Design and Development of Continuous Passive Motion (CPM) for Fingers and Wrist Grounded-Exoskeleton Rehabilitation System
Abstract
1. Introduction
2. Technical and Clinical Requirements
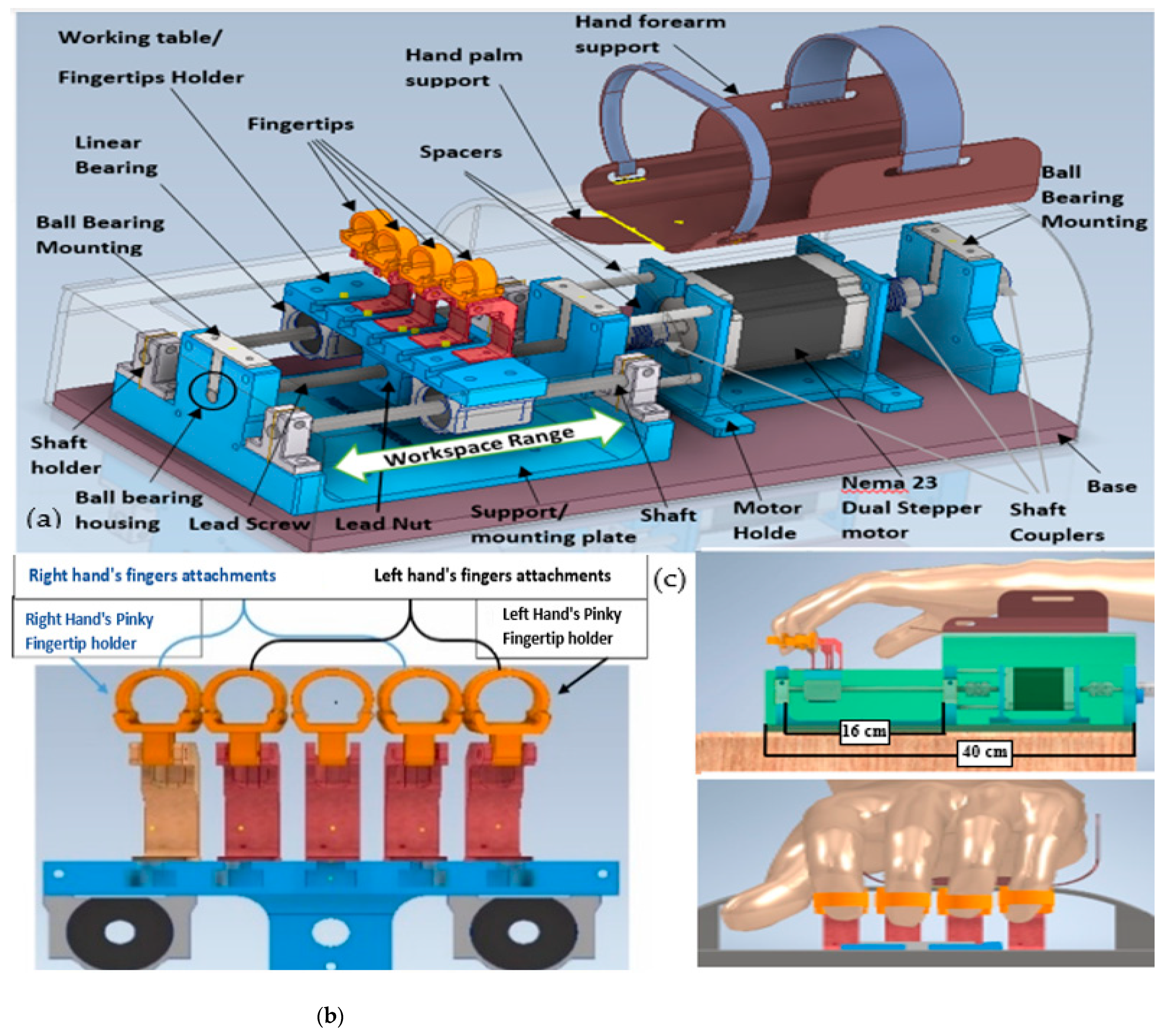
3. Design of FWRMS
3.1. Fingers Mechanism Design
3.2. Wrist and Forearm Mechanism Design
4. The Hardware Design and Implementation
- A display unit, a 16 × 2 LCD (Liquid Crystal Display), it is a low-cost, fundamental display module. Technically, it has 16-pin, which can display 16 characters per line; each character appeared in a 5 × 7 pixel matrix. Functionally, it displays the preprogrammed therapy characters; unlike other primary display devices such as seven segments, there is no limitation of displaying unique and custom characters; as a function, all the control parameters display through it.
- Three pushbuttons with LEDs, and a buzzer, is implemented. The first pushbutton is employed to start the therapy. The second pushbutton is utilized to pause/resume the therapy execution session temporarily, and the third pushbutton deactivates the actuation unit and cancels the therapy immediately; this is important as a safety feature at the hardware level. Furthermore, whenever a pushbutton clicks, the buzzer gives a piping sound to notify and confirm the taken action.
- An incremental rotary encoder with a center pushbutton was employed. It is efficient input hardware for mechatronics systems, where the angular position is required; they come with buttons attached. It can be clicked by pressing the knob and is recognized by the Arduino just as any other pushbutton; the advantage of using rotary encoders is that their rotation is limitless. We have used a 24-pulse mechanical, incremental rotary encoder with a pushbutton switch to scroll through software designed menus and select control parameters options.
- The master controller, an Arduino nano based on an AT-mega328P microcontroller, owns the advantage of low cost, compact size 18 × 35 mm, persuasive functions, and lightweight 7 g, which significantly reduce the size and weight of the portable control. Arduino nano consists of 8 analog input pinouts, operates by 5 VDC operating voltage, 1 KB EEPROM, 2 KB SRAM, and 32 KB flash memory. The required current per each I/O pinouts is 40 mA. All input data and output commands of the first subsystem’s pushbuttons, LEDs, buzzer, and LCD, and the incremental rotary encoder are controlled through it.
- Concerning the actuator, a hybrid bipolar dual shaft stepper motor is utilized in the actuation unit. A step motor is a brushless DC electric motor which often employed in the biomechatronics system [38]. Precise positioning control in medical devices is essential. We utilized a NEMA23 stepper motor with the configurations of; a two-phase motor with four leading wires, holding torque 1.3N.m, rated current 4A, rated voltage 3.3 VDC, external dimensions 56.4 × 56.4 × 56 mm, and the dual shaft dimensions are 8 mm 6.35 mm. Figure 3 shows a 3D motor model and motor’s implementation. Generally, brushless motors use non-periodic movements; it breaks a full revolution into several equivalent steps; hence the step motor does not rotate but steps, the step angle of used motor is 1.8 degrees per step.
- Regarding single driving, A 2m542 stepper driver is used to control our system’s desired steps. The 2m542 driver has the specifications of; supply voltage of 24 VDC, a pulse input frequency ranged from 0 to 200 kHz, micro-steps up to 25600 steps/rev, with the implementation of a robust 32-bit Digital Signal Processing (DSP) processor. The DSP acquires the control command and converts it to the operation command of the motor. As per the motor operation commands, the DSP transmits the required signals to the drive circuit. This technology considerably enhances the stepper motor’s efficiency and reduces motor’s vibration, with the benefits of low noise, less current, and lower heating. Moreover, this technology can accomplish smoother motion performance at low speeds by significantly minimizing variations from the desired motor speed.
- Moreover, mechanical limit switches, as implied by their name, limit switch are electromechanical devices. Mechanical limit switches have the advantage of high accuracy and repeatability, reliable, and low power consumption devices. We implemented two limit switches to control the worktable’s mechanical functional range. The switches were engaged in controlling the travel worktable limit, to ensure that the worktable will be within the preselected fingers F/E displacement range.
5. Software Control Method Implementation
- A confirmation warning will emerge from the sub-menu after selecting the desired trainable joint from the main menu, asking whether or not to confirm the type of therapy chosen; this is an additional safety feature at the software level.
- After confirming the chosen F/E range, the second step is to select the desired ROM degrees for the wrist and forearm joints or minimum and maximum displacement to flex and extend the wrist and forearm therapy fingers. FWRMS controller is programmed to provide the Wrist F/E with controllable rotational ROMs ranged from 0°–78°/0°–68°, Wrist R/U from 0°–28°/0°–38°, while Forearm S/P from 0°–88°/0°–83°. as shown in Table 2. To achieve these degrees, we implemented a position control method for the stepper motor. The necessary steps of the position control method of the used stepper motor include: Since the used motor step angle is 1.8 degrees, which means, for each step, the motor rotates 1.8 degrees; by using a simple calculation, the number of steps produced by the motor that needed to achieve the desired degrees for example 45 degrees can be determined (revolution Steps for each revolution = 45/step angle, (45/1.8 = 25 Steps for each revolution). practically, in order to achieve the desired 45 degrees, the motor driver sends 25 pulses into the step pin. Therefore, in the backend code, the developed loop has 25 iterations, so the step pin for pulse generation is adjusted on a high state and then low for each iteration. Once the 25 derived steps are executed, we add a two-second delay and then alter the rotation orientation by turning the direction pin on a low state pin, in that method we could also control the repeat numbers of the motor execution. In the developed menu items the users can select the number of the desired repeats ranged between 0–200 repeat. This method of was applied at all therapy execution control, and FWRMS took advantage of these steps to control and set the desired angular position.
- However, for the execution method for finger F/E is specified, the machine will look to extend the fingers to a desired maximum value of extension; until it is executed, it will look then to the desired flexion minimum value. The controller checks whether the fingers have reached those limits by tracking the stepper motor steps via the stepper driver to control the desired steps. Therefore, no position feedback sensor is required, which significantly reduces our proposed system’s overall cost. However, for the finger’s F/E, we have limited the derived steps numbers in the developed main menu to match the worktable’s functional range. As demonstrated in in Figure 10 the displacement range is between (0 to −8 cm) for fingers flexion (0 to +8 cm) for fingers extension. These ranges of displacement are enough for the functional ranges of different fingers.
- It is crucial to define a starting position, which is considered as a safety position. Therefore, before and after any therapy session, the exoskeleton fingertips return to their starting position (zero position). Figure 10 demonstrates the summarized block diagram of the control working flow. The actuator starts spinning until it reaches the worktable’s maximum workspace physically clicks the first limit (+LS) switch. Once the +LS signals are registered, the controller disables the motor’s driver’s pulse signal. Eventually, that leads to stop the stepper motor, then return to the motor start spinning again in the opposite direction until the worktable reaches the other limit switch (−LS); once the −LS signal confirmed, the motor driver provides the stepper motor with the required pules until it reaches the predefined zero-position.
- In the developed menu items, the users can adjust the therapy speed. We have implemented the speed control to drive the desired therapy speed of the desired therapy. The digital step motor driver’s pulse frequency (Hz) determines the motor speed. The pulse frequency induces the oscillatory motion. It specifies how quick to replicate a move of a certain step in a certain time. The motor pule frequency can be transformed into rpm, which indicates the revolving motion. Therefore, to regulate the stepper motor rotation speed can only be done by modifying the motor driver pule frequencies or the input pulse numbers. However, it is essential to limit the maximum motor speed on the software level; we have limited the selectable speed range for the wrist F/E, R/U, and Forearm S/P between 0–10 rpm. On the other side, the required speed for fingers F/E mechanism is higher than the other joints; because of the leading screw and nut’s transmission mechanism, we have limited it to the range between 0–80 rpm, which is enough to drive smooth motion for the fingers. Table 2 summarized these technical configurations of the proposed FWRMS; it is worth to mention these specifications were selected based on the suggestions of professional therapists at the Rehabilitation Clinic, University of Debrecen.
6. Experimental Tests
6.1. Experimental Procedure
6.2. Experimental Results and Discussion
7. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADL | Activity Daily Living |
| DOF | Degree of Freedom |
| AAM | Active Assistive Motion |
| CPM | Continuous Passive Motion |
| EMG | Electromyographic |
| FPGA | Field-Programmable Gate Array |
| DIP | Distal Interphalangeal |
| PIP | Proximal Interphalangeal |
| MCP | Metacarpophalangeal |
| SPRM | Symmetric Pinion and Rack Mechanism |
| PC | Personal Computer |
| PLA | Polylactic Acid |
| ROM | Range of Motion |
| UART | Universal Asynchronous Receiver/Transmitter |
| LCD | Liquid Crystal Display |
| EEPROM | Electrically Erasable Programmable Read-Only Memory |
| DSP | Digital Signal Processing |
| RMSE | Root Mean Square Error |
| SRAM | Static Random-Access Memory |
| THT | Through-Hole Technology |
| PCB | Printed Circuit Board |
| MC | Mechanical Connections |
| RPM | Revolution Per Minute |
| bps | Bits Per Second |
References
- World Health Organization. Global Health Estimates; World Health Organization: Geneva, Switzerland, 2012; Available online: http://www.who.int/healthinfo/global_burden_disease/en/ (accessed on 6 December 2020).
- World Health Organisation. STEPwise Approach to Stroke Surveillance. 2011. Available online: http://www.who.int/chp/steps/stroke/en/index.html (accessed on 6 December 2020).
- Harwin, W.S.; Patton, J.L.; Edgerton, V.R. Challenges and Opportunities for Robot-Mediated Neurorehabilitation. Proc. IEEE 2006, 94, 1717–1726. [Google Scholar] [CrossRef]
- Bos, R.A.; Haarman, C.J.W.; Stortelder, T.; Nizamis, K.; Herder, J.L.; Stienen, A.H.A.; Plettenburg, D.H. A structured overview of trends and technologies used in dynamic hand orthoses. J. Neuroeng. Rehabil. 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- Ueki, S.; Kawasaki, H.; Ito, S.; Nishimoto, Y.; Abe, M.; Aoki, T.; Ishigure, Y.; Ojika, T.; Mouri, T. Development of a Hand-Assist Robot with Multi-Degrees-of-Freedom for Rehabilitation Therapy. IEEE ASME Trans. Mechatron. 2012, 17, 136–146. [Google Scholar] [CrossRef]
- Wyndaele, J.J. Color atlas of human anatomy. Spinal Cord 1995, 47, 838. [Google Scholar] [CrossRef][Green Version]
- NHS Choices Symptoms Stroke. Available online: https://www.nhs.uk/conditions/stroke/symptoms/ (accessed on 24 October 2020).
- Chu, C.-Y.; Patterson, R.M. Soft robotic devices for hand rehabilitation and assistance: A narrative review. J. Neuroeng. Rehabil. 2018, 15. [Google Scholar] [CrossRef]
- Chen, B.; Zi, B.; Wang, Z.; Qin, L.; Liao, W.-H. Knee exoskeletons for gait rehabilitation and human performance augmentation: A state-of-the-art. Mech. Mach. Theory 2019, 134, 499–511. [Google Scholar] [CrossRef]
- Maciejasz, P.; Eschweiler, J.; Gerlach-Hahn, K.; Jansen-Troy, A.; Leonhardt, S. A survey on robotic devices for upper limb rehabilitation. J. Neuroeng. Rehabil. 2014, 11, 3. [Google Scholar] [CrossRef]
- Riener, R.; Nef, T.; Colombo, G. Robot-aided neurorehabilitation of the upper extremities. Med. Biol. Eng. Comput. 2005, 43, 2–10. [Google Scholar] [CrossRef]
- Marchal-Crespo, L.; Reinkensmeyer, D.J. Review of control strategies for robotic movement training after neurologic injury. J. Neuroeng. Rehabil. 2009, 6. [Google Scholar] [CrossRef]
- Kawasaki, H.; Ito, S.; Ishigure, Y.; Nishimoto, Y.; Aoki, T.; Mouri, T.; Sakaeda, H.; Abe, M. Development of a Hand Motion Assist Robot for Rehabilitation Therapy by Patient Self-Motion Control. In Proceedings of the 2007 IEEE 10th International Conference on Rehabilitation Robotics, Noordwijk, The Netherlands, 13–15 June 2007. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Wei, R.; Perez, M.; Shepard, B.; Koeneman, E.; Koeneman, J.; He, J. RUPERT: An exoskeleton robot for assisting rehabilitation of arm functions. In Proceedings of the 2008 Virtual Rehabilitation, Vancouver, BC, Canada, 25–27 August 2008. [Google Scholar] [CrossRef]
- Lambercy, O.; Dovat, L.; Gassert, R.; Burdet, E.; Teo, C.L.; Milner, T. A Haptic Knob for Rehabilitation of Hand Function. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Palsbo, S.E.; Hood-Szivek, P. Effect of Robotic-Assisted Three-Dimensional Repetitive Motion to Improve Hand Motor Function and Control in Children with Handwriting Deficits: A Nonrandomized Phase 2 Device Trial. Am. J. Occup. Ther. 2012, 66, 682–690. [Google Scholar] [CrossRef] [PubMed]
- AbdulKareem, A.H.; Adila, A.S.; Husi, G. Recent trends in robotic systems for upper-limb stroke recovery: A low-cost hand and wrist rehabilitation device. In Proceedings of the 2018 2nd International Symposium on Small-scale Intelligent Manufacturing Systems (SIMS), Cavan, Ireland, 16–18 April 2018. [Google Scholar] [CrossRef]
- Ho, T.-Y.; Chen, Y.-J.; Chen, P.-H. The design and implementation of a motor drive for foot rehabilitation. Comput. Electr. Eng. 2016, 56, 795–806. [Google Scholar] [CrossRef]
- The Amadeo® System, Tyromotion. Available online: http://www.tyromotion.com/en/products/amadeo/ (accessed on 6 December 2020).
- Patterson Companies, Inc.—Home. Available online: http://www.sammonspreston.com/app.aspx?cmd=get_product&id=91378 (accessed on 6 December 2020).
- Koeneman, E.J.; Schultz, R.S.; Wolf, S.L.; Herring, D.E.; Koeneman, J.B. A pneumatic muscle hand therapy device. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5 September 2004. [Google Scholar] [CrossRef]
- Nef, T.; Guidali, M.; Klamroth-Marganska, V.; Riener, R. ARMin—Exoskeleton Robot for Stroke Rehabilitation. IFMBE Proc. 2009, 127–130. [Google Scholar] [CrossRef]
- Sanchez, R.; Reinkensmeyer, D.; Shah, P.; Liu, J.; Rao, S.; Smith, R.; Cramer, S.; Rahman, T.; Bobrow, J. Monitoring functional arm movement for home-based therapy after stroke. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5 September 2004. [Google Scholar] [CrossRef]
- Hesse, S.; Schulte-Tigges, G.; Konrad, M.; Bardeleben, A.; Werner, C. Robot-assisted arm trainer for the passive and active practice of bilateral forearm and wrist movements in hemiparetic subjects11An organization with which 1 or more of the authors is associated has received or will receive financial benefits from a commercial party having a direct financial interest in the results of the research supporting this article. Arch. Phys. Med. Rehabil. 2003, 84, 915–920. [Google Scholar] [CrossRef]
- Cordo, P.; Lutsep, H.; Cordo, L.; Wright, W.G.; Cacciatore, T.; Skoss, R. Assisted Movement with Enhanced Sensation (AMES): Coupling Motor and Sensory to Remediate Motor Deficits in Chronic Stroke Patients. Neurorehabilit. Neural Repair 2008, 23, 67–77. [Google Scholar] [CrossRef]
- Takahashi, C.D.; Der-Yeghiaian, L.; Le, V.; Motiwala, R.R.; Cramer, S.C. Robot-based hand motor therapy after stroke. Brain 2008, 131, 425–437. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Mikami, Y.; Watanabe, K.; Sankai, Y. Five-fingered assistive hand with mechanical compliance of human finger. In Proceedings of the 2008 IEEE International Conference on Robotics and Automation, Pasadena, CA, USA, 19–23 May 2008. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, S.; Sun, Q. Development and Assist-As-Needed Control of an End-Effector Upper Limb Rehabilitation Robot. Appl. Sci. 2020, 10, 6684. [Google Scholar] [CrossRef]
- da Silva, L.D.L.; Pereira, T.F.; Leithardt, V.R.Q.; Seman, L.O.; Zeferino, C.A. Hybrid Impedance-Admittance Control for Upper Limb Exoskeleton Using Electromyography. Appl. Sci. 2020, 10, 7146. [Google Scholar] [CrossRef]
- Zhang, F.; Lin, L.; Yang, L.; Fu, Y. Design of an Active and Passive Control System of Hand Exoskeleton for Rehabilitation. Appl. Sci. 2019, 9, 2291. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Cui, Y.; Dong, M.; Fang, B.; Zhang, P. Design and performance analysis of a parallel wrist rehabilitation robot (PWRR). Robot. Auton. Syst. 2020, 125, 103390. [Google Scholar] [CrossRef]
- Pang, Z.; Wang, T.; Wang, Z.; Yu, J.; Sun, Z.; Liu, S. Design and Analysis of a Wearable Upper Limb Rehabilitation Robot with Characteristics of Tension Mechanism. Appl. Sci. 2020, 10, 2101. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Zi, B.; Cao, Z.; Ding, H. Development of an active and passive finger rehabilitation robot using pneumatic muscle and magnetorheological damper. Mech. Mach. Theory 2020, 147, 103762. [Google Scholar] [CrossRef]
- Fischer, H.C.; Stubblefield, K.; Kline, T.; Luo, X.; Kenyon, R.V.; Kamper, D.G. Hand Rehabilitation Following Stroke: A Pilot Study of Assisted Finger Extension Training in a Virtual Environment. Top. Stroke Rehabil. 2007, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Almusawi, H.; Afghan, S.A.; Géza, H. Designing the Mechanical Parts of a Low-Cost Hand Rehabilitation CPM Device for Stroke Patients. Innov. Eng. Entrep. 2018, 60–66. [Google Scholar] [CrossRef]
- Andrews, J.G.; Youm, Y. A biomechanical investigation of wrist kinematics. J. Biomech. 1979, 12, 83–93. [Google Scholar] [CrossRef]
- Gull, M.A.; Bai, S.; Bak, T. A Review on Design of Upper Limb Exoskeletons. Robotics 2020, 9, 16. [Google Scholar] [CrossRef]
- Ragazzo, F. Review on Upper Limb Continuous Passive Motion Devices. MATEC Web Conf. 2016, 53, 01062. [Google Scholar] [CrossRef]
- Charmant, J. Kinovea 0.9.3, Computer Software 2020. Available online: https://www.kinovea.org/ (accessed on 6 December 2020).
- Puig-Diví, A.; Escalona-Marfil, C.; Padullés-Riu, J.M.; Busquets, A.; Padullés-Chando, X.; Marcos-Ruiz, D. Validity and reliability of the Kinovea program in obtaining angles and distances using coordinates in 4 perspectives. PLoS ONE 2019, 14, e0216448. [Google Scholar] [CrossRef]
- Moral-Muñoz, J.A.; Esteban-Moreno, B.; Arroyo-Morales, M.; Cobo, M.J.; Herrera-Viedma, E. Agreement Between Face-to-Face and Free Software Video Analysis for Assessing Hamstring Flexibility in Adolescents. J. Strength Cond. Res. 2015, 29, 2661–2665. [Google Scholar] [CrossRef]
- Guzmán-Valdivia, C.H.; Blanco-Ortega, A.; Oliver-Salazar, M.A.; Carrera-Escobedo, J.L. Therapeutic motion analysis of lower limbs using Kinovea. Int. J. Soft Comput. Eng. 2013, 3, 2231–2307. [Google Scholar]
- Youssef, A.R. Photogrammetric Quantification of Forward Head Posture is Side Dependent in Healthy Participants and Patients with Mechanical Neck Pain. Int. J. Physiother. 2016, 3. [Google Scholar] [CrossRef]
- Fernández-González, P.; Koutsou, A.; Cuesta-Gómez, A.; Carratalá-Tejada, M.; Miangolarra-Page, J.C.; Molina-Rueda, F. Reliability of Kinovea® Software and Agreement with a Three-Dimensional Motion System for Gait Analysis in Healthy Subjects. Sensors 2020, 20, 3154. [Google Scholar] [CrossRef] [PubMed]














| System | No. of Actuated Movements | Training Mode | Transmission Schematic | Type of Actuator | Mechanical Structure Type | Supported Movements |
|---|---|---|---|---|---|---|
| [29] | 2 | Passive | parallel mechanism | 2* Servo motors | Exoskeleton | Elbow (F/E) and shoulder (F/E) |
| [30] | 1 | Passive and active | SPRM) with the parallel mechanism | DC motor | Exoskeleton | 1 finger |
| [31] | 2 | Passive and active | parallel mechanism | 2* pneumatic actuator | End-effector | Wrist (F/E, R/U) |
| [32] | 6 | CPM | rope + toothed belt | DC motors Harmonic motors | End-effector and exoskeleton | shoulder (F/E, I/E, A/A), elbow (F/E, I/E); wrist (F/E). |
| [33] | 1 | Active and Passive | Cable-driven and differential rotation | PMS | exoskeleton | 1 finger (F/E) |
| Our FWRMS | 7 | CPM | Indirect screw nut mechanism and rotational | 1* Electrical Stepper motor | End-effector and grounded-exoskeleton | 4 Fingers (F/E), Wrist (F/E, R/U) Forearm (S/P) |
| Joint | Joint ROM | FWRMS ROM | Repeat Time | Speed |
|---|---|---|---|---|
| Wrist F/E | 0°–80°/0°–70° | 0°–78°/0°–68° | 0–200 repeat | 0–10 RPM |
| Wrist R/U | 0°–30°/0°–40° | 0°–28°/0°–38° | 0–200 repeat | 0–10 RPM |
| Finger F/E DIP | 0°–0°/0°–80° | 0°–0°/0°–80° | 0–200 repeat | 0–80 RPM |
| Forearm S/P | 0°–90°/0°–85° | 0°–88°/0°–83° | 0–200 repeat | 0–10 RPM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almusawi, H.; Husi, G. Design and Development of Continuous Passive Motion (CPM) for Fingers and Wrist Grounded-Exoskeleton Rehabilitation System. Appl. Sci. 2021, 11, 815. https://doi.org/10.3390/app11020815
Almusawi H, Husi G. Design and Development of Continuous Passive Motion (CPM) for Fingers and Wrist Grounded-Exoskeleton Rehabilitation System. Applied Sciences. 2021; 11(2):815. https://doi.org/10.3390/app11020815
Chicago/Turabian StyleAlmusawi, Husam, and Géza Husi. 2021. "Design and Development of Continuous Passive Motion (CPM) for Fingers and Wrist Grounded-Exoskeleton Rehabilitation System" Applied Sciences 11, no. 2: 815. https://doi.org/10.3390/app11020815
APA StyleAlmusawi, H., & Husi, G. (2021). Design and Development of Continuous Passive Motion (CPM) for Fingers and Wrist Grounded-Exoskeleton Rehabilitation System. Applied Sciences, 11(2), 815. https://doi.org/10.3390/app11020815






