Improved Delivery of Remedial Agents Using Surface Foam Spraying with Vertical Holes into Unsaturated Diesel-Contaminated Soil for Total Petroleum Hydrocarbon Removal
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Used for This Study
2.2. Preparation of Remediation Solutions
2.3. Oxidant and Bioaugmentation Foam Generation and Characterization
2.4. Soil Column and Vertical Holes in the Soil Column
2.5. Sampling and Measurements
2.6. Statistical Analysis
3. Results and Discussion
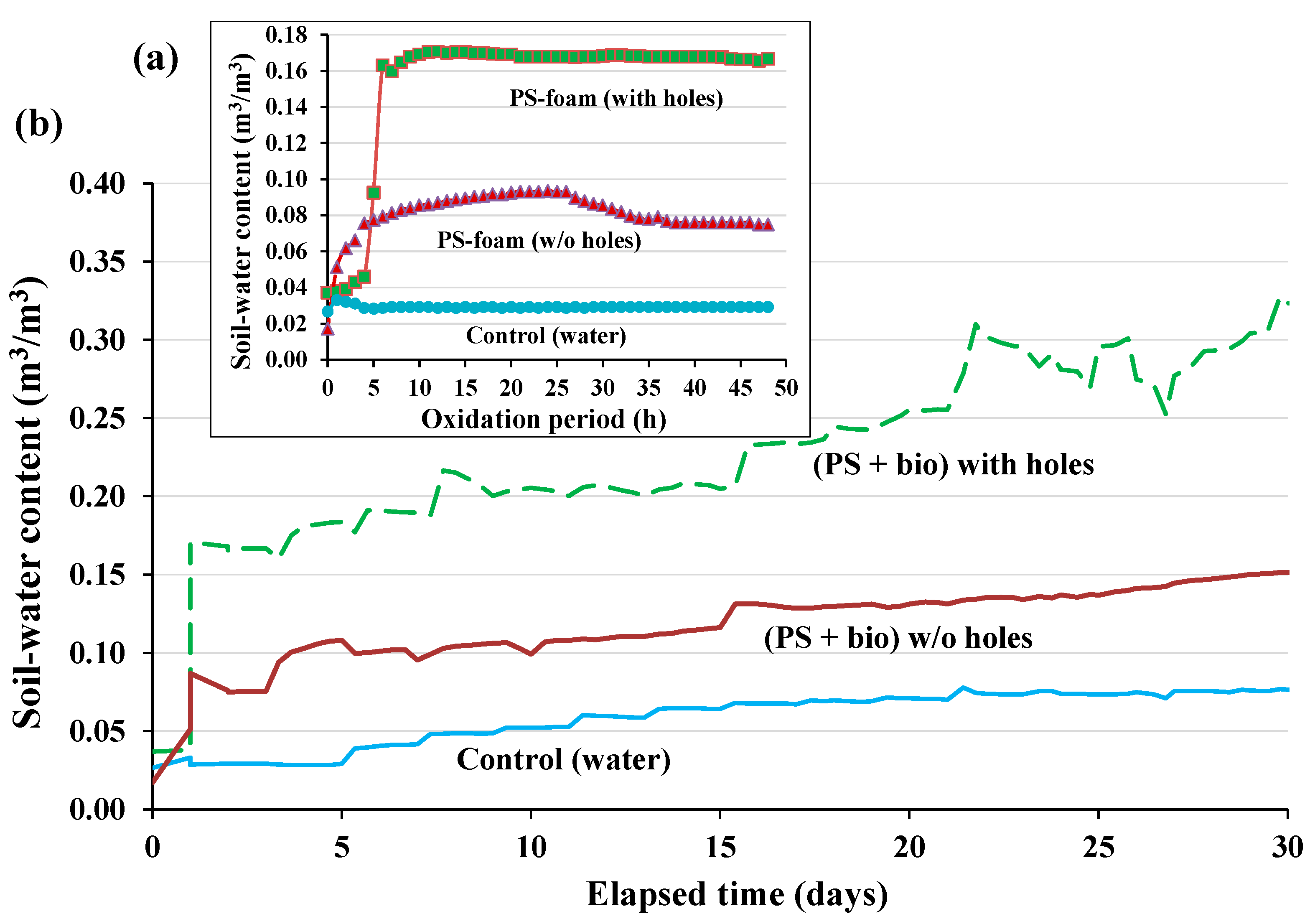
3.1. Volumetric Soil Water Content (VWC) during Soil Column Experiment
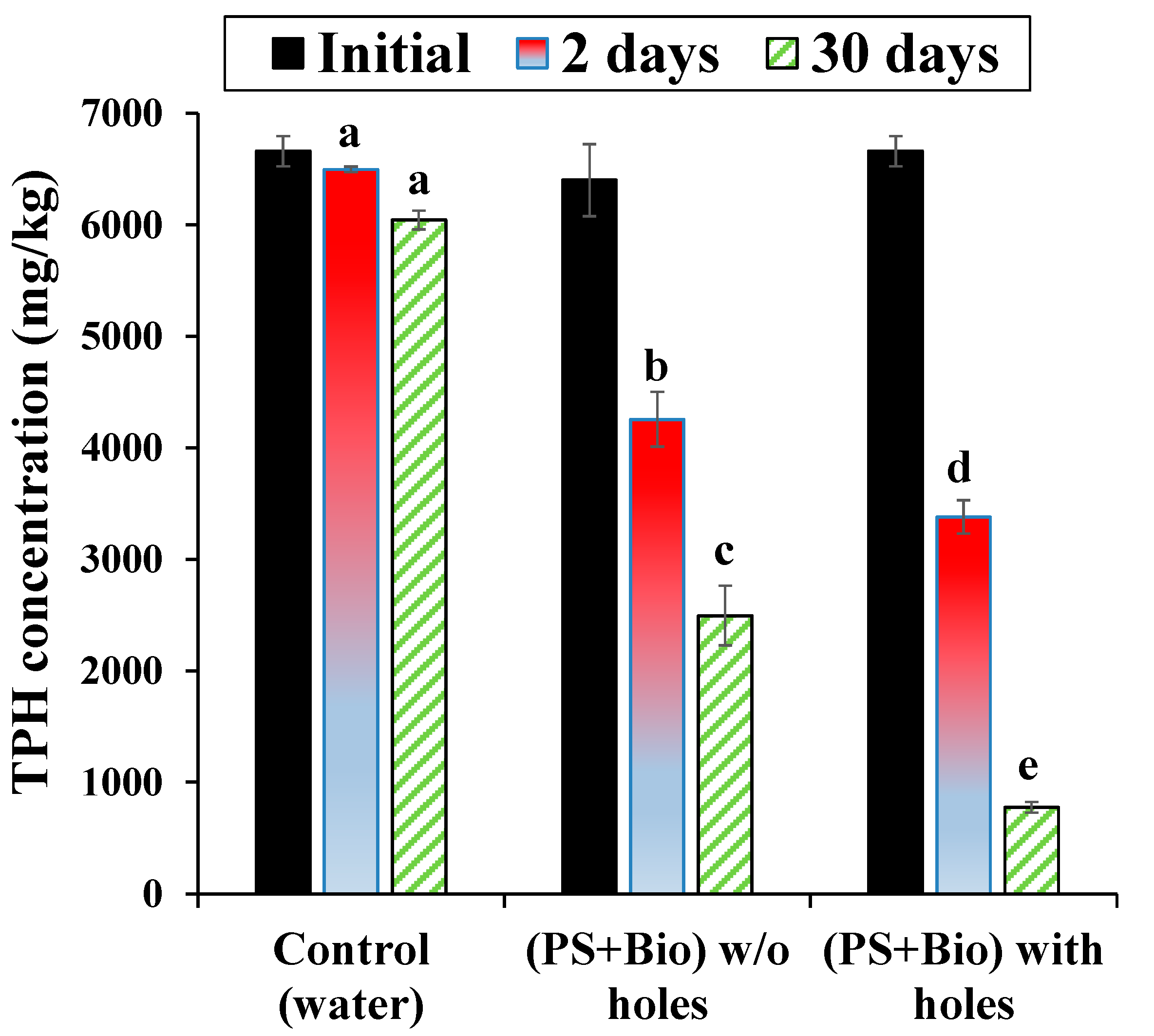
3.2. TPH Degradation in the Soil Column
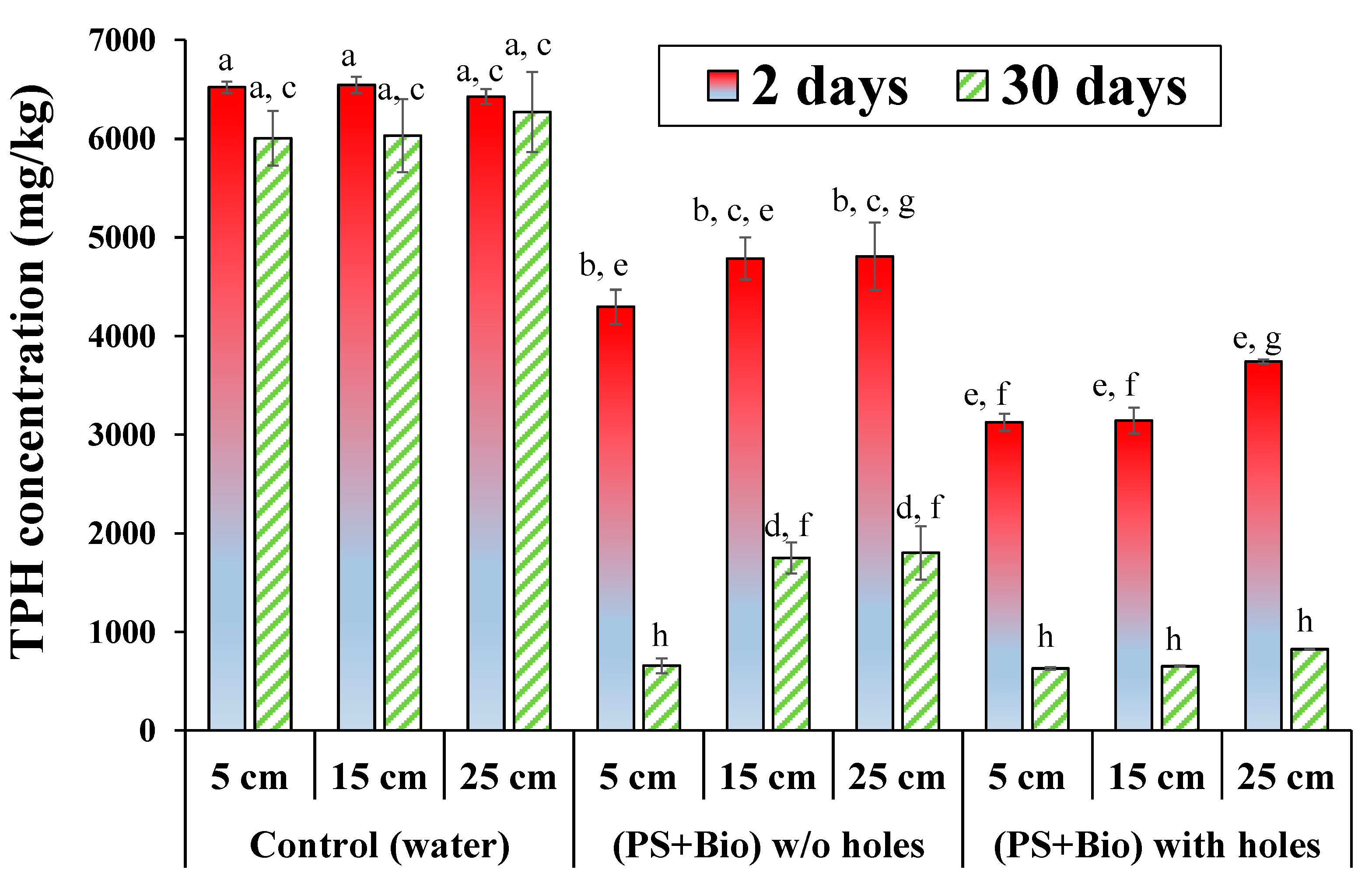
3.3. Vertical TPH Removal at Three Depths in Soil Column
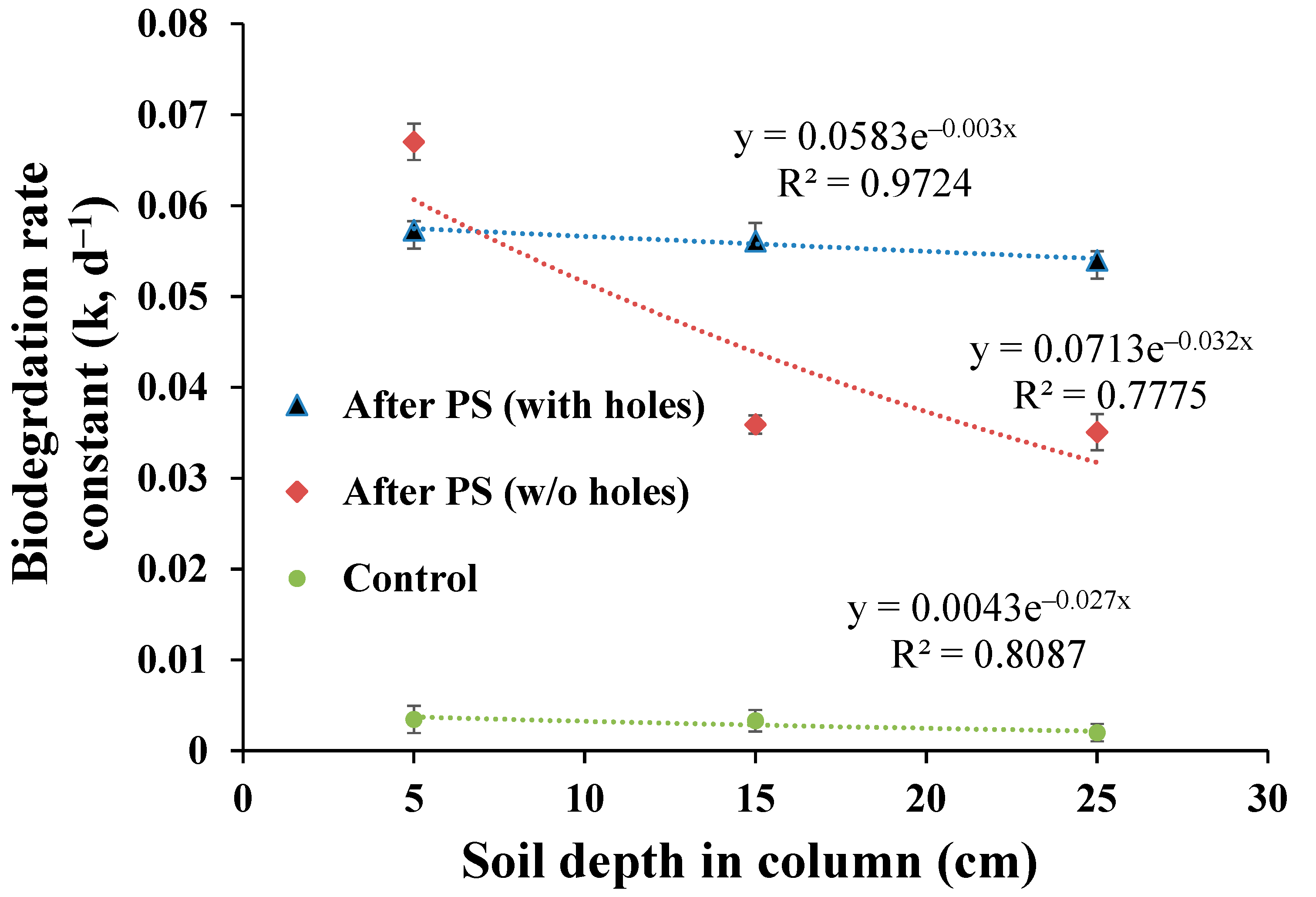
3.4. Vertical Biodegradation Kinetics of TPH in a Soil Column
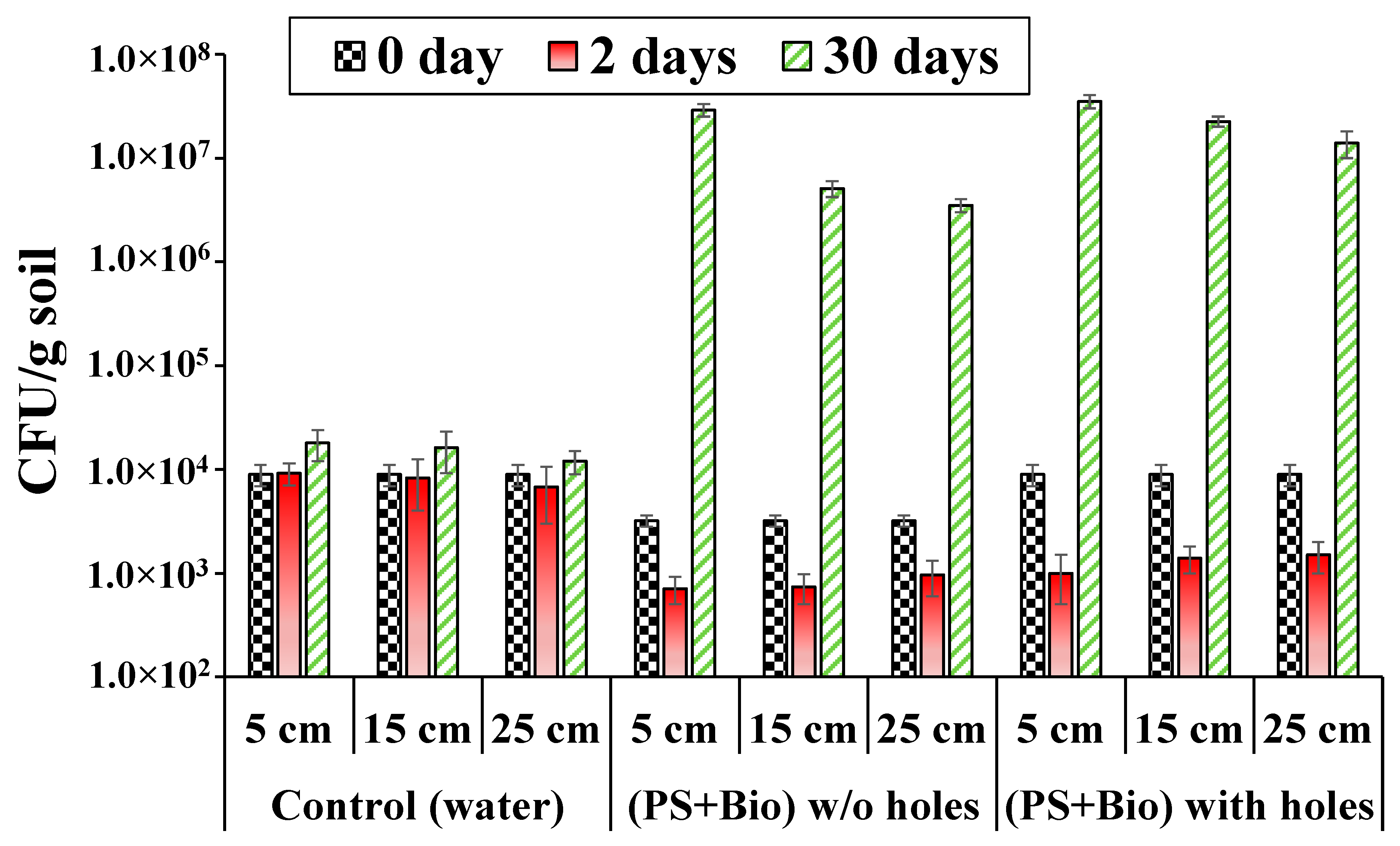
3.5. Changes in Microbial Population
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jeong, S.-W. Estimation of remediation cost for reducing cancer and non-cancer risk of a fuel-contaminated site. J. Korean Soc. Environ. Eng. 2019, 41, 286–291. [Google Scholar] [CrossRef] [Green Version]
- Hadley, P.W.; Harclerode, M. Green remediation or sustainable remediation: Moving from dialogue to common practice. Remediat. J. 2015, 25, 95–115. [Google Scholar] [CrossRef]
- Mohan, S.V.; Kisa, T.; Ohkuma, T.; Kanaly, R.A.; Shimizu, Y. Bioremediation technologies for treatment of PAH-contaminated soil and strategies to enhance process efficiency. Rev. Environ. Sci. Biotechnol. 2006, 5, 347–374. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, J.; Chen, Z. Remediation of water contaminated with diesel oil using a coupled process: Biological degradation followed by heterogeneous Fenton-like oxidation. Chemosphere 2017, 183, 286–293. [Google Scholar] [CrossRef]
- Sirtori, C.; Zapata, A.; Oller, I.; Gernjak, W.; Agüera, A.; Malato, S. Decontamination industrial pharmaceutical wastewater by combining solar photo-Fenton and biological treatment. Water Res. 2009, 43, 661–668. [Google Scholar] [CrossRef]
- Gautam, P.; Bajagain, R.; Jeong, S.-W. Soil infiltration capacity of chemical oxidants used for risk reduction of soil contamination. Ecotoxicol. Environ. Saf. 2019, 183, 109548. [Google Scholar] [CrossRef]
- Reddy, K.R. Technical challenges to in-situ remediation of polluted sites. Geotech. Geol. Eng. 2010, 28, 211–221. [Google Scholar] [CrossRef]
- Goi, A.; Trapido, M.; Kulik, N. Contaminated soil remediation with hydrogen peroxide oxidation. World Acad. Sci. Eng. Technol. 2009, 52, 185–189. [Google Scholar]
- Lim, M.W.; Von Lau, E.; Poh, P.E. A comprehensive guide of remediation technologies for oil contaminated soil—Present works and future directions. Mar. Pollut. Bull. 2016, 109, 14–45. [Google Scholar] [CrossRef] [PubMed]
- Kakosová, E.; Hrabák, P.; Černík, M.; Novotný, V.; Czinnerová, M.; Trögl, J.; Popelka, J.; Kuráň, P.; Zoubková, L.; Vrtoch, Ľ. Effect of various chemical oxidation agents on soil microbial communities. Chem. Eng. J. 2017, 314, 257–265. [Google Scholar] [CrossRef]
- Polli, F.; Zingaretti, D.; Crognale, S.; Pesciaroli, L.; D’Annibale, A.; Petruccioli, M.; Baciocchi, R. Impact of the Fenton-like treatment on the microbial community of a diesel-contaminated soil. Chemosphere 2018, 191, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Goi, A.; Kulik, N.; Trapido, M. Combined chemical and biological treatment of oil contaminated soil. Chemosphere 2006, 63, 1754–1763. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.B. Remediation of weathered petroleum oil-contaminated soil using a combination of biostimulation and modified Fenton oxidation. Int. Biodeterior. Biodegrad. 2012, 70, 89–95. [Google Scholar] [CrossRef]
- Kim, I.; Lee, M. Pilot scale feasibility study for in-situ chemical oxidation using H2O2 solution conjugated with biodegradation to remediate a diesel contaminated site. J. Hazard. Mater. 2012, 241–242, 173–181. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, Z.; Qiao, W.; Wei, X.; Guan, Y.; Ma, Q.; Guan, Y. Remediation of petroleum-contaminated soil after composting by sequential treatment with Fenton-like oxidation and biodegradation. Bioresour. Technol. 2010, 101, 2106–2113. [Google Scholar] [CrossRef]
- Silva-Castro, G.A.; Rodelas, B.; Perucha, C.; Laguna, J.; González-López, J.; Calvo, C. Bioremediation of diesel-polluted soil using biostimulation as post-treatment after oxidation with Fenton-like reagents: Assays in a pilot plant. Sci. Total Environ. 2013, 445–446, 347–355. [Google Scholar] [CrossRef]
- Sutton, N.B.; Grotenhuis, T.; Rijnaarts, H.H.M. Impact of organic carbon and nutrients mobilized during chemical oxidation on subsequent bioremediation of a diesel-contaminated soil. Chemosphere 2014, 97, 64–70. [Google Scholar] [CrossRef]
- Zhong, L.; Szecsody, J.E.; Zhang, F.; Mattigod, S.V. Foam delivery of amendments for vadose zone remediation: Propagation performance in unsaturated sediments. Vadose Zone J. 2010, 9, 757–767. [Google Scholar] [CrossRef]
- Shen, X.; Zhao, L.; Ding, Y.; Liu, B.; Zeng, H.; Zhong, L.; Li, X. Foam, a promising vehicle to deliver nanoparticles for vadose zone remediation. J. Hazard. Mater. 2011, 186, 1773–1780. [Google Scholar] [CrossRef]
- Zhong, L.; Qafoku, N.P.; Szecsody, J.E.; Dresel, P.E.; Zhang, Z.F. Foam delivery of calcium polysulfide to the vadose zone for chromium (VI) immobilization: A laboratory evaluation. Vadose Zone J. 2009, 8, 976–985. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.W.; Corapcioglu, M.Y.; Roosevelt, S.E. Micromodel study of surfactant foam remediation of residual trichloroethylene. Environ. Sci. Technol. 2000, 34, 3456–3461. [Google Scholar] [CrossRef]
- Srirattana, S.; Piaowan, K.; Lowry, G.V.; Phenrat, T. Electromagnetic induction of foam-based nanoscale zerovalent iron (NZVI) particles to thermally enhance non-aqueous phase liquid (NAPL) volatilization in unsaturated porous media: Proof of concept. Chemosphere 2017, 183, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Bajagain, R.; Park, Y.; Jeong, S.W. Feasibility of oxidation-biodegradation serial foam spraying for total petroleum hydrocarbon removal without soil disturbance. Sci. Total Environ. 2018, 626, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Bajagain, R.; Lee, S.; Jeong, S.-W. Application of persulfate-oxidation foam spraying as a bioremediation pretreatment for diesel oil-contaminated soil. Chemosphere 2018, 207, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, D.K.; Bajagain, R.; Jeong, S.-W.; Kim, J. Biodegradation of diesel oil and n-alkanes (C18, C20, and C22) by a novel strain Acinetobacter sp. K-6 in unsaturated soil. Environ. Eng. Res. 2020, 25, 290–298. [Google Scholar] [CrossRef]
- Bajagain, R.; Gautam, P.; Jeong, S.-W. Biodegradation and post-oxidation of fuel-weathered field soil. Sci. Total Environ. 2020, 734, 139452. [Google Scholar] [CrossRef]
- Mulligan, C.N.; Eftekhari, F. Remediation with surfactant foam of PCP-contaminated soil. Eng. Geol. 2003, 70, 269–279. [Google Scholar] [CrossRef]
- Jeong, S.W.; Jeong, J.; Kim, J. Simple surface foam application enhances bioremediation of oil-contaminated soil in cold conditions. J. Hazard. Mater. 2015, 286, 164–170. [Google Scholar] [CrossRef]
- Longpré-Girard, M.; Martel, R.; Robert, T.; Lefebvre, R.; Lauzon, J.M.; Thomson, N. Surfactant foam selection for enhanced light non-aqueous phase liquids (LNAPL) recovery in contaminated aquifers. Transp. Porous Media 2020, 131, 65–84. [Google Scholar] [CrossRef]
- Jing, J.; Sun, J.; Zhang, M.; Wang, C.; Xiong, X.; Hu, K. Preparation and rheological properties of a stable aqueous foam system. RSC Adv. 2017, 7, 39258–39269. [Google Scholar] [CrossRef] [Green Version]
- Ranjani, I.S.; Ramamurthy, K. Relative assessment of density and stability of foam produced with four synthetic surfactants. Mater. Struct. 2010, 43, 1317–1325. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Zheng, C.; Wang, D.; Zhan, H. Effect of temperature on foaming ability and foam stability of typical surfactants used for foaming agent. J. Surfactants Deterg. 2017, 20, 615–622. [Google Scholar] [CrossRef]
- NIER. Korean Soil Analysis Methods; National Institute of Environmental Research: Incheon, Korea, 2013. [Google Scholar]
- Taki, G.; Islam, M.N.; Park, S.J.; Park, J.H. Optimization of operating parameters to remove and recover crude oil from contaminated soil using subcritical water extraction process. Environ. Eng. Res. 2018, 23, 175–180. [Google Scholar] [CrossRef]
- Bajagain, R.; Gautam, P.; Jeong, S.-W. Degradation of petroleum hydrocarbons in unsaturated soil and effects on subsequent biodegradation by potassium permanganate. Environ. Geochem. Health 2019, 42, 1705–1714. [Google Scholar] [CrossRef]
- Gautam, P.; Bajagain, R.; Jeong, S.-W. Combined effects of soil particle size with washing time and soil-to-water ratio on removal of total petroleum hydrocarbon from fuel contaminated soil. Chemosphere 2020, 250, 126206. [Google Scholar] [CrossRef]
- Nimmo, J.R. Preferential flow occurs in unsaturated conditions. Hydrol. Process. 2012, 26, 786–789. [Google Scholar] [CrossRef]
- Jarvis, N.; Koestel, J.; Larsbo, M. Understanding preferential flow in the vadose zone: Recent advances and future prospects. Vadose Zone J. 2016, 15, 1–11. [Google Scholar] [CrossRef]
- USEPA. Engineering Forum Issue: Considerations in Deciding to Treat Contaminated Unsaturated Soils In Situ; EPA/540/S-94/500; Office of Research and Development: Washington, DC, USA, 1993. [Google Scholar]
- Zhu, H.; Aitken, M.D. Surfactant-enhanced desorption and biodegradation of polycyclic aromatic hydrocarbons in contaminated soil. Environ. Sci. Technol. 2010, 44, 7260–7265. [Google Scholar] [CrossRef] [Green Version]
- Chemlal, R.; Abdi, N.; Lounici, H.; Drouiche, N.; Pauss, A.; Mameri, N. Modeling and qualitative study of diesel biodegradation using biopile process in sandy soil. Int. Biodeterior. Biodegrad. 2013, 78, 43–48. [Google Scholar] [CrossRef]
- Suja, F.; Rahim, F.; Taha, M.R.; Hambali, N.; Rizal Razali, M.; Khalid, A.; Hamzah, A. Effects of local microbial bioaugmentation and biostimulation on the bioremediation of total petroleum hydrocarbons (TPH) in crude oil contaminated soil based on laboratory and field observations. Int. Biodeterior. Biodegrad. 2014, 90, 115–122. [Google Scholar] [CrossRef]
- Vincent, A.O.; Felix, E.; Ize-Iyamu, O.K.; Daniel, E.E. Microbial degradation and its kinetics on crude oil polluted soil. Res. J. Chem. Sci. 2011, 1, 8–14. [Google Scholar]
- Yudono, B.; Said, M.; Sabaruddin, K.; Napoleon, A.; Fanani, Z. Kinetics approach of biodegradation of petroleum contaminated soil by using indigenous isolated bacteria. J. Trop. Soils 2013, 16, 33–38. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajagain, R.; Gautam, P.; Jeong, S.-W. Improved Delivery of Remedial Agents Using Surface Foam Spraying with Vertical Holes into Unsaturated Diesel-Contaminated Soil for Total Petroleum Hydrocarbon Removal. Appl. Sci. 2021, 11, 781. https://doi.org/10.3390/app11020781
Bajagain R, Gautam P, Jeong S-W. Improved Delivery of Remedial Agents Using Surface Foam Spraying with Vertical Holes into Unsaturated Diesel-Contaminated Soil for Total Petroleum Hydrocarbon Removal. Applied Sciences. 2021; 11(2):781. https://doi.org/10.3390/app11020781
Chicago/Turabian StyleBajagain, Rishikesh, Prakash Gautam, and Seung-Woo Jeong. 2021. "Improved Delivery of Remedial Agents Using Surface Foam Spraying with Vertical Holes into Unsaturated Diesel-Contaminated Soil for Total Petroleum Hydrocarbon Removal" Applied Sciences 11, no. 2: 781. https://doi.org/10.3390/app11020781






