Modification of Nanocrystalline Porous Cu2-xSe Films during Argon Plasma Treatment
Abstract
1. Introduction
2. Experimental Details
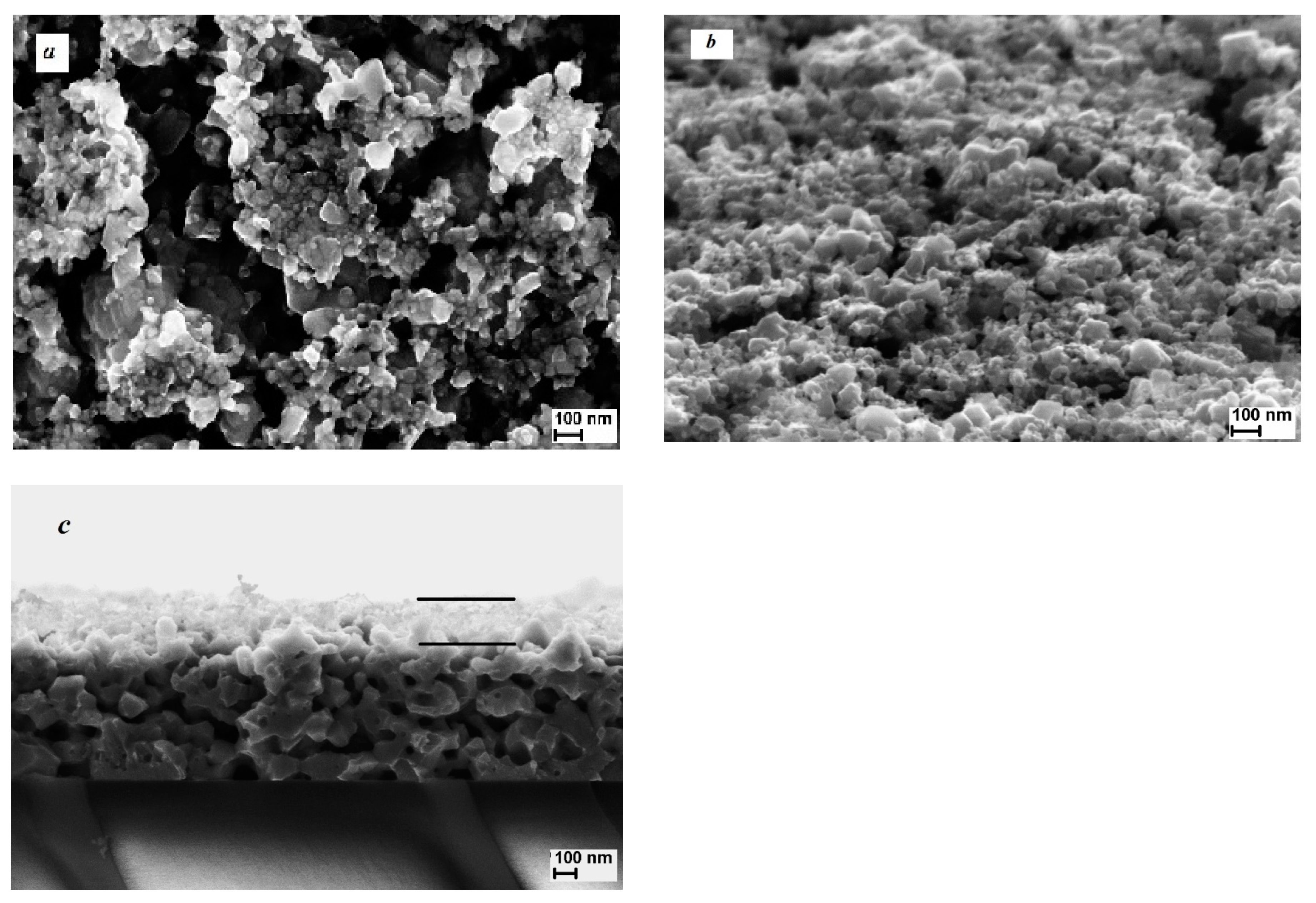
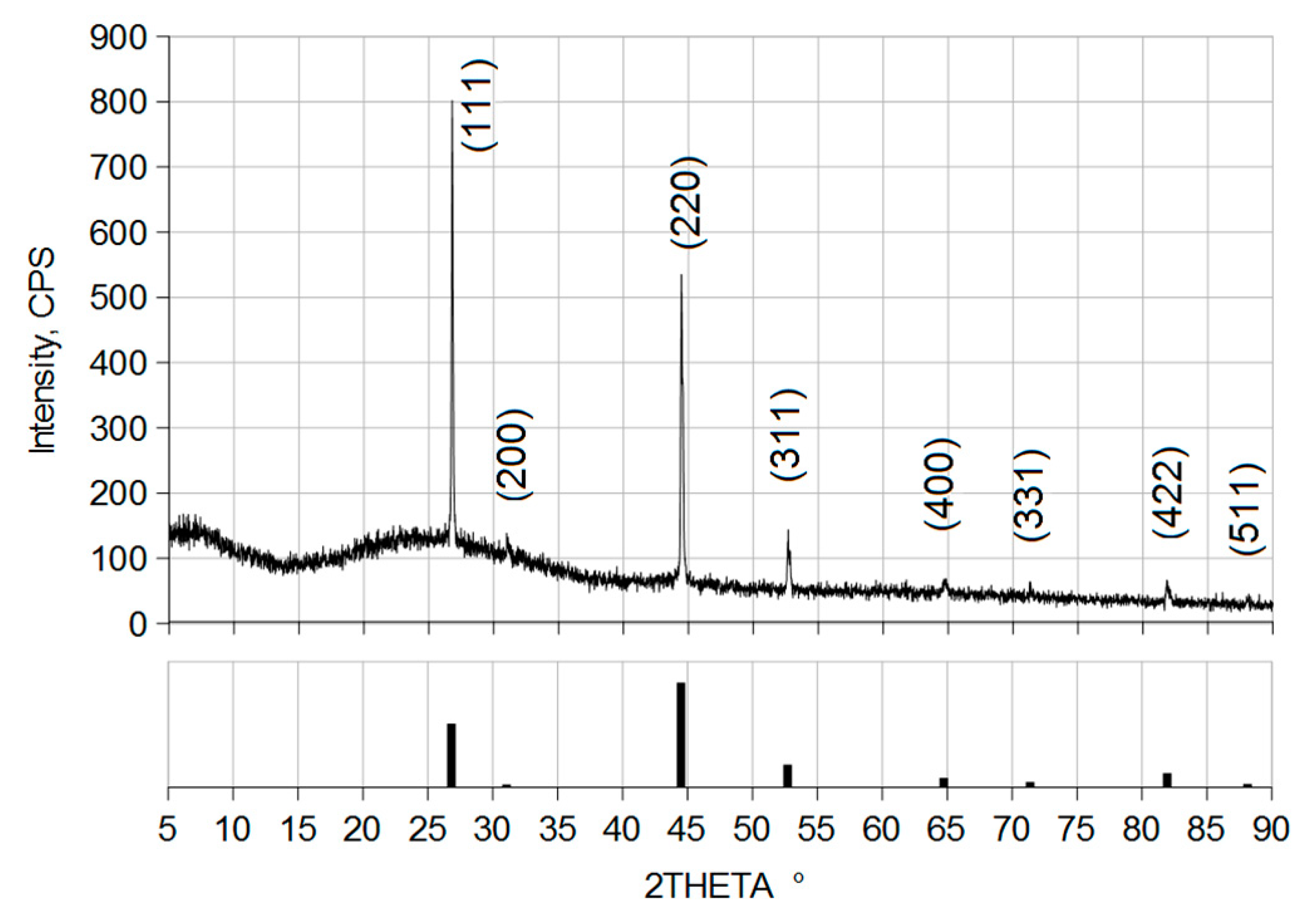
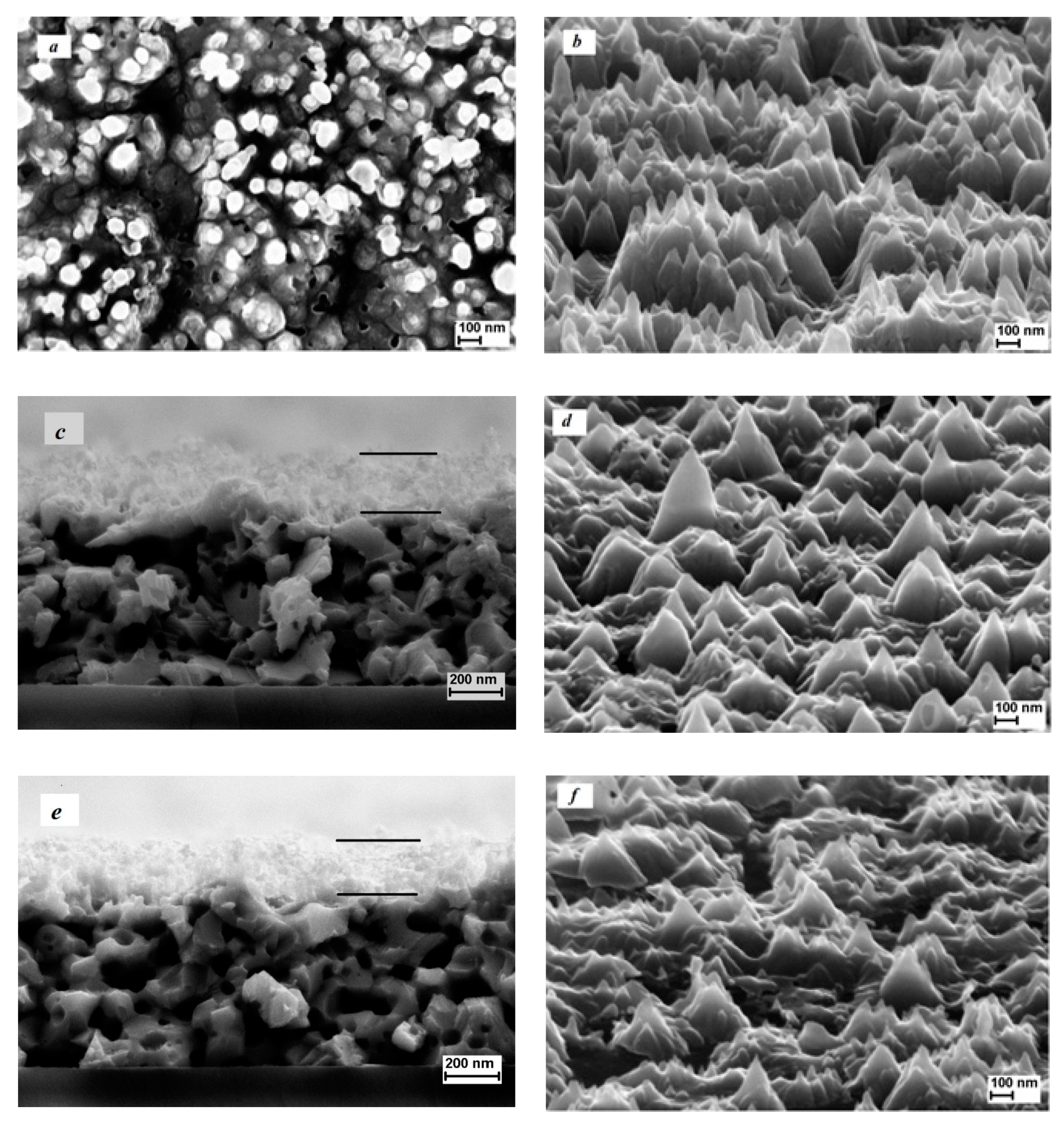
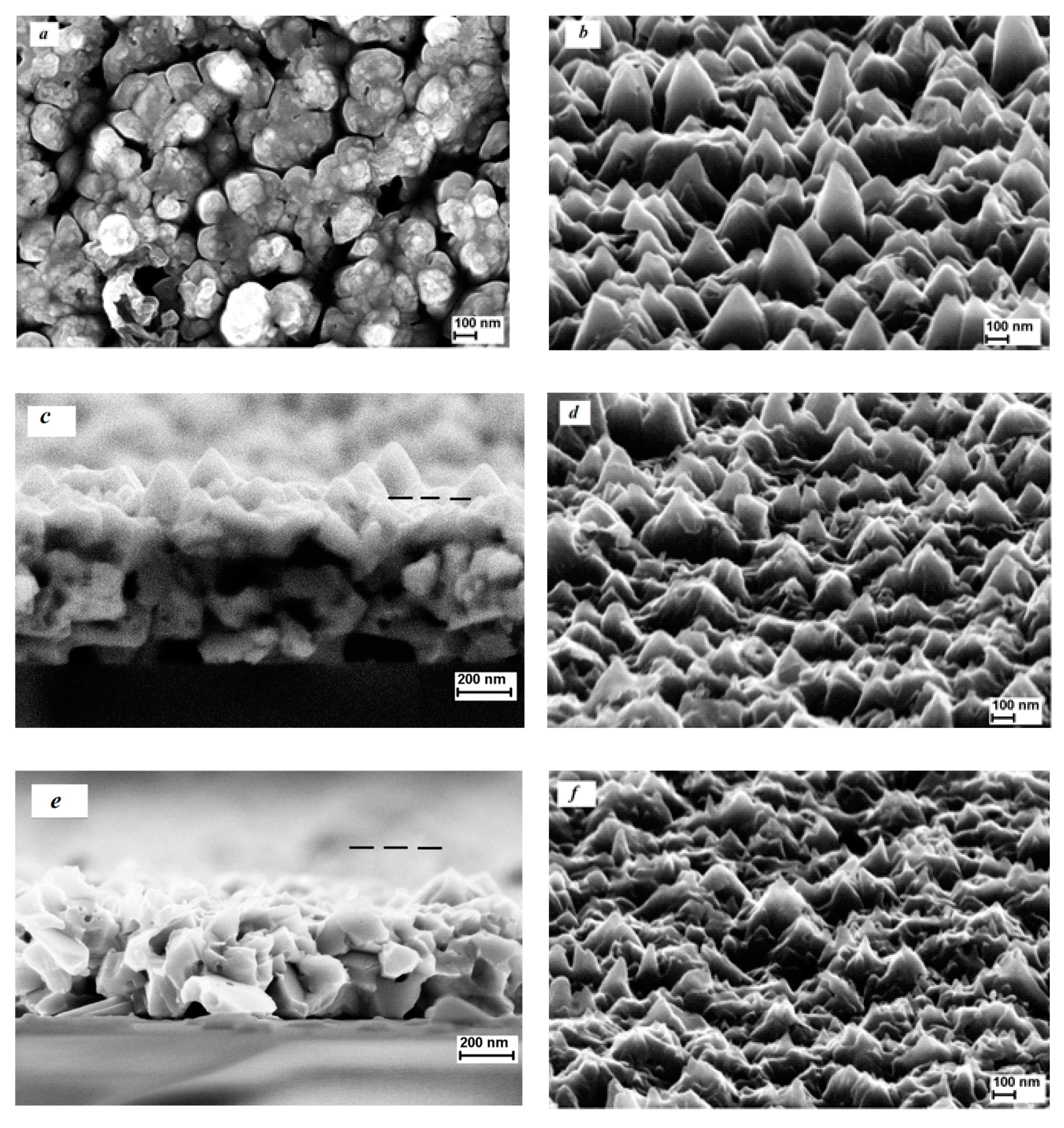
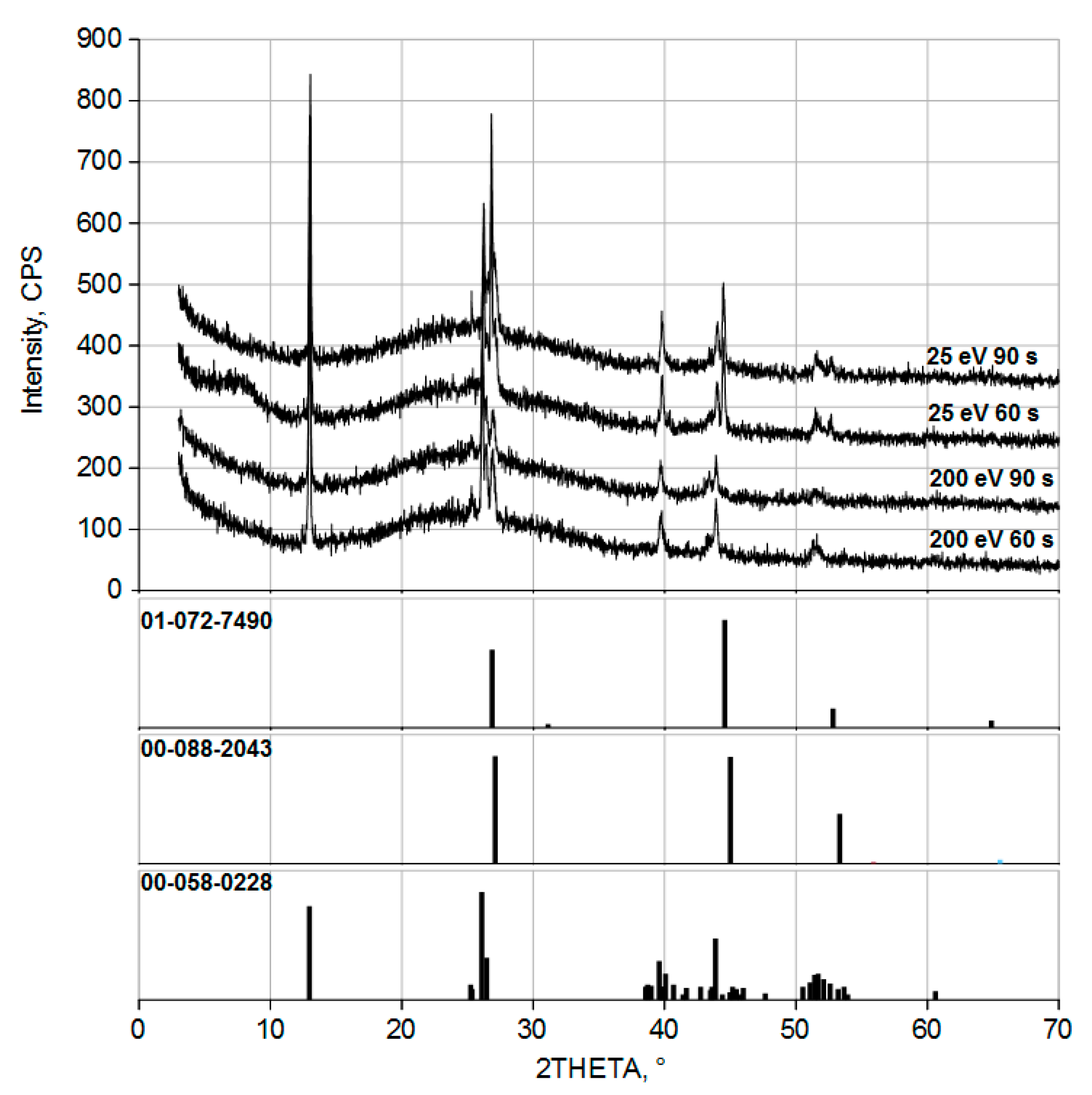
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Yang, J.; Wu, T.; Li, L.; Luo, W.; Jiang, W.; Wang, L. Nanostructured binary copper chalcogenides: Synthesis strategies and common applications. Nanoscale 2018, 10, 15130–15163. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.A.; Hussain, I. Copper selenide thin films from growth to applications. Solid State Sci. 2020, 100, 106101. [Google Scholar] [CrossRef]
- Gan, X.Y.; Keller, E.L.; Warkentin, C.; Crawford, S.; Frontiera, R.; Millstone, J.E. Plasmon-Enhanced Chemical Conversion Using Copper Selenide Nanoparticles. Nano Lett. 2019, 19, 2384–2388. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.C.; Li, H.; Yao, C.; Zhan, Z.; Yu, W.; Yu, Z.; Guo, C. Structural and compositional control in copper selenide nanocrystals for light-induced self-repairable electrodes. Nano Energy 2018, 51, 774–785. [Google Scholar] [CrossRef]
- Chen, Y.H.; Davoisne, C.; Tarascon, J.M.; Guery, C. Growth of single-crystal copper sulfide thin films via electrodeposition in ionic liquid media for lithium ion batteries. J. Mater. Chem. 2012, 22, 5295–5299. [Google Scholar] [CrossRef]
- Chen, X.Q.; Li, Z.; Bai, Y.; Sun, Q.; Wang, L.Z.; Dou, S.X. Room-Temperature Synthesis of Cu2-xE (E=S, Se) Nanotubes with Hierarchical Architecture as High-Performance Counter Electrodes of Quantum-Dot-Sensitized Solar Cells. Chem. Eur. J. 2015, 21, 1055–1063. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, C.; Zheng, C.; Xi, Y.; Wan, B. Synthesis and Thermoelectric Property of Cu2-xSe Nanowires. J. Phys. Chem. C 2010, 114, 14849–14853. [Google Scholar] [CrossRef]
- Chen, D.H.; Chen, G.; Jin, R.C.; Xu, H.M. Self-decorated Cu2-xSe nanosheets as anode materials for Li ion batteries and electrochemical hydrogen storage. Cryst. Eng. Comm. 2014, 16, 2810–2817. [Google Scholar] [CrossRef]
- Choi, J.; Kang, N.; Yang, H.Y.; Kim, H.; Son, S.U. Colloidal Synthesis of Cubic-Phase Copper Selenide Nanodiscs and Their Optoelectronic Properties. Chem. Mater. 2010, 22, 3586–3588. [Google Scholar] [CrossRef]
- Cao, H.L.; Qian, X.F.; Zai, J.T.; Yin, J.; Zhu, Z.K. Conversion of Cu2O nanocrystals into hollow Cu2-xSe nanocages with the preservation of morphologies. Chem. Commun. 2006, 43, 4548–4550. [Google Scholar] [CrossRef]
- Cho, A.; Ahn, S.; Yun, J.H.; Gwak, J.; Ahn, S.K.; Shin, K.; Yoo, J.; Song, H.; Yoon, K. The growth of Cu2-xSe thin films using nanoparticles. Thin Solid Films 2013, 546, 299–307. [Google Scholar] [CrossRef]
- Zimin, S.P.; Amirov, I.I.; Naumov, V.V.; Guseva, K.E. The Formation of Hollow Lead Structures on the Surface of PbSe Films Treated in Argon Plasma. Tech. Phys. Lett. 2018, 44, 518–521. [Google Scholar] [CrossRef]
- Zimin, S.P.; Gorlachev, E.S.; Mokrov, D.A.; Amirov, I.I.; Naumov, V.V.; Gremenok, V.F.; Juskenas, R.; Skapas, M.; Kim, W.Y.; Bente, K.; et al. Surface nanostructuring of CuIn1−xGaxSe2 films using argon plasma treatment. Semicond. Sci. Technol. 2017, 32, 075014. [Google Scholar] [CrossRef]
- Rasool, S.; Saritha, K.; Ramakrishna Reddy, K.T.; Tivanov, M.S.; Gremenok, V.F.; Zimin, S.P.; Pipkova, A.S.; Mazaletskiy, L.A.; Amirov, I.I. Annealing and plasma treatment effect on structural, morphological and topographical properties of evaporated β-In2S3 films. Mater. Res. Express 2020, 7, 016431. [Google Scholar] [CrossRef]
- Kim, S.; Kim, N.-H. Impurity Phases and Optoelectronic Properties of CuSbSe2 Thin Films Prepared by Cosputtering Process for Absorber Layer in Solar Cells. Coatings 2020, 10, 1209. [Google Scholar] [CrossRef]
- Zimin, S.; Gorlachev, E.; Amirov, I. Inductively Coupled Plasma Sputtering: Structure of IV-VI Semiconductors. In Encyclopedia of Plasma Technology, 1st ed.; Shohet, J.L., Ed.; CRC Press: New York, NY, USA, 2017; pp. 679–691. Available online: https://www.routledgehandbooks.com/doi/10.1081/E-EPLT-120053966 (accessed on 1 November 2020).
- Ghosh, A.; Kulsi, C.; Banerjee, D.; Mondal, A. Galvanic synthesis of Cu2-xSe thin films and their photocatalytic and thermoelectric properties. Appl. Surf. Sci. 2016, 369, 525–534. [Google Scholar] [CrossRef]
- Zhou, R.; Huang, Y.; Zhou, J.; Niu, H.; Wan, L.; Li, Y.; Xu, J.; Xu, J. Copper selenide (Cu3Se2 and Cu2-xSe) thin films: Electrochemical deposition and electrocatalytic application in quantum dot-sensitized solar cells. Dalton Trans. 2018, 47, 16587–16595. [Google Scholar] [CrossRef]
- Ramesh, K.; Bharathi, B.; Thanikaikarasan, S.; Mahalingam, T.; Sebastian, P.J. Growth and Characterization of Electroplated Copper Selenide Thin Films. J. New Mater. Electrochem. Syst. 2013, 16, 127–132. [Google Scholar] [CrossRef]
- Fedorova, E.A.; Maskaeva, L.N.; Markov, V.F.; Bamburov, V.G.; Voronin, V.I. Morphology and Thermal Stability of Thin Cu1.8Se Films Produced by Chemical Deposition. Inorg. Mater. 2019, 55, 106–115. [Google Scholar] [CrossRef]
- Liu, K.; Jing, M.; Zhang, L.; Li, J.; Shi, L. Characterization of the phases and morphology in synthesizing Cu2-xSe and CuSe films. Integr. Ferroelectr. 2018, 189, 71–77. [Google Scholar] [CrossRef]
- Bhuse, V.M.; Hankare, P.P.; Garadkar, K.; Khomane, A. A simple, convenient, low temperature route to grow polycrystalline copper selenide thin films. Mater. Chem. Phys. 2003, 80, 82–88. [Google Scholar] [CrossRef]
- Carter, G.; Navinsek, B.; Whitton, H.L. Heavy ion sputtering induced surface topography development. In Sputtering by Particle Bombardment II; Berish, R., Ed.; Topics in Applied Physics; Springer: Berlin/Heidelberg, Germany, 1983; Volume 52, pp. 231–269. [Google Scholar] [CrossRef]
- Whitton, J.L. Experimental Studies of Morphology Development. In Erosion and Growth of Solids Stimulated by Atom and Ion Beams, 1st ed.; Kiriakidis, G., Garter, G., Whitton, J.L., Eds.; Nato Science Series E; Springer: Dordrecht, The Netherlands, 1986; Volume 112, pp. 151–173. [Google Scholar] [CrossRef]
- Krasheninnikov, A.V.; Nordlund, K. Ion and electron irradiation-induced effects in nanostructured materials. J. Appl. Phys. 2010, 107, 071301. [Google Scholar] [CrossRef]
- Meinander, K.; Nordlund, K. Irradiation-induced densification of cluster-assembled thin films. Phys. Rev. B 2009, 79, 045411. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chen, C.-H.; Chen, S.-Y.; Yen, Y.-T.; Kuo, W.-C.; Liao, Y.-K.; Juang, J.-Y.; Kuo, H.-C.; Lai, C.-H.; Chen, L.-J.; et al. Large Scale Single-Crystal Cu(In,Ga)Se2 Nanotip Arrays for High Efficiency Solar Cell. Nano Lett. 2011, 11, 4443–4448. [Google Scholar] [CrossRef] [PubMed]
- Zimin, S.P.; Gorlachev, E.S.; Mokrov, D.A.; Amirov, I.I.; Gremenok, V.F.; Ivanov, V.A. Specific Features of Vapor–Liquid–Solid Nanostructure Growth on the Surface of SnS Films during Plasma Treatment. Semiconductors 2017, 51, 1728–1731. [Google Scholar] [CrossRef]
- Begrambekov, L.; Grunin, A.; Zakharov, A. Powder modification under influence of heat, electric field and particle irradiation. Nucl. Instrum. Methods B 2015, 354, 282–286. [Google Scholar] [CrossRef]
- Sigmund, P. Elements of Sputtering Theory. In Nanofabrication by Ion-Beam Sputtering, 1st ed.; Som, T., Kanjilal, D., Eds.; Pan Stanford Publishing: Singapore, 2013; pp. 1–40. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 76th ed.; CRC Press: New York, NY, USA, 1995; pp. 3–320. [Google Scholar]
- Piacente, V.; Scardala, P. A study on the vaporization of copper(II) selenide. J. Mater. Sci. Lett. 1994, 13, 1343–1345. Available online: http://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=4245620 (accessed on 1 November 2020). [CrossRef]
- García, V.M.; Guerrero, L.; Nair, M.T.S.; Nair, P.K. Effect of thermal processing on optical and electrical properties of copper selenide thin films. Superf. Vacío 1999, 9, 213–218. [Google Scholar]
- Liew, J.Y.C.; Talib, Z.A.; Zainal, Z.; Kamarudin, M.A.; Osman, N.H.; Lee, H.K. Structural and transport mechanism studies of copper selenide nanoparticles. Semicond. Sci. Technol. 2019, 34, 125017. [Google Scholar] [CrossRef]
- Geng, Z.; Shi, D.; Shi, L.; Li, Y.; Snyder, G.J.; Lam, K. Conventional sintered Cu2-xSe thermoelectric material. J. Materiomics 2019, 5, 626–633. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimin, S.P.; Amirov, I.I.; Vasilev, S.V.; Fedorov, I.S.; Mazaletskiy, L.A.; Kim, N.-H. Modification of Nanocrystalline Porous Cu2-xSe Films during Argon Plasma Treatment. Appl. Sci. 2021, 11, 612. https://doi.org/10.3390/app11020612
Zimin SP, Amirov II, Vasilev SV, Fedorov IS, Mazaletskiy LA, Kim N-H. Modification of Nanocrystalline Porous Cu2-xSe Films during Argon Plasma Treatment. Applied Sciences. 2021; 11(2):612. https://doi.org/10.3390/app11020612
Chicago/Turabian StyleZimin, Sergey P., Ildar I. Amirov, Sergey V. Vasilev, Ivan S. Fedorov, Leonid A. Mazaletskiy, and Nam-Hoon Kim. 2021. "Modification of Nanocrystalline Porous Cu2-xSe Films during Argon Plasma Treatment" Applied Sciences 11, no. 2: 612. https://doi.org/10.3390/app11020612
APA StyleZimin, S. P., Amirov, I. I., Vasilev, S. V., Fedorov, I. S., Mazaletskiy, L. A., & Kim, N.-H. (2021). Modification of Nanocrystalline Porous Cu2-xSe Films during Argon Plasma Treatment. Applied Sciences, 11(2), 612. https://doi.org/10.3390/app11020612





