.; Welling, G.W.; Weenk, G.; Hellingwerf, K.J. Specific detection and analysis of a probiotic Bifidobacterium strain in infant feces. Appl. Environ. Microbiol. 1996, 62, 3668–3672. [Google Scholar] [CrossRef] [Green Version]
- Moens, F.; Duysburgh, C.; van den Abbeele, P.; Morera, M.; Marzorati, M. Lactobacillus rhamnosus GG and Saccharomyces cerevisiae boulardii exert synergistic antipathogenic activity in vitro against enterotoxigenic Escherichia coli. Benef. Microbes 2019, 10, 923–935. [Google Scholar] [CrossRef] [Green Version]
- De Weirdt, R.; Possemiers, S.; Vermeulen, G.; Moerdijk-Poortvliet, T.C.; Boschker, H.T.; Verstraete, W.; Van de Wiele, T. Human faecal microbiota display variable patterns of glycerol metabolism. FEMS Microbiol. Ecol. 2010, 74, 601–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinttilä, T.; Kassinen, A.; Malinen, E.; Krogius, L.; Palva, A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 2004, 97, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Verstrepen, L.; Ghyselinck, J.; Albers, R.; Marzorati, M.; Mercenier, A. A Novel Non-Digestible, Carrot-Derived Polysaccharide (cRG-I) Selectively Modulates the Human Gut Microbiota while Promoting Gut Barrier Integrity: An Integrated in Vitro Approach. Nutrients 2020, 12, 917. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, D.K. What is the proper way to apply the multiple comparison test? Korean J. Anesthesiol. 2018, 71, 353–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semjonovs, P.; Shakizova, L.; Denina, I.; Kozlinskis, E.; Unite, D. Development of a Fructan-supplemented Synbiotic Cabbage Juice Beverage Fermented by Bifidobacterium lactis Bb12. Res. J. Microbiol. 2014, 9, 129–141. [Google Scholar] [CrossRef] [Green Version]
- Valdés-Varela, L.; Ruas-Madiedo, P.; Gueimonde, M. In vitro fermentation of different fructo-oligosaccharides by Bifidobacterium strains for the selection of synbiotic combinations. Int. J Food Microbiol. 2017, 242, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Vernazza, C.L.; Gibson, G.R.; Rastall, R.A. Carbohydrate preference, acid tolerance and bile tolerance in five strains of Bifidobacterium. J. Appl. Microbiol. 2006, 100, 846–853. [Google Scholar] [CrossRef]
- Akalin, A.S.; Erişir, D. Effects of inulin and oligofructose on the rheological characteristics and probiotic culture survival in low-fat probiotic ice cream. J. Food Sci. 2008, 73, M184–M188. [Google Scholar] [CrossRef]
- Bedani, R.; Rossi, E.A.; Isay Saad, S.M. Impact of inulin and okara on Lactobacillus acidophilus La-5 and Bifidobacterium animalis Bb-12 viability in a fermented soy product and probiotic survival under in vitro simulated gastrointestinal conditions. Food Microbiol. 2013, 34, 382–389. [Google Scholar] [CrossRef] [Green Version]
- Falony, G.; Lazidou, K.; Verschaeren, A.; Weckx, S.; Maes, D.; De Vuyst, L. In vitro kinetic analysis of fermentation of prebiotic inulin-type fructans by Bifidobacterium species reveals four different phenotypes. Appl. Environ. Microbiol. 2009, 75, 454–461. [Google Scholar] [CrossRef] [Green Version]
- Van der Meulen, R.; Makras, L.; Verbrugghe, K.; Adriany, T.; De Vuyst, L. In vitro kinetic analysis of oligofructose consumption by Bacteroides and Bifidobacterium spp. indicates different degradation mechanisms. Appl. Environ. Microbiol. 2006, 72, 1006–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moens, F.; Verce, M.; De Vuyst, L. Lactate- and acetate-based cross-feeding interactions between selected strains of lactobacilli, bifidobacteria and colon bacteria in the presence of inulin-type fructans. Int. J. Food Microbiol. 2017, 241, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Kolida, S.; Gibson, G.R. Prebiotic capacity of inulin-type fructans. J. Nutr. 2007, 137, 2503s–2506s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belenguer, A.; Duncan, S.H.; Calder, A.G.; Holtrop, G.; Louis, P.; Lobley, G.E.; Flint, H.J. Two Routes of Metabolic Cross-Feeding between Bifidobacterium adolescentis and Butyrate-Producing Anaerobes from the Human Gut. Appl. Environ. Microbiol. 2006, 72, 3593–3599. [Google Scholar] [CrossRef] [Green Version]
- De Vuyst, L.; Moens, F.; Selak, M.; Rivière, A.; Leroy, F. Summer Meeting 2013: Growth and physiology of bifidobacteria. J. Appl. Microbiol. 2014, 116, 477–491. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Srutkova, D.; Schwarzer, M.; Hudcovic, T.; Zakostelska, Z.; Drab, V.; Spanova, A.; Rittich, B.; Kozakova, H.; Schabussova, I. Bifidobacterium longum CCM 7952 Promotes Epithelial Barrier Function and Prevents Acute DSS-Induced Colitis in Strictly Strain-Specific Manner. PLoS ONE 2015, 10, e0134050. [Google Scholar] [CrossRef]
- Furrie, E.; Macfarlane, S.; Kennedy, A.; Cummings, J.H.; Walsh, S.V.; O’Neil, D.A.; Macfarlane, G.T. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: A randomised controlled pilot trial. Gut 2005, 54, 242–249. [Google Scholar] [CrossRef]
- Yu, R.; Zuo, F.; Ma, H.; Chen, S. Exopolysaccharide-Producing Bifidobacterium adolescentis Strains with Similar Adhesion Property Induce Differential Regulation of Inflammatory Immune Response in Treg/Th17 Axis of DSS-Colitis Mice. Nutrients 2019, 11, 782. [Google Scholar] [CrossRef] [Green Version]
- Rossi, M.; Corradini, C.; Amaretti, A.; Nicolini, M.; Pompei, A.; Zanoni, S.; Matteuzzi, D. Fermentation of fructooligosaccharides and inulin by bifidobacteria: A comparative study of pure and fecal cultures. Appl. Environ. Microbiol. 2005, 71, 6150–6158. [Google Scholar] [CrossRef] [Green Version]
- Sheridan, P.O.; Martin, J.C.; Lawley, T.D.; Browne, H.P.; Harris, H.M.B.; Bernalier-Donadille, A.; Duncan, S.H.; O’Toole, P.W.; Scott, K.P.; Flint, H.J. Polysaccharide utilization loci and nutritional specialization in a dominant group of butyrate-producing human colonic Firmicutes. Microb Genom 2016, 2, e000043. [Google Scholar] [CrossRef] [PubMed]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Zhang, M.; Yang, X.; Hong, N.; Yu, C. Faecalibacterium prausnitzii upregulates regulatory T cells and anti-inflammatory cytokines in treating TNBS-induced colitis. J. Crohn’s Colitis 2013, 7, e558–e568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Liu, F.; Ling, Z.; Tong, X.; Xiang, C. Human Intestinal Lumen and Mucosa-Associated Microbiota in Patients with Colorectal Cancer. PLoS ONE 2012, 7, e39743. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Crouse, A.; Chevallier, L.; Pontier, S.M.; Alzahrani, A.; Silué, N.; Campbell-Valois, F.X.; Montagutelli, X.; Gruenheid, S.; Malo, D. Enterobacteria and host resistance to infection. Mamm. Genome 2018, 29, 558–576. [Google Scholar] [CrossRef]
- Daguet, D.; Pinheiro, I.; Verhelst, A.; Possemiers, S.; Marzorati, M. Arabinogalactan and fructooligosaccharides improve the gut barrier function in distinct areas of the colon in the Simulator of the Human Intestinal Microbial Ecosystem. J. Funct. Foods 2016, 20, 369–379. [Google Scholar] [CrossRef]



| Code | Commercial Name | Supplier | Purity Fructan | Sugars 1 | DP |
|---|---|---|---|---|---|
| OF1 | Frutalose® OFP | Sensus, The Netherlands | 89 | 8 | 4 |
| OF2 | Orafti®P95 | Beneo, Belgium | 93.2–97.5 | 2.5–6.8 | 4–5 |
| OF/IN1 | Fibrulose® F97 | Cosucra, Belgium | 97 ± 2 | 3 ± 2 | 5.5 |
| OF/IN2 | Orafti® Synergy1 | Beneo, Belgium | 92 ± 2 | 8 ± 2 | 14.5 |
| IN1 | Fibruline® Instant | Cosucra, Belgium | >90 | <10 | 9 |
| IN2 | Orafti® GR | Beneo, Belgium | >90 | <10 | 10 |
| IN3 | Frutafit® IQ | Sensus, The Netherlands | >90 | <10 | 8–13 |
| Endpoint | Blank | OF1 | OF2 | OF/IN1 | OF/IN2 | IN1 | IN2 | IN3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BB-12 | - | BB-12 | - | BB-12 | - | BB-12 | - | BB-12 | - | BB-12 | - | BB-12 | - | BB-12 | |
| pH | 0.00 | −0.93 | 0.07 | −0.97 | 0.02 | −0.86 | 0.01 | −0.78 | 0.05 | −0.68 | −0.03 | −0.80 | 0.00 | −0.77 | 0.01 |
| Gas (kPa) | 2.4 | 28.1 | 2.9 | 21.5 | 4.6 | 30.6 | 3.3 | 48.9 | −1.3 | 45.9 | 1.0 | 45.0 | 0.7 | 43.8 | 2.2 |
| Acetate (mM) | 0.6 | 20.4 | −0.2 | 20.1 | 4.3 | 22.5 | 1.0 | 23.6 | −0.3 | 24.6 | −2.8 | 26.3 | −4.2 | 24.5 | 1.0 |
| Lactate (mM) | 0.1 | 15.1 | −0.9 | 15.1 | −1.2 | 14.1 | −1.2 | 7.8 | 0.3 | 7.6 | 0.5 | 8.1 | 0.2 | 9.2 | −0.2 |
| Propionate (mM) | 0.4 | 0.2 | 0.4 | 0.6 | 0.9 | 1.0 | 0.1 | 4.3 | 1.2 | 5.5 | −0.1 | 4.4 | 0.3 | 3.4 | 1.2 |
| Butyrate (mM) | 0.1 | 2.4 | 0.6 | 0.2 | 0.3 | 1.9 | 1.1 | 5.5 | 1.4 | 5.9 | 1.4 | 4.2 | 1.9 | 5.6 | −0.1 |
| bCFA (mM) | 0.1 | −2.8 | 0.0 | −2.8 | 0.0 | −2.8 | 0.1 | −2.7 | 0.0 | −2.7 | 0.1 | −2.8 | 0.1 | −2.7 | 0.1 |
| Phylum | Blank | OF1 | OF2 | OF/IN1 | OF/IN2 | IN1 | IN2 | IN3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BB-12 | - | BB-12 | - | BB-12 | - | BB-12 | - | BB-12 | - | BB-12 | - | BB-12 | - | BB-12 | |
| Actinobacteria | 0.19 | 0.63 | 0.30 | 0.67 | 0.17 | 0.64 | 0.22 | 0.64 | 0.26 | 0.63 | 0.22 | 0.58 | 0.30 | 0.65 | 0.27 |
| Bacteroidetes | −0.01 | −0.54 | 0.02 | −0.52 | 0.09 | −0.45 | 0.03 | −0.09 | 0.15 | 0.09 | 0.02 | −0.05 | 0.02 | −0.17 | 0.13 |
| Firmicutes | 0.01 | 0.10 | −0.03 | 0.19 | −0.11 | 0.01 | −0.05 | 0.20 | 0.12 | 0.23 | 0.06 | 0.15 | 0.06 | 0.11 | 0.04 |
| Proteobacteria | 0.00 | −0.04 | −0.09 | −0.07 | −0.14 | 0.04 | −0.08 | 0.08 | −0.08 | 0.05 | −0.03 | 0.04 | −0.05 | 0.07 | −0.04 |
| Verrucomicrobia | 0.14 | −0.10 | −0.02 | 0.02 | 0.06 | 0.04 | −0.06 | −0.05 | 0.17 | 0.05 | 0.03 | 0.02 | 0.16 | 0.09 | 0.08 |
| Phylum | Family | Blank | OF1 | OF2 | OF/IN1 | OF/IN2 | IN1 | IN2 | IN3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BB-12 | - | BB-12 | - | BB-12 | - | BB-12 | - | BB-12 | - | BB-12 | - | BB-12 | - | BB-12 | ||
| Actinobacteria | Bifidobacteriaceae | 0.54 | 1.20 | 0.29 | 1.26 | 0.17 | 1.05 | 0.35 | 1.06 | 0.38 | 0.99 | 0.41 | 0.94 | 0.46 | 0.97 | 0.46 |
| Coriobacteriaceae | −0.10 | 0.14 | 0.32 | 0.16 | 0.20 | 0.30 | 0.09 | 0.15 | 0.08 | −0.01 | 0.25 | −0.02 | 0.27 | 0.16 | 0.21 | |
| Eggerthellaceae | −0.02 | 0.12 | 0.28 | 0.08 | 0.16 | 0.39 | 0.05 | 0.46 | 0.01 | 0.42 | 0.04 | 0.41 | 0.08 | 0.51 | 0.06 | |
| Bacteroidetes | Bacteroidaceae | −0.03 | −0.82 | 0.01 | −0.82 | 0.09 | −0.58 | 0.04 | −0.13 | 0.16 | 0.14 | 0.03 | 0.01 | 0.00 | −0.15 | 0.11 |
| Marinifilaceae | 0.10 | −0.18 | 0.03 | −0.33 | 0.24 | −0.05 | −0.09 | −0.13 | 0.13 | −0.23 | −0.06 | −0.09 | 0.16 | −0.06 | 0.02 | |
| Muribaculaceae | 0.09 | 0.10 | −0.05 | 0.19 | −0.04 | 0.00 | 0.04 | 0.08 | −0.23 | −0.05 | −0.04 | 0.06 | 0.12 | 0.08 | 0.01 | |
| Prevotellaceae | −0.02 | −0.06 | 0.07 | 0.28 | 0.08 | −0.08 | −0.01 | 0.03 | −0.10 | −0.18 | 0.00 | 0.12 | −0.03 | −0.24 | 0.18 | |
| Rikenellaceae | −0.07 | 0.18 | 0.01 | 0.21 | 0.01 | 0.15 | 0.00 | 0.03 | 0.11 | 0.03 | 0.09 | 0.07 | 0.07 | 0.09 | 0.11 | |
| Tannerellaceae | −0.03 | −1.16 | 0.01 | −1.21 | 0.05 | −0.94 | −0.06 | −0.90 | 0.23 | −0.93 | 0.08 | −0.88 | 0.14 | −0.84 | 0.07 | |
| Firmicutes | Enterococcaceae | 0.06 | 1.00 | −0.14 | 0.63 | 0.33 | 0.52 | 0.07 | 0.34 | −0.14 | 0.26 | −0.01 | 0.22 | 0.03 | 0.17 | 0.07 |
| Erysipelotrichaceae | −0.04 | −0.19 | −0.10 | −0.20 | −0.06 | −0.19 | −0.08 | −0.26 | 0.34 | −0.02 | 0.17 | −0.15 | 0.34 | −0.26 | 0.49 | |
| Lachnospiraceae | 0.01 | −0.25 | −0.07 | −0.18 | −0.15 | −0.25 | 0.02 | 0.19 | 0.03 | 0.24 | −0.08 | 0.17 | −0.01 | 0.12 | 0.08 | |
| Lactobacillaceae | 0.00 | 2.13 | 0.12 | 1.92 | 0.53 | 1.71 | 0.03 | 0.25 | −0.12 | 0.08 | −0.04 | 0.57 | −0.34 | 0.85 | −0.52 | |
| Ruminococcaceae | 0.01 | −0.29 | −0.05 | −0.35 | 0.04 | −0.36 | −0.03 | 0.11 | 0.10 | 0.09 | 0.16 | 0.00 | 0.06 | −0.14 | 0.02 | |
| Streptococcaceae | 0.13 | 1.38 | −0.05 | 1.28 | 0.29 | 1.28 | −0.09 | 1.11 | 0.36 | 1.07 | 0.07 | 1.00 | 0.33 | 0.97 | 0.29 | |
| Veillonellaceae | 0.08 | 0.08 | 0.05 | 0.25 | −0.04 | 0.38 | −0.03 | 0.70 | 0.02 | 0.62 | 0.18 | 0.67 | 0.09 | 0.60 | 0.05 | |
| Proteobacteria | Burkholderiaceae | 0.07 | 0.39 | −0.01 | 0.35 | −0.07 | 0.43 | −0.02 | 0.58 | 0.01 | 0.51 | 0.02 | 0.49 | 0.09 | 0.48 | 0.14 |
| Desulfovibrionaceae | 0.02 | −0.29 | 0.01 | −0.15 | −0.11 | −0.08 | 0.11 | −0.10 | −0.07 | −0.21 | 0.20 | −0.18 | 0.04 | −0.08 | −0.15 | |
| Enterobacteriaceae | −0.02 | −0.20 | −0.24 | −0.21 | −0.26 | −0.07 | −0.31 | −0.21 | −0.21 | −0.21 | −0.11 | −0.16 | −0.21 | −0.14 | −0.19 | |
| Verrucomicrobia | Akkermansiaceae | 0.14 | −0.10 | −0.02 | 0.02 | 0.06 | 0.04 | −0.06 | −0.05 | 0.17 | 0.05 | 0.03 | 0.02 | 0.16 | 0.09 | 0.08 |
| Phylum | Family | OTU# | Related Species | Blank | OF1 | OF2 | OF/IN1 | OF/IN2 | IN1 | IN2 | IN3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BB-12 | - | BB-12 | - | BB-12 | - | BB-12 | - | BB-12 | - | BB-12 | - | BB-12 | - | BB-12 | ||||
| Actinobacteria | Bifidobacteriaceae | 2 | B. adolescentis | −0.07 | 1.15 | −0.24 | 1.18 | −0.26 | 0.99 | −0.13 | 1.08 | −0.18 | 1.01 | −0.09 | 0.96 | −0.08 | 0.98 | −0.08 |
| 5 | B. animalis | 2.19 | 0.00 | 2.99 | 0.00 | 2.82 | 0.00 | 2.85 | 0.00 | 2.81 | 0.00 | 2.64 | 0.00 | 2.72 | 0.00 | 2.73 | ||
| 21 | B. longum | −0.07 | 0.98 | −0.32 | 1.06 | −0.31 | 0.76 | −0.32 | 0.51 | −0.16 | 0.43 | −0.08 | 0.48 | −0.14 | 0.48 | −0.19 | ||
| Coriobacteriaceae | 20 | Collinsella aerofaciens | −0.10 | 0.14 | 0.32 | 0.16 | 0.20 | 0.29 | 0.10 | 0.15 | 0.07 | −0.02 | 0.26 | −0.03 | 0.28 | 0.16 | 0.21 | |
| Eggerthellaceae | 4 | Senegalimassilia sp. | 0.01 | −0.01 | 0.15 | 0.01 | 0.03 | 0.07 | 0.13 | 0.34 | −0.07 | 0.24 | 0.01 | 0.24 | 0.04 | 0.29 | 0.03 | |
| 7 | Ellagibacter isourolithinifaciens | 0.04 | 0.71 | 0.23 | 0.72 | 0.22 | 0.96 | 0.00 | 0.78 | 0.06 | 0.89 | −0.05 | 1.01 | −0.11 | 0.91 | 0.13 | ||
| Bacteroidetes | Bacteroidaceae | 9 | Bacteroides caccae | −0.02 | −0.40 | 0.04 | −0.48 | 0.15 | −0.27 | 0.12 | 0.81 | 0.09 | 1.00 | −0.01 | 0.75 | 0.01 | 0.46 | 0.20 |
| 6 | Bacteroides dorei | −0.05 | −0.95 | 0.07 | −1.02 | 0.17 | −0.60 | 0.03 | −0.60 | 0.16 | −0.53 | −0.01 | −0.46 | 0.07 | −0.46 | 0.08 | ||
| 11 | B.uniformis | −0.03 | −0.72 | 0.01 | −0.71 | 0.02 | −0.71 | 0.09 | 0.07 | −0.04 | 0.12 | −0.03 | −0.11 | −0.01 | −0.17 | −0.05 | ||
| Rikenellaceae | 13 | Alistipes onderdonkii | −0.08 | 0.63 | 0.21 | 1.10 | −0.14 | 0.71 | −0.21 | 0.16 | 0.06 | 0.13 | 0.04 | 0.22 | −0.05 | 0.28 | −0.04 | |
| Firmicutes | Lactobacillaceae | 19 | Lactobacillus fermentum | 0.00 | 1.94 | 0.10 | 1.76 | 0.56 | 1.59 | 0.00 | 0.18 | −0.08 | 0.02 | −0.02 | 0.47 | −0.28 | 0.81 | −0.51 |
| Lachnospiraceae | 10 | Blautia faecis | 0.11 | −0.31 | 0.00 | −0.28 | −0.03 | −0.05 | −0.20 | 0.45 | 0.05 | 0.68 | −0.15 | 0.55 | −0.12 | 0.57 | −0.07 | |
| 23 | butyrate−producing SR1/5 | 0.01 | 0.02 | 0.01 | 0.08 | −0.03 | 0.20 | −0.13 | 0.22 | 0.04 | 0.25 | −0.01 | 0.30 | 0.00 | 0.23 | −0.04 | ||
| 16 | Dorea longicatena | −0.14 | −0.26 | 0.05 | −0.41 | 0.08 | −0.30 | 0.05 | −0.23 | 0.14 | −0.16 | −0.03 | −0.30 | 0.08 | −0.23 | 0.01 | ||
| 17 | Clostridium bolteae/clostridioforme | −0.06 | −0.52 | 0.09 | −0.81 | −0.02 | −0.09 | 0.05 | 0.28 | 0.00 | 0.21 | 0.06 | 0.24 | −0.03 | 0.26 | 0.01 | ||
| Ruminococcaceae | 12 | Faecalibacterium prausnitzii | 0.06 | −0.45 | 0.04 | −0.43 | −0.03 | −0.43 | 0.02 | −0.23 | 0.31 | −0.10 | 0.14 | −0.31 | 0.06 | −0.45 | 0.22 | |
| 8 | Faecalibacterium prausnitzii | 0.01 | 0.25 | −0.24 | 0.29 | −0.06 | 0.29 | −0.02 | 1.28 | 0.05 | 1.23 | 0.24 | 1.09 | 0.25 | 0.82 | −0.04 | ||
| Veillonellaceae | 18 | Dialister succinatiphilus | 0.06 | 0.21 | 0.05 | 0.33 | −0.07 | 0.48 | −0.06 | 0.62 | 0.02 | 0.57 | 0.11 | 0.62 | 0.01 | 0.61 | 0.00 | |
| Proteobacteria | Burkholderiaceae | 3 | Sutterella wadsworthensis | 0.06 | 0.47 | −0.13 | 0.32 | 0.05 | 0.47 | 0.05 | 1.07 | −0.02 | 1.03 | −0.02 | 0.99 | 0.01 | 0.77 | 0.29 |
| Enterobacteriaceae | 1 | Escherichia coli | −0.02 | −0.20 | −0.24 | −0.21 | −0.26 | −0.07 | −0.31 | −0.21 | −0.21 | −0.21 | −0.11 | −0.16 | −0.21 | −0.14 | −0.19 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van den Abbeele, P.; Duysburgh, C.; Ghyselinck, J.; Goltz, S.; Berezhnaya, Y.; Boileau, T.; De Blaiser, A.; Marzorati, M. Fructans with Varying Degree of Polymerization Enhance the Selective Growth of Bifidobacterium animalis subsp. lactis BB-12 in the Human Gut Microbiome In Vitro. Appl. Sci. 2021, 11, 598. https://doi.org/10.3390/app11020598
Van den Abbeele P, Duysburgh C, Ghyselinck J, Goltz S, Berezhnaya Y, Boileau T, De Blaiser A, Marzorati M. Fructans with Varying Degree of Polymerization Enhance the Selective Growth of Bifidobacterium animalis subsp. lactis BB-12 in the Human Gut Microbiome In Vitro. Applied Sciences. 2021; 11(2):598. https://doi.org/10.3390/app11020598
Chicago/Turabian StyleVan den Abbeele, Pieter, Cindy Duysburgh, Jonas Ghyselinck, Shellen Goltz, Yulia Berezhnaya, Thomas Boileau, Anke De Blaiser, and Massimo Marzorati. 2021. "Fructans with Varying Degree of Polymerization Enhance the Selective Growth of Bifidobacterium animalis subsp. lactis BB-12 in the Human Gut Microbiome In Vitro" Applied Sciences 11, no. 2: 598. https://doi.org/10.3390/app11020598
APA StyleVan den Abbeele, P., Duysburgh, C., Ghyselinck, J., Goltz, S., Berezhnaya, Y., Boileau, T., De Blaiser, A., & Marzorati, M. (2021). Fructans with Varying Degree of Polymerization Enhance the Selective Growth of Bifidobacterium animalis subsp. lactis BB-12 in the Human Gut Microbiome In Vitro. Applied Sciences, 11(2), 598. https://doi.org/10.3390/app11020598




