Coal Fly Ash–Clay Based Geopolymer-Incorporating Electric Arc Furnace Dust (EAFD): Leaching Behavior and Geochemical Modeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Sample Preparation
2.3. Geopolymer Characterization
2.4. Leaching Tests
2.5. Geochemical Modeling
3. Results and Discussion
3.1. Geopolymer Characterization
3.2. Leaching Tests
3.2.1. Mobilization and Availability of Metals
3.2.2. pH dependence Leaching Behavior
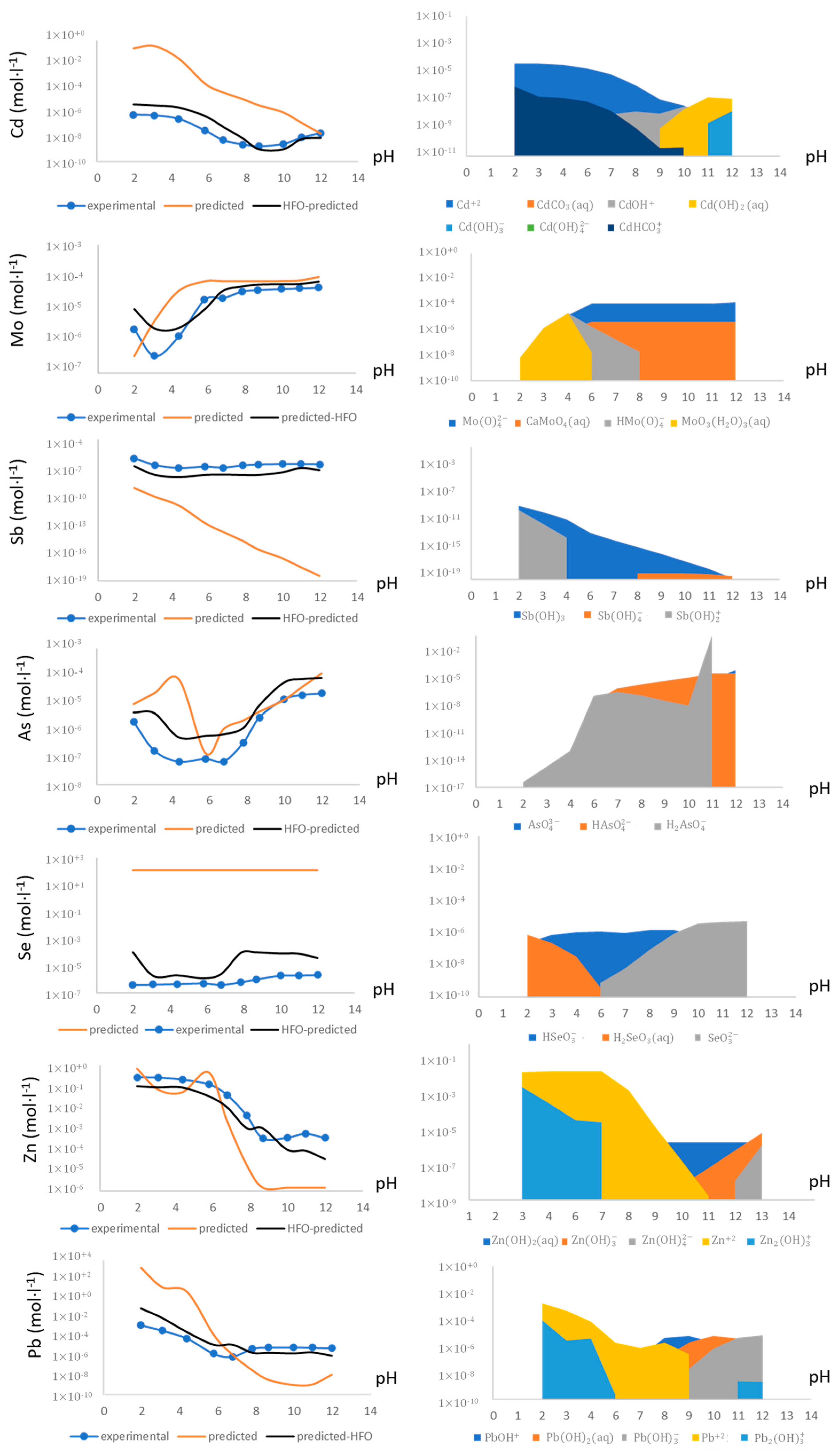
3.3. Geochemical Modeling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Buzin, P.J.W.K.; Heck, N.C.; Vilela, A.C.F. EAF dust: An overview on the influences of physical, chemical and mineral features in its recycling and waste incorporation routes. J. Mater. Res. Technol. 2017, 6, 194–202. [Google Scholar] [CrossRef]
- Sebag, M.G.; Korzenowski, C.; Bernardes, A.M.; Vilela, A.C. Evaluation of environmental compatibility of EAFD using different leaching standards. J. Hazard. Mater. 2009, 166, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Global Steel Dust Ltd. Available online: https://www.bayt.com/en/company/global-steel-dust-gulf-llc-1503840/ (accessed on 20 November 2020).
- Fuessle, R.W.; Taylor, M.A. Long-Term Solidification/Stabilization and Toxicity Characteristic Leaching Procedure for an Electric Arc Furnace Dust. J. Envviron. Eng. 2004, 130, 492–498. [Google Scholar] [CrossRef]
- Sofilić, T.; Rastovčan-Mioč, A.; Cerjan-Stefanović, Š.; Novosel-Radović, V.; Jenko, M. Characterization of steel mill electric-arc furnace dust. J. Hazard. Mater. 2004, 109, 59–70. [Google Scholar] [CrossRef]
- Maslehuddin, M.; Awan, F.R.; Shameem, M.; Ibrahim, M.; Ali, M.R. Effect of electric arc furnace dust on the properties of OPC and blended cement concretes. Constr. Build. Mater. 2011, 25, 308–312. [Google Scholar] [CrossRef]
- The European Commission. Commission Decision 2014/955/EU-List of Waste; The European Commission: Brussels, Belgium, 2014. [Google Scholar]
- Andres, A.; Ruiz-Labrador, B.; Coz, A.; Viguri, J. Testing of solidified-stabilized products at industrial-scale prior to disposal using new compliance leaching tests. In Proceedings of the WASCON Congress, Belgrade, Serbia, 30 May–2 June 2006. [Google Scholar]
- Coz, A.; Ruiz-Labrador, B.; Viguri, J.; Andres, A. Factorial Experimental Design of Batch Leaching Tests in Stabilised/Solidified Metallic Waste. In Proceedings of the ISWA World Congress, Amsterdam, The Netherland, 24–27 September 2007. [Google Scholar]
- Remus, R.; Aguado, M.; Roudier, S.; Delgado, L. Best Available Techniques (BAT) Reference Document for Iron and Steel Production; 212AD; Publications Office of the European Union: Luxembourg, 2012. [Google Scholar]
- Lanzerstorfer, C. Electric arc furnace (EAF) dust: Application of air classification for improved zinc enrichment in in-plant recycling. J. Clean. Prod. 2018, 174, 1–6. [Google Scholar] [CrossRef]
- Elías, X. Residuos Sólidos Urbanos y Fangos Depuradora; Ed. Díaz de Santos: Madrid, Spain, 2012. [Google Scholar]
- Kavouras, P.; Kehagias, T.; Tsilika, I.; Kaimakamis, G.; Chrissafis, K.; Kokkou, S.; Papadopoulos, D.; Karakostas, T. Glass-ceramic materials from electric arc furnace dust. J. Hazard. Mater. 2007, 139, 424–429. [Google Scholar] [CrossRef]
- Magalhães, M.d.S.; Faleschini, F.; Pellegrino, C.; Brunelli, K. Influence of alkali addition on the setting and mechanical behavior of cement pastes and mortars with electric arc furnace dust. Constr. Build. Mater. 2019, 214, 413–419. [Google Scholar] [CrossRef]
- Massarweh, O.; Maslehuddin, M.; Al-Dulaijan, S.U.; Shameem, M.; Ahmad, S. Development of a concrete set retarder utilizing electric arc furnace dust. Constr. Build. Mater. 2020, 255, 119378. [Google Scholar] [CrossRef]
- Nikolic, I.; Durovic, D.; Blecic, D.; Zejak, R.; Karanovic, L.; Mitsche, S.; Radmilovic, V.R. Geopolymerization of coal fly ash in the presence of electric arc furnace dust. Miner. Eng. 2013, 49, 24–32. [Google Scholar] [CrossRef]
- Arnold, M.C.; de Vargas, A.S.; Bianchini, L. Study of electric-arc furnace dust (EAFD) in fly ash and rice husk ash-based geopolymers. Adv. Powder Technol. 2017, 28, 2023–2034. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Labrincha, J.A.; Leonelli, C.; Palomo, A.; Chindaprasirt, P. Handbook of Alkali-Activated Cements, Mortars and Concretes; Woodhead Publishing, Ltd.: Sawston, UK, 2014. [Google Scholar]
- Davidovits, J. Geopolymer: Chemistry and Applications; Institute Geopolymere: Saint-Quentin, France, 2011. [Google Scholar]
- Provis, J.L.; Van Deventer, J.S.J. Geopolymers: Structures, Processing, Properties and Industrial Applications; Woodhead Publishing: Sawston, UK, 2009. [Google Scholar]
- Imtiaz, L.; Rehman, S.K.U.; Ali Memon, S.; Khizar Khan, M.; Faisal Javed, M. A Review of Recent Developments and Advances in Eco-Friendly Geopolymer Concrete. Appl. Sci. 2020, 10, 7838. [Google Scholar] [CrossRef]
- Fang, W.; Qi, G.; Wei, Y.; Kosson, D.S.; van der Sloot, H.A.; Liu, J. Leaching characteristic of toxic trace elements in soils amended by sewage sludge compost: A comparison of field and laboratory investigations. Envviron. Pollut. 2018, 237, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Vollpracht, A.; van der Sloot, H.A. pH dependent leaching characterization of major and trace elements from fly ash and metakaolin geopolymers. Cem. Concr. Res. 2019, 125, 105889. [Google Scholar] [CrossRef]
- Saveyn, H.; Eder, P.; Garbarino, E.; Muchova, L.; Hjelmar, O.; Van Der Sloot, H.A.; Comans, R.; Van Zomeren, A.; Hyks, J.; Oberender, A. Study on Methodological Aspects Regarding Limit Values for Pollutants in Aggregates in the Context of the Possible Development of End-of-Waste Criteria under the EU Waste Framework Directive. 2014. Available online: https://ec.europa.eu/jrc/en/publication/eur-scientific-and-technical-research-reports/study-methodological-aspects-regarding-limit-values-pollutants-aggregates-context-possible (accessed on 20 November 2020).
- Bandow, N.; Gartiser, S.; Ilvonen, O.; Schoknecht, U. Evaluation of the impact of construction products on the environment by leaching of possibly hazardous substances. Envviron. Sci. Eur. 2018, 30, 14. [Google Scholar] [CrossRef] [Green Version]
- European Parliament and Council. Directive (EU) 2018/850 of the European Parliament and of the Council of 30 May 2018 amending Directive 1999/31/EC on the landfill of waste. Off. J. Eur. Union 2018, L150, 100–108. [Google Scholar]
- EN 12920:2007+A1:2009. Characterization of waste-Methodology for the Determination of the Leaching Behaviour of Waste under Specified Condition 2009. Spanish Technical Standard. Available online: http://www.une.org (accessed on 20 September 2019).
- Grathwohl, P.; Susset, B. Comparison of percolation to batch and sequential leaching tests: Theory and data. Waste Manag. 2009, 29, 2681–2688. [Google Scholar] [CrossRef]
- Quina, M.J.; Bordado, J.C.M.; Quinta-Ferreira, R.M. The influence of pH on the leaching behaviour of inorganic components from municipal solid waste APC residues. Waste Manag. 2009, 29, 2483–2493. [Google Scholar] [CrossRef]
- Kosson, D.S.; Van der Sloot, H.A.; Sanchez, F.; Garrabrants, A.C. An Integrated Framework for Evaluating Leaching in Waste Management and Utilization of Secondary Materials. Envviron. Eng. Sci. 2002, 19, 159–204. [Google Scholar] [CrossRef] [Green Version]
- Coz, A.; Ruiz-Labrador, B.; Alonso-Santurde, R.; Coronado, M.; Andres, A. Leaching behaviour methodology as a tool for stabilised/solidified metallic waste characterisation. In Proceedings of the WASCON 2012 Conference Proceedings, Göteborg, Sweden, 30 May–1 June 2012. [Google Scholar]
- Hjelmar, O.; Wahlström, M.; Van Zomeren, A.; Kalbe, U.; Grathwohl, P.; Abdelghafour, M.; Schiopu, N. Robustness Validation of TS-2 and TS-3 Developed by CEN/TC351/WG1 to Assess Release from Products to Soil, Surface Water and Groundwater. 2013. Available online: https://www.nen.nl/media/Overig/WG_1_Robustness_Validation_Report_-_TS-2_and_TS-3_-_Leaching_methods.pdf (accessed on 20 January 2020).
- Jarošíková, A.; Ettler, V.; Mihaljevič, M.; Kříbek, B.; Mapani, B. The pH-dependent leaching behavior of slags from various stages of a copper smelting process: Environmental implications. J. Envviron. Manag. 2017, 187, 178–186. [Google Scholar] [CrossRef]
- Pandey, B.; Kinrade, S.D.; Catalan, L.J.J. Effects of carbonation on the leachability and compressive strength of cement-solidified and geopolymer-solidified synthetic metal wastes. J. Envviron. Manag. 2012, 101, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Van der Sloot, H.A.; Kosson, D.S.; van Zomeren, A. Leaching, geochemical modelling and field verification of a municipal solid waste and a predominantly non-degradable waste landfill. Waste Manag. 2017, 63, 74–95. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, J.; Wang, Z.; Zeng, Y.; Ren, Q. Long-term leaching characterization and geochemical modeling of chromium released from AOD slag. Envviron. Sci. Pollut. Res. 2020, 27, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Fernández Pereira, C.; Luna, Y.; Querol, X.; Antenucci, D.; Vale, J. Waste stabilization/solidification of an electric arc furnace dust using fly ash-based geopolymers. Fuel 2009, 88, 1185–1193. [Google Scholar] [CrossRef]
- Almalkawi, A.T.; Balchandra, A.; Soroushian, P. Potential of Using Industrial Wastes for Production of Geopolymer Binder as Green Construction Materials. Constr. Build. Mater. 2019, 220, 516–524. [Google Scholar] [CrossRef]
- Wang, L.; Geddes, D.A.; Walkley, B.; Provis, J.L.; Mechtcherine, V.; Tsang, D.C.W. The role of zinc in metakaolin-based geopolymers. Cem. Concr. Res. 2020, 136, 106194. [Google Scholar] [CrossRef]
- Tome, S.; Etoh, M.A.; Etame, J.; Sanjay, K. Characterization and leachability behaviour of geopolymer cement synthesised from municipal solid waste incinerator fly ash and volcanic ash blend. Recycling 2018, 3, 50. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.G.; Ke, Y.; Min, X.B.; Liang, Y.J.; Wang, Z.B.; Li, Y.C.; Fei, L.W.; Xu, H.; Jiang, G.H. Cotreatment of MSWI fly ash and granulated lead smelting slag using a geopolymer system. Int. J. Environ Res. Public Health. 2019, 16, 156. [Google Scholar] [CrossRef] [Green Version]
- Azarsa, P.; Gupta, R. Freeze-Thaw performance characterization and leachability of potassium-based geopolymer concrete. J. Compos. Sci. 2020, 4, 45. [Google Scholar] [CrossRef]
- Loncnar, M.; van der Sloot, H.A.; Mladenovič, A.; Zupančič, M.; Kobal, L.; Bukovec, P. Study of the leaching behaviour of ladle slags by means of leaching tests combined with geochemical modelling and mineralogical investigations. J. Hazard. Mater. 2016, 317. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Q.; Xue, J. Reuse of hazardous electrolytic manganese residue: Detailed leaching characterization and novel application as a cementitious material. Resour. Conserv. Recycl. 2020, 154, 104645. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Q.; Jamro, I.A.; Li, R.; Li, Y.; Li, S.; Luan, J. Geochemical modeling and assessment of leaching from carbonated municipal solid waste incinerator (MSWI) fly ash. Environ. Sci. Pollut. Res. 2016, 23, 12107–12119. [Google Scholar] [CrossRef] [PubMed]
- Nikolići, I.; Dsignurović, D.; Tadić, M.; Blečić, D.; Radmilović, V. Immobilization of zinc from metallurgical waste and water solutions using geopolymerization technology. E3S Web Conf. 2013, 1, 41026. [Google Scholar] [CrossRef] [Green Version]
- Coronado, M. Foundry Wastes as New Resources in Ceramic Processes. Ph.D. Thesis, Universidad de Cantabria, Cantabria, Spain, 2014. [Google Scholar]
- De Vargas, A.S.; Dal Molin, D.C.C.; Vilela, A.C.F.; Silva, F.J.D.; Pavão, B.; Veit, H. The effects of Na2O/SiO2 molar ratio, curing temperature and age on compressive strength, morphology and microstructure of alkali-activated fly ash-based geopolymers. Cem. Concr. Compos. 2011, 33, 653–660. [Google Scholar] [CrossRef]
- Izquierdo, M.; Querol, X.; Davidovits, J.; Antenucci, D.; Nugteren, H.; Fernández-Pereira, C. Coal fly ash-slag-based geopolymers: Microstructure and metal leaching. J. Hazard. Mater. 2009, 166, 561–566. [Google Scholar] [CrossRef]
- Álvarez-Ayuso, E.; Querol, X.; Plana, F.; Alastuey, A.; Moreno, N.; Izquierdo, M.; Font, O.; Moreno, T.; Diez, S.; Vázquez, E.; et al. Environmental, physical and structural characterisation of geopolymer matrixes synthesised from coal (co-)combustion fly ashes. J. Hazard. Mater. 2008, 154, 175–183. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Macphee, D.E.; Lachowski, E.E. Fixing arsenic in alkali-activated cementitious matrices. J. Am. Ceram. Soc. 2005, 88, 1122–1126. [Google Scholar] [CrossRef]
- Maldonado-Alameda, À.; Giro-Paloma, J.; Alfocea-Roig, A.; Formosa, J.; Chimenos, J.M. Municipal Solid Waste Incineration Bottom Ash as Sole Precursor in the Alkali-Activated Binder Formulation. Appl. Sci. 2020, 10, 4129. [Google Scholar] [CrossRef]
- Zhang, P.; Muhammad, F.; Yu, L.; Xia, M.; Lin, H.; Huang, X.; Jiao, B.; Shiau, Y.C.; Li, D. Self-cementation solidification of heavy metals in lead-zinc smelting slag through alkali-activated materials. Constr. Build. Mater. 2020, 249, 118756. [Google Scholar] [CrossRef]
- Abdel-Gawwad, H.A.; Mohammed, M.S.; Zakey, S.E. Preparation, performance, and stability of alkali-activated-concrete waste-lead-bearing sludge composites. J. Clean. Prod. 2020, 259, 120924. [Google Scholar] [CrossRef]
- Heasman, L.; van der Sloot, H.; Quevauviller, H. Harmonization of Leaching/Extraction tests. Stud. Environ. Sci. 1997, 70. [Google Scholar]
- Xu, P.; Zhao, Q.; Qiu, W.; Xue, Y. The evaluation of the heavy metal leaching behavior of MSWI-FA added alkali-activated materials bricks by using different leaching test methods. Int. J. Environ. Res. Public Health 2019, 16, 1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Cetin, B.; Likos, W.J.; Edil, T.B. Impacts of pH on leaching potential of elements from MSW incineration fly ash. Fuel 2016, 184, 815–825. [Google Scholar] [CrossRef]
- Engelsen, C.J.; Van Der Sloot, H.A.; Wibetoe, G.; Justnes, H.; Lund, W.; Stoltenberg-Hansson, E. Leaching characterisation and geochemical modelling of minor and trace elements released from recycled concrete aggregates. Cem. Concr. Res. 2010, 40, 1639–1649. [Google Scholar] [CrossRef] [Green Version]
- Komonweeraket, K.; Benson, C.H.; Edil, T.B.; Bleam, W.F. Leaching behavior and mechanisms controlling the release of elements from soil stabilized with fly ash. In Proceedings of the Geo-Frontiers 2011: Advances in Geotechnical Engineering, Dallas, TX, USA, 13–16 March 2011; pp. 1101–1110. [Google Scholar]
- Cui, Y.; Chen, J.; Zhang, Y.; Peng, D.; Huang, T.; Sun, C. pH-Dependent Leaching Characteristics of Major and Toxic Elements from Red Mud. Int. J. Environ. Res. Public Health 2019, 16, 2046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
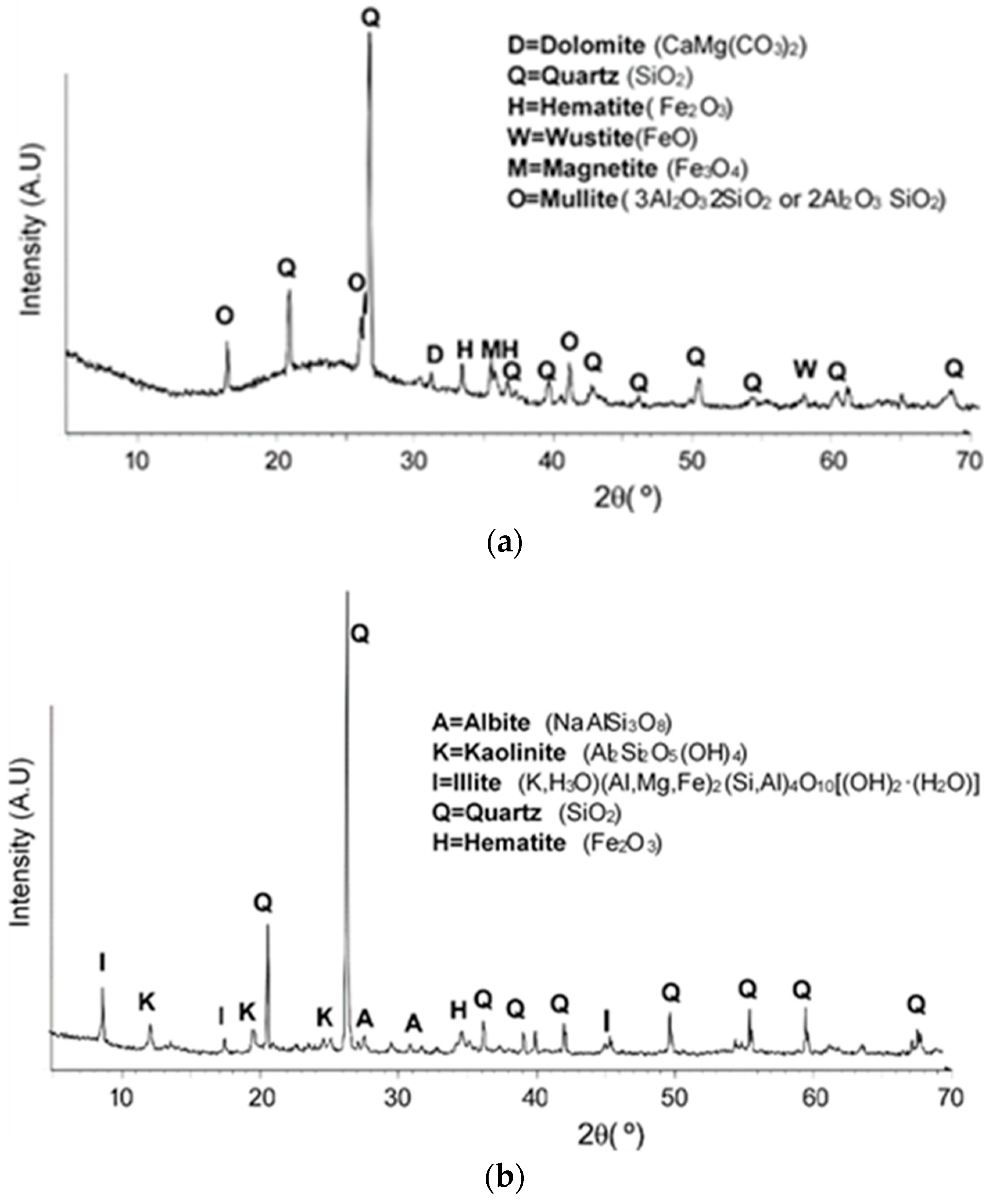
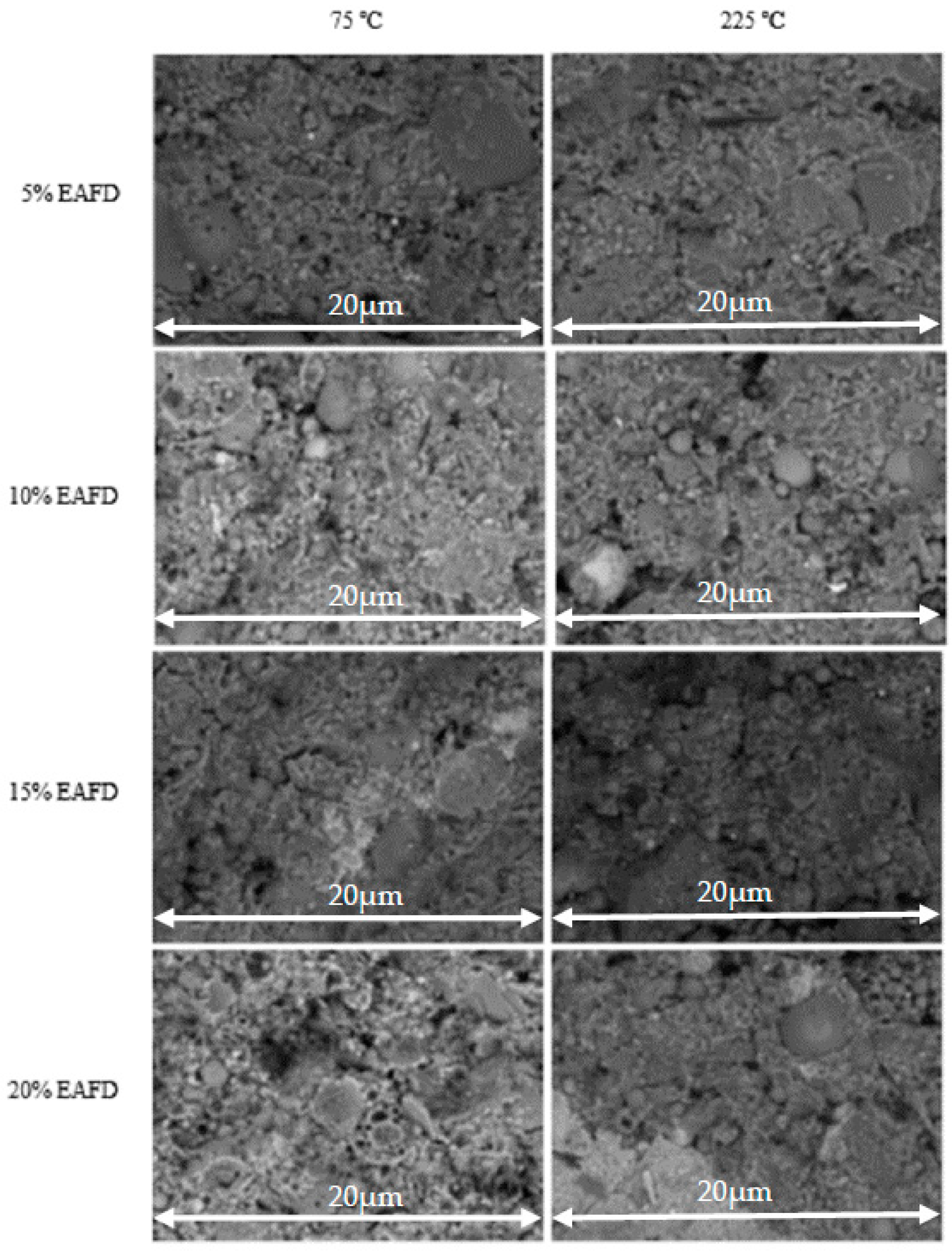
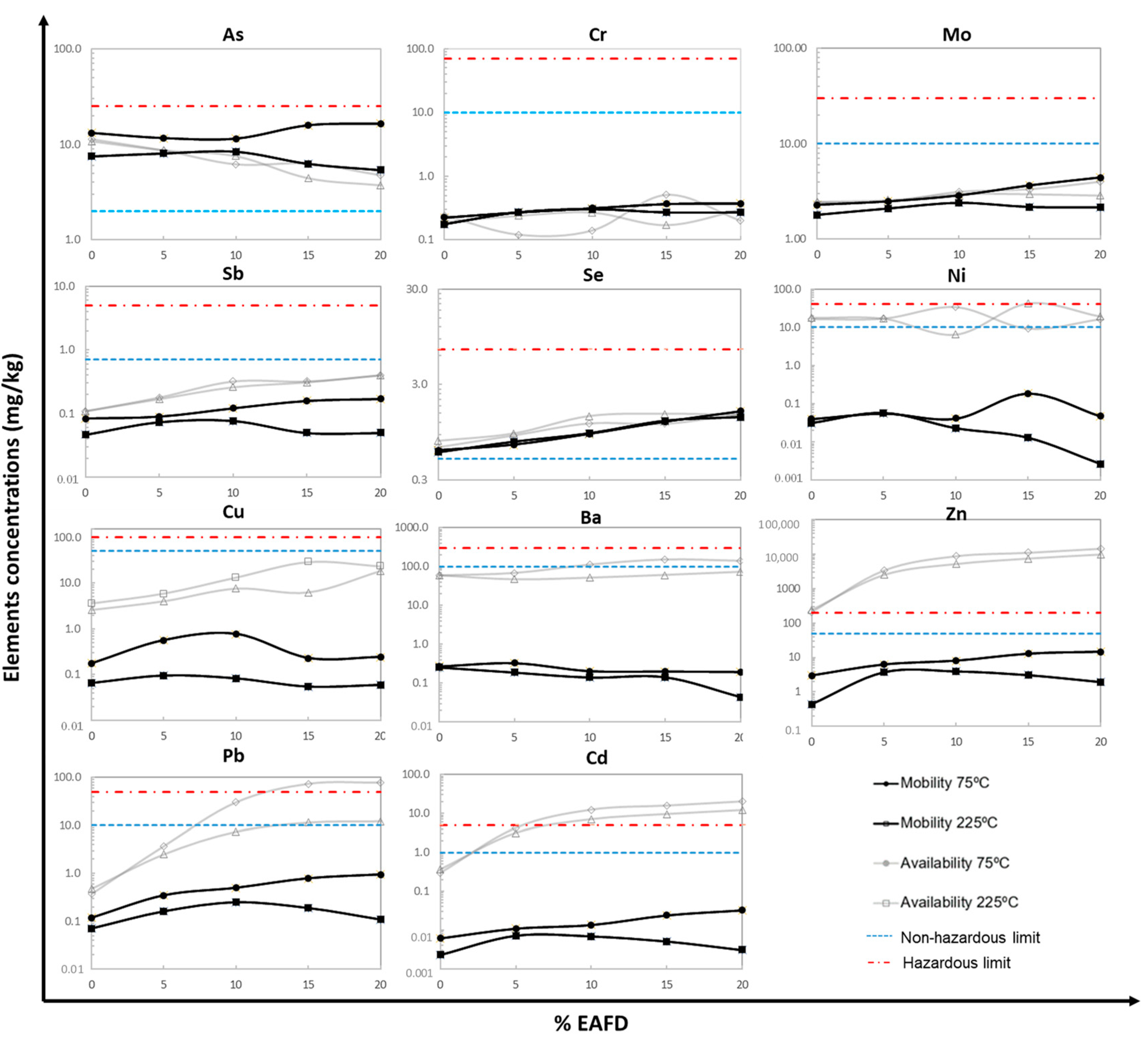

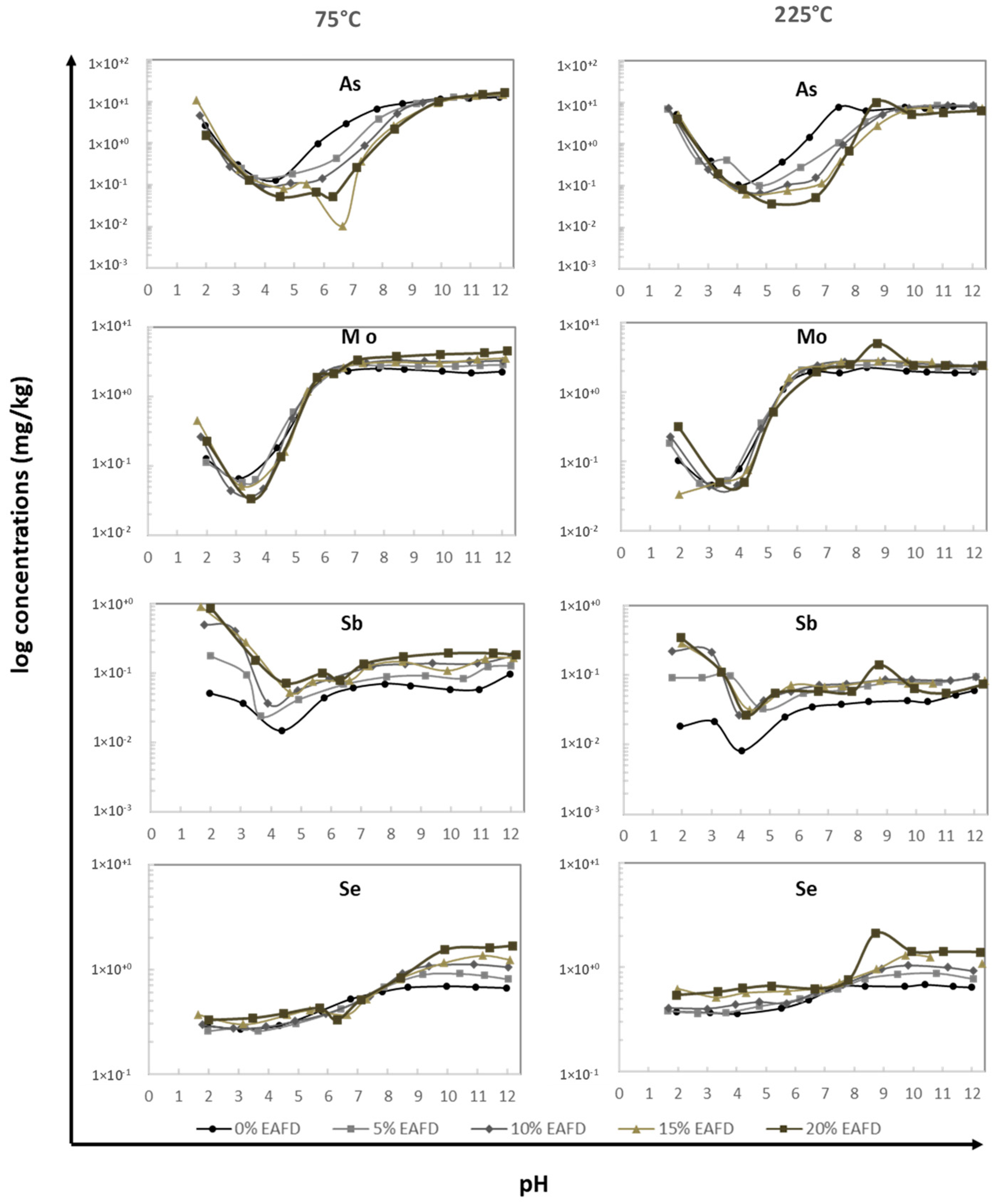
| Raw Materials | FA | Clay | EAFD |
|---|---|---|---|
| Composition (wt %) | |||
| SiO2 | 52.38 | 64.22 | 4.18 |
| Al2O3 | 21.32 | 16.93 | 0.98 |
| Fe2O3 | 6.88 | 5.94 | 33.36 |
| K2O | 2.50 | 3.03 | 1.40 |
| CaO | 6.23 | 0.52 | 6.71 |
| MgO | 2.48 | 0.89 | 2.38 |
| Na2O | 2.02 | 0.58 | 1.88 |
| TiO2 | 0.92 | 0.86 | 0.09 |
| MnO | 0.07 | 0.06 | 2.42 |
| P2O5 | 0.82 | 0.08 | 0.97 |
| LOI | 5.39 | 5.78 | 11.33 |
| Composition (mg/kg) | |||
| As | 44 | 30 | 39.50 |
| Ba | 2662.25 | 483 | 477 |
| Cd | 1.10 | 0.50 | 260 |
| Cr | 76.25 | 46 | 2040 |
| Cu | 85.75 | 27 | 2030 |
| Hg | n.d. | n.d. | n.d. |
| Mo | 18 | 2 | 35.10 |
| Ni | 129 | 29 | 150 |
| Pb | 52.50 | 28 | 23,000 |
| Sb | 7.80 | 2.5 | 136 |
| Se | 0.55 | 0.02 | 38.70 |
| Zn | 291.25 | 139 | 259,000 |
| % EAFD | Water Absorption (%) | Flexural Strength (MPa) | ||
|---|---|---|---|---|
| 75 °C | 225 °C | 75 °C | 225 °C | |
| 0 | 5.41 ± 0.15 | 5.89 ± 0.25 | 11.72 ± 2.83 | 8.73 ± 2.93 |
| 5 | 5.00 ± 0.26 | 5.62 ± 0.18 | 17.02 ± 4.92 | 8.72 ± 3.19 |
| 10 | 5.00 ± 0.21 | 5.71 ± 0.24 | 16.42 ± 1.82 | 11.67 ± 4.48 |
| 15 | 4.69 ± 0.20 | 6.64 ± 0.33 | 16.56 ± 2.12 | 16.15 ± 7.98 |
| 20 | 4.44 ± 0.22 | 7.27 ± 0.29 | 16.06 ± 0.93 | 18.92 ± 2.49 |
| Main Species |
|---|
| MoO42−; Sb(OH)3; Sb(OH)2+; Pb2+; Cd2+; AsO43−;HSeO31−; SeO32−; SeO42−; HSeO41−; Zn2+ |
| Elements |
| Mo, Sb, Pb, Cd, As, Se, Zn |
| Mineral Phases * |
| Powellite (CaMoO4); Senarmontite (Sb2O3); Valentinite (Sb2O3); Antimony (VI) Oxide (Sb2O4); Lead(II) hydroxide (Pb2(OH)2); Otavite (CdCO3); Arsenic(V) oxide (As2O5); Arsenates CO3(As2O4)2; Metal Arsenates (Ca3(AsO4)2·4H2O, Pb3(AsO4)2; Zn3AsO4·5H2O); Zincite (ZnO); Selenium(IV) oxide (SeO2); Selenium(VI) oxide (SeO3). |
| Mineral Adsorption |
| Diffuse layer Surface complexation model of Fe- and Al-/hydro) oxides Surface (HFO) mineral adsorption |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cifrian, E.; Dacuba, J.; Llano, T.; Díaz-Fernández, M.d.C.; Andrés, A. Coal Fly Ash–Clay Based Geopolymer-Incorporating Electric Arc Furnace Dust (EAFD): Leaching Behavior and Geochemical Modeling. Appl. Sci. 2021, 11, 810. https://doi.org/10.3390/app11020810
Cifrian E, Dacuba J, Llano T, Díaz-Fernández MdC, Andrés A. Coal Fly Ash–Clay Based Geopolymer-Incorporating Electric Arc Furnace Dust (EAFD): Leaching Behavior and Geochemical Modeling. Applied Sciences. 2021; 11(2):810. https://doi.org/10.3390/app11020810
Chicago/Turabian StyleCifrian, Eva, Juan Dacuba, Tamara Llano, María del Carmen Díaz-Fernández, and Ana Andrés. 2021. "Coal Fly Ash–Clay Based Geopolymer-Incorporating Electric Arc Furnace Dust (EAFD): Leaching Behavior and Geochemical Modeling" Applied Sciences 11, no. 2: 810. https://doi.org/10.3390/app11020810





