Management of the First Feeding of Dorada Brycon sinuensis with Two Species of Cladocerans
Abstract
:1. Introduction
2. Materials and Methods
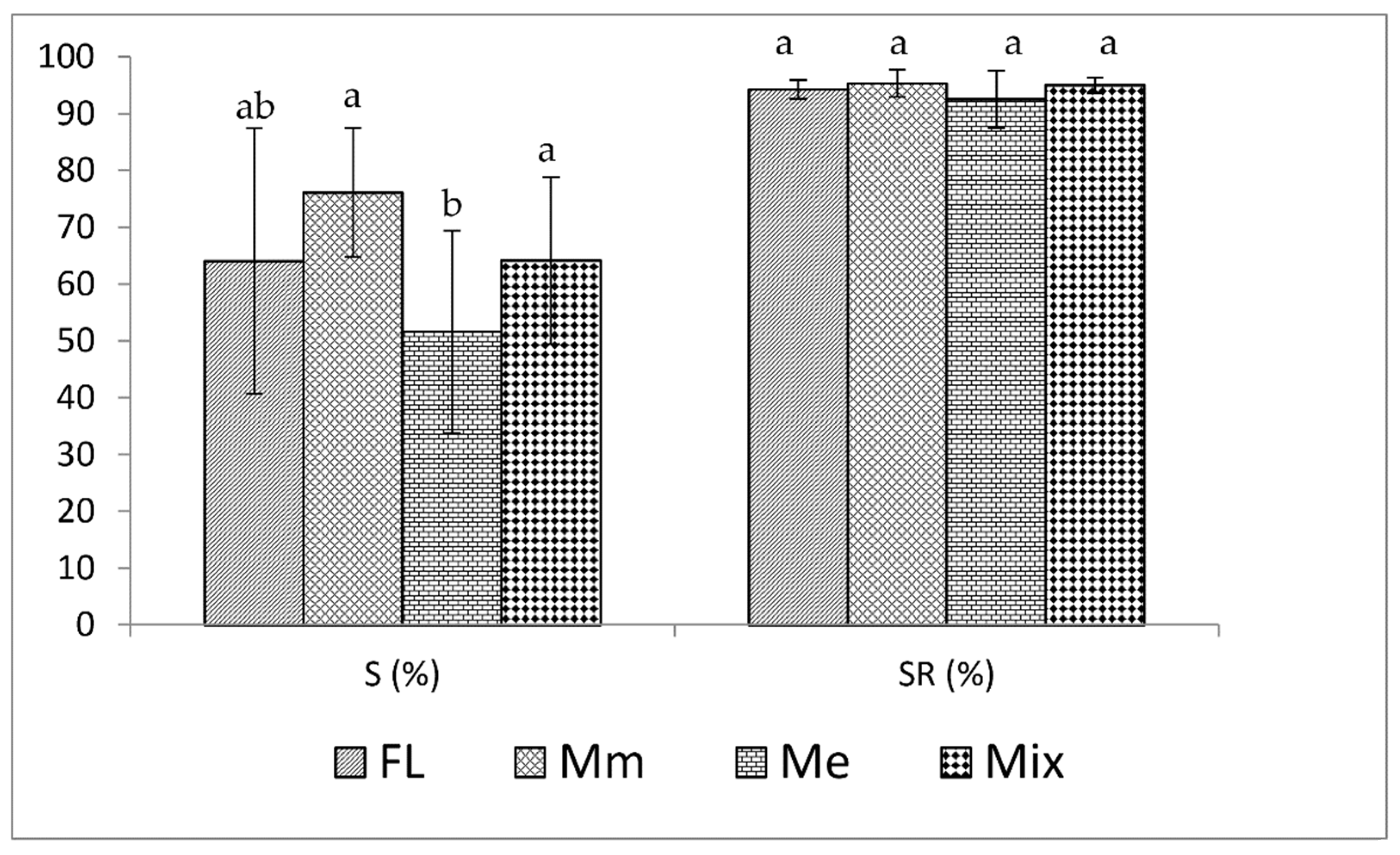
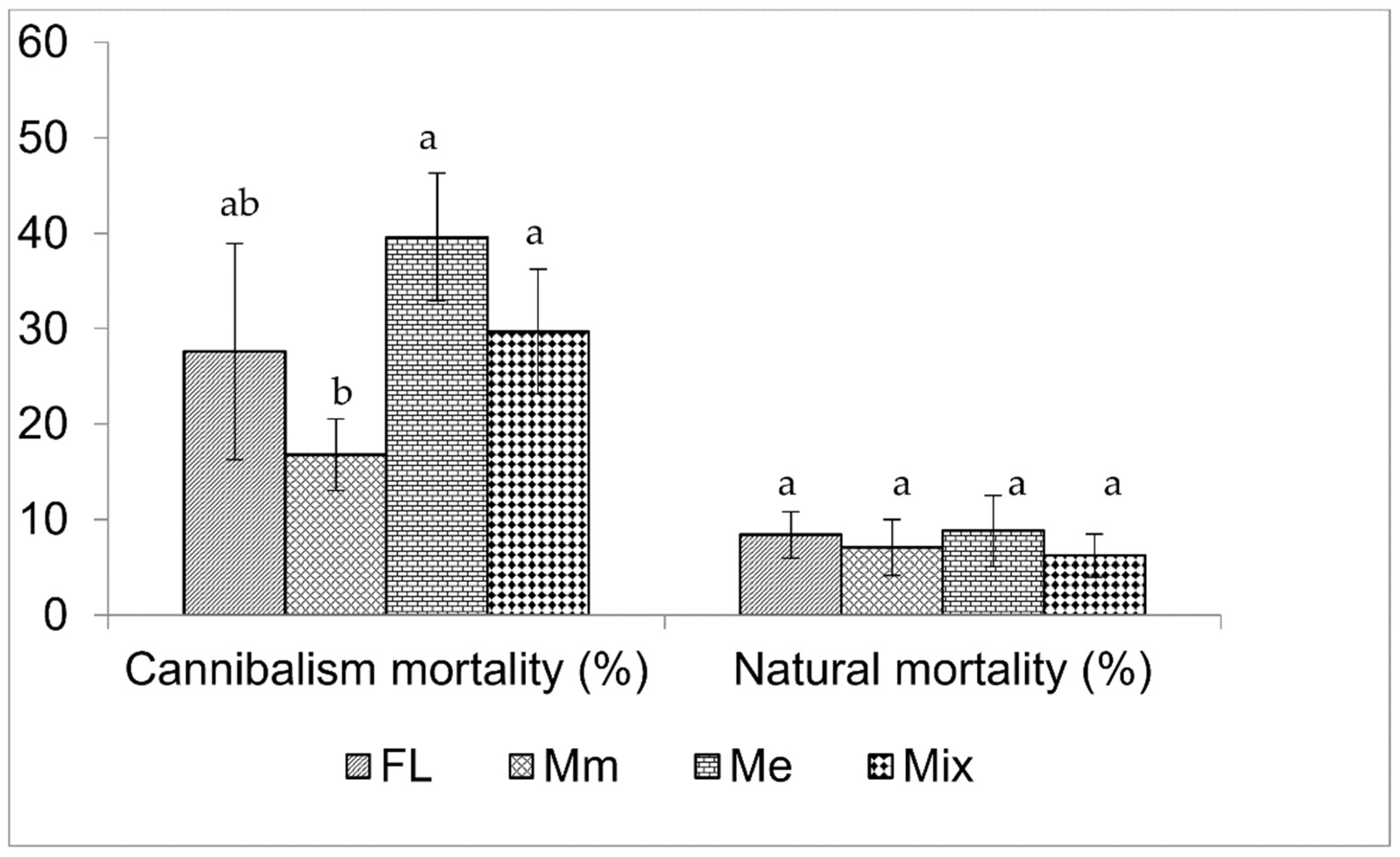
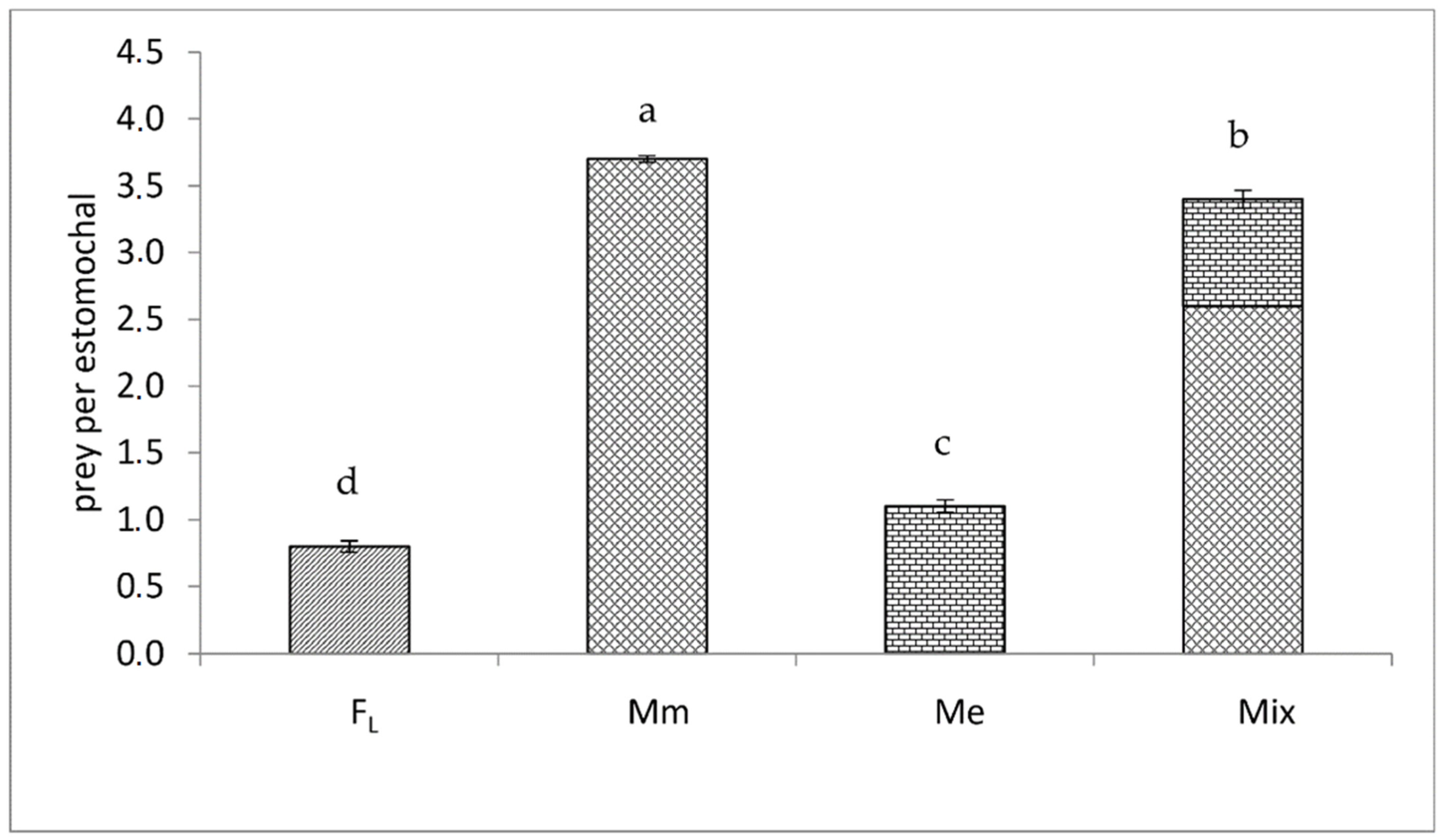
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mojica, J.I.E.; Usma Oviedo, J.U.E.; Alvarez León, R.E.; Lasso, C.A. Libro rojo de peces Dulceacuícolas de Colombia (2012); Instituto de Investigación de Recursos Biológicos, Alexander von Humboldt: Bogotá, Colombia, 2012. [Google Scholar]
- Atencio-García, V.J.; Pertuz Buelvas, V.M.; Pérez Espitia, F.; Ortiz Mestra, R.; Carrasco, S.C.P. Manejo de la primera alimentación de dorada Brycon sinuensis ofreciendo larvas de bocachico Prochilodus magdalenae. Rev. Colomb. Cienc. Pecu. 2010, 23, 317–324. [Google Scholar]
- Atencio-García, V.; Zaniboni-Filho, E.; Pardo-Carrasco, S.; Arias-Castellanos, A. Influência da primeira alimentação na larvicultura e alevinagem do yamú Brycon siebenthalae (Characidae). Acta Sci. Anim. Sci. 2003, 25, 61–72. [Google Scholar] [CrossRef]
- Atencio-García, V.J.; Zaniboni Filho, E. El canibalismo en la larvicultura de peces. Rev. MVZ Córdoba 2006, 11, 9–19. [Google Scholar] [CrossRef]
- da Silva, A.F.L.; Russo, M.R.; de Araújo Ramos, L.; Rocha, A.S. Feeding of larvae of the hybrid surubim Pseudoplatystoma sp. under two conditions of food management. Acta Sci. Biol. Sci. 2013, 35, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Ferraz, F.B.; Gomes, L.C. Social relationship as inducer of immunological and stress responses in matrinxã (Brycon amazonicus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 153, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Baras, E.; Lucas, M. Individual growth trajectories of sibling Brycon moorei raised in isolation since egg stage, and their relationship with aggressive behaviour. J. Fish Biol. 2010, 77, 985–997. [Google Scholar] [CrossRef] [PubMed]
- de Souza, E.C.M.; da Silva, J.P.; Villacorta-Correa, M.A.; Carvalho, T.B. Aggressiveness and locomotion activity related to hatching time in Matrinxã, Brycon amazonicus (Spix and Agassiz, 1829). Appl. Anim. Behav. Sci. 2014, 157, 146–151. [Google Scholar] [CrossRef]
- Marciales Caro, L.J.; DíazOlarte, J.J.; Medina Robles, V.M.; Cruz Casallas, P.E. Evaluación del crecimiento y sobrevivencia de larvas de bagre rayado Pseudoplatystoma fasciatum (Linneaus, 1766) alimentadas con alimento vivo natural y enriquecido con ácidos grasos. Rev. Colomb. Cienc. Pecu. 2010, 23, 308–316. [Google Scholar]
- Prieto-Guevara, M.; Atencio-García, V. Zooplancton en la larvicultura de peces neotropicales. [Zooplankton in larviculture of neotropical fishes.]. Rev. Med. Vet. Zootec. Córdoba 2008, 13, 1415–1425. [Google Scholar] [CrossRef] [Green Version]
- Prieto-Guevara, M.; De la Cruz, L.; Morales, M. Cultivo experimental del cladocero Moina sp alimentado con Ankistrodesmus sp y Saccharomyces cereviseae. Revista MVZ Córdoba 2006, 11, 705–714. [Google Scholar] [CrossRef]
- Morales-Ventura, J.; Nandini, S.; Sarma, S.; Castellanos-Páez, M.E. Demography of zooplankton (Anuraeopsis fissa, Brachionus rubens and Moina macrocopa) fed Chlorella vulgaris and Scenedesmus acutus cultured on different media. Rev. Biol. Trop. 2012, 60, 955–965. [Google Scholar] [CrossRef] [Green Version]
- Persson, J.; Vrede, T. Polyunsaturated fatty acids in zooplankton: Variation due to taxonomy and trophic position. Fresh Water Biol. 2006, 51, 887–900. [Google Scholar] [CrossRef]
- Masclaux, H.; Bec, A.; Kainz, M.J.; Perriere, F.; Desvilettes, C.; Bourdier, G. Accumulation of polyunsaturated fatty acids by cladocerans: Effects of taxonomy, temperature and food. Fresh Water Biol. 2012, 57, 696–703. [Google Scholar] [CrossRef]
- Hartwich, M.; Martin-Creuzburg, D.; Wacker, A. Seasonal changes in the accumulation of polyunsaturated fatty acids in zooplankton. J. Plankton Res. 2013, 35, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Gama-Flores, J.L.; Huidobro-Salas, M.E.; Sarma, S.; Nandini, S.; Zepeda-Mejia, R.; Gulati, R.D. Temperature and age affect the life history characteristics and fatty acid profiles of Moina macrocopa (Cladocera). J. Therm. Biol. 2015, 53, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Abaho, I.; Bwanika, G.; Walekhwa, P.; Arinaitwe, A.V.I.; Kwetegyeka, J.S. Fatty acid profiles and growth of African catfish (Clarias gariepinus, Burchell, 1822) larvae fed on freshwater rotifer (s) and Artemia as live starter feeds. Int. J. Fish Aquat. Stud. 2016, 4, 189–196. [Google Scholar] [CrossRef]
- Das, P.; Mandal, S.C.; Bhagabati, S.; Akhtar, M.; Singh, S. Important live food organisms and their role in aquaculture. Front. Aquac. 2012, 5, 69–86. [Google Scholar] [CrossRef]
- Mitra, G.; Mukhopadhyay, P.; Ayyappan, S. Biochemical composition of zooplankton community grown in freshwater earthen ponds: Nutritional implication in nursery rearing of fish larvae and early juveniles. Aquaculture 2007, 272, 346–360. [Google Scholar] [CrossRef]
- Taghavi, D.; Farhadian, O.; Soofiani, N.M.; Keivany, Y. Effects of different light/dark regimes and algal food on growth, fecundity, ephippial induction and molting of freshwater cladoceran, Ceriodaphnia quadrangula. Aquaculture 2013, 410, 190–196. [Google Scholar] [CrossRef]
- Nandini, S.; Alonso-Soto, R.; Sarma, S. Growth of Plankton (Scenedesmus acutus (Chlorophyceae) and Moina macracopa (Cladocera)) on domestic wastewater. CLEAN–Soil Air Water 2013, 41, 11–15. [Google Scholar] [CrossRef]
- Kumar, S.; Srivastava, A.; Chakrabarti, R. Study of digestive proteinases and proteinase inhibitors of Daphnia carinata. Aquaculture 2005, 243, 367–372. [Google Scholar] [CrossRef]
- Beerli, E.L.; Logato, P.V.R.; Freitas, R.T.F.d. Alimentação e comportamento de larvas de pacu, Piaractus mesopotamicus (Holmberg, 1887). Ciênc. Agrotecnol. 2004, 28, 149–155. [Google Scholar] [CrossRef] [Green Version]
- David, C.; Lenis, G.; Castañeda, G.; Lopera, A.; Restrepo, L.F. La dieta usada en la primera alimentación afecta la ganancia de peso y longitud total de larvas de cachama blanca (Piaractus brachypomus). Rev. Colomb. Cienc. Pecu. 2011, 24, 48–53. [Google Scholar]
- Menossi, O.C.C.; Takata, R.; Sánchez-Amaya, M.I.; Freitas, T.M.d.; Yúfera, M.; Portella, M.C. Crescimento e estruturas do sistema digestório de larvas de pacu alimentadas com dieta microencapsulada produzida experimentalmente. Rev. Bras. Zootec. 2012, 41, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Simhachalam, G.; Kumar, N.S.; Rao, K.G. Biochemical composition and nutritional value of Streptocephalus simplex as live feed in ornamental fish culture. J. Basic Appl. Zool. 2015, 72, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Sampaio, A.C.S. Desenvolvimento Inicial e Comportamento Alimentar da Matrinxã Brycon Amazonicus (GUNTHER, 1869), em Laboratório. Master’s Thesis, Universidade Federal Do Rio Grande, Rio Grande, Brasil, 2010. [Google Scholar]
- Tesser, M.B.; Portella, M.C. Ingestão de ração e comportamento de larvas de pacu em resposta a estímulos químicos e visuais. Rev. Bras. Zootec. 2006, 35, 1887–1892. [Google Scholar] [CrossRef] [Green Version]
- Prieto-Guevara, M.J.; Logato, P.V.R.; Moraes, G.F.d.; Okamura, D.; Araújo, F.G.d. Tipo de alimento, sobrevivência e desempenho inicial de pós-larvas de pacu (Piaractus mesopotamicus). Cienc. Agrotecnol. 2006, 30, 1002–1007. [Google Scholar] [CrossRef] [Green Version]
- Sars, G.O. Contributions to the Knowledge of the Fresh-water “Entomostraca” of South America, as Shown by Artificial Hatching from Dried Material. Arch. Mathemetik Natuwidenskab 1901, 23, 1–102. [Google Scholar]
- AOAC International. Official methods of analysis of AOAC International, 21st ed.; AOAC International: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Hopkins, K.D. Reporting fish growth: A review of the basics 1. J. World Aquac. Soc. 1992, 23, 173–179. [Google Scholar] [CrossRef]
- Sousa, F.D.R.; Elmoor-Loureiro, L.M.A. Cladóceros fitófilos (Crustacea, Branchiopoda) do Parque Nacional das Emas, estado de Goiás. Biota Neotrop. 2008, 8, 159–166. [Google Scholar] [CrossRef]
- Diniz, L.P.; Elmoor-Loureiro, L.M.A.; Almeida, V.L.d.S.; Melo Júnior, M.d. Cladocera (Crustacea, Branchiopoda) of a temporary shallow pond in the Caatinga of Pernambuco, Brazil. Nauplius 2013, 21, 65–78. [Google Scholar] [CrossRef] [Green Version]
- Kestemont, P.; Jourdan, S.; Houbart, M.; Mélard, C.; Paspatis, M.; Fontaine, P.; Cuvier, A.; Kentouri, M.; Baras, E. Size heterogeneity, cannibalism and competition in cultured predatory fish larvae: Biotic and abiotic influences. Aquaculture 2003, 227, 333–356. [Google Scholar] [CrossRef]
- Acosta-Muñoz, A.H.; Ortega-Montenegro, C.; Sanguino-Ortiz, R.; Ceballos-Ruiz, B.L.; López-Macias, J.N. Evaluación de tres tipos de alimento como dieta en post-larvas de Sábalo Amazónico, Brycon melanopterus, (Cope, 1872). Vet. Zootec. 2010, 4, 42–50. [Google Scholar]
- Prieto-Guevara, M.; Hernández, J.; Gómez, C.; Pardo, S.; Atencio-Garcia, V.; Rosa, P.V. Efecto de tres tipos de presas vivas en la larvicultura de bagre blanco (Sorubim cuspicaudus). Rev. Med. Vet. Zootec. Córdoba 2013, 18, 3790–3798. [Google Scholar] [CrossRef] [Green Version]
- Barton, B.A.J. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef]
- Hori, T.S.; Avilez, I.M.; Iwama, G.K.; Johnson, S.C.; Moraes, G.; Afonso, L.O.J. Impairment of the stress response in matrinxã juveniles (Brycon amazonicus) exposed to low concentrations of phenol. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2008, 147, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.F.; Qin, J. Prey size selection and cannibalistic behaviour of juvenile barramundi Lates calcarifer. J. Fish Biol. 2015, 86, 1549–1566. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Merlano, J.A.; Otero-Paternina, A.M.; Corredor-Santamaría, W.; Medina-Robles, V.M.; Cruz-Casallas, P.E.; Velasco-Santamaría, Y.M. Utilización de organismos vivos como primera alimentación de larvas de yaque (Leiarius marmoratus) bajo condiciones de laboratorio. Orinoquia 2010, 14, 45–58. [Google Scholar]
- Janakiraman, A.; Altaff, K. Koi carp (Cyprinus carpio) larval rearing with different zooplankton live feeds to evaluate their suitability and growth performance. Int. J. Res. Fish. Aquac. 2014, 4, 181–185. [Google Scholar]
- Giacomini, H.C.; Shuter, B.J.; Lester, N.P. Predator bioenergetics and the prey size spectrum: Do foraging costs determine fish production? J. Theor. Biol. 2013, 332, 249–260. [Google Scholar] [CrossRef]
- Iles, A.C.; Rasmussen, J.B.J.F.B. Indirect effects of metal contamination on energetics of yellow perch (Perca flavescens) resulting from food web simplification. Fresh Water Biol. 2005, 50, 976–992. [Google Scholar] [CrossRef]
- Kaufman, S.D.; Gunn, J.M.; Morgan, G.E.; Couture, P. Muscle enzymes reveal walleye (Sander vitreus) are less active when larger prey (cisco, Coregonus artedi) are present. Can. J. Fish. Aquat. Sci. 2006, 63, 970–979. [Google Scholar] [CrossRef]
- Luna-Figueroa, J.; Vargas, Z.d.J.; Figueroa, T. Alimento vivo como alternativa en la dieta de larvas y juveniles de Pterophyllum scalare (Lichtenstein, 1823). Av. Investig. Agropecu. 2010, 14, 63–72. [Google Scholar]
- Aya, F.A.; Corpuz, M.; Garcia, L. Diet composition, feed preferences and mouth morphology of early stage silver therapon (Leiopotherapon plumbeus, K ner 1864) larvae reared in outdoor tanks. J. Appl. Ichthyol. 2015, 31, 77–82. [Google Scholar] [CrossRef]
- Islam, M.; Hassan, M.; Begum, M.; Punom, N.; Begum, M.; Sultana, N.; Rahman, M. Effects of feeding zooplankton, Moina macrocopa (Straus, 1820) on the growth of Nile tilapia Oreochromis niloticus L. Bangladesh J. Sci. Ind. Res. 2017, 52, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Das, S.K.; Tiwari, V.; Venkateshwarlu, G.; Reddy, A.; Parhi, J.; Sharma, P.; Chettri, J. Growth, survival and fatty acid composition of Macrobrachium rosenbergii (de Man, 1879) post larvae fed HUFA-enriched Moina micrura. Aquaculture 2007, 269, 464–475. [Google Scholar] [CrossRef]
- Rivera-Narváez, C.M.; Botero-Aguirre, M. Alimento vivo enriquecido con ácidos grasos para el desarrollo larvario de peces. Rev. Colomb. Cienc. Pecu. 2009, 22, 607–618. [Google Scholar]
- Botero, M. Comportamiento de los peces en la búsqueda y la captura del alimento. Rev. Colomb. Cienc. Pecu. 2004, 17, 63–75. [Google Scholar]
- Vandewalle, P.; Germeau, G.; Besancenet, P.; Parmentier, E.; Baras, E. Early development of the head skeleton in Brycon moorei (Pisces, Ostariophysi, Characidae). J. Fish Biol. 2005, 66, 996–1024. [Google Scholar] [CrossRef]
- Leonardo, A.F.G.; Hoshiba, M.A.; Senhorini, J.A.; Urbinati, E.C. Canibalismo em larvas de matrinxã, Brycon cephalus, após imersão dos ovos à diferentes concentrações de triiodotironina (T3). Boletim Inst. Pesca São Paulo 2018, 34, 231–239. [Google Scholar]
- Maciel, C.M.R.R.; Lanna, E.A.T.; Maciel Junior, A.; Donzele, J.L.; Neves, C.A.; Menin, E. Morphological and behavioral development of the piracanjuba larvae. Rev. Bras. Zootec. 2010, 39, 961–970. [Google Scholar] [CrossRef] [Green Version]
- Neumann, E. Desenvolvimento inicial de jatuarana, Brycon amazonicus (Teleostei, Characidae). Ph.D. Thesis, Universidade Estadual Paulista, Sao Paulo, Brasil, 2008. [Google Scholar]
| Parameters | Treatments | |||
|---|---|---|---|---|
| FL | Mm | Me | Mix | |
| WG (mg) | 1.5 ± 0.4 a | 0.5 ± 0.1 ab | 0.3 ± 0.1 b | 0.4 ± 0.0 b |
| LG (mm) | 1.6 ± 0.1 a | 0.9 ± 0.2 b | 0.9 ± 0.3 b | 1.0 ± 0.3 ab |
| G (%/h) | 3.2 ± 0.4 a | 1.3 ± 0.2 b | 0.8 ± 0.2 b | 1.1 ± 0.1 b |
| Treatments | ||||
|---|---|---|---|---|
| Proximate Analysis * | FL | Mm | Me | Mix |
| Crude protein | 52.3 ± 0.1 e | 57.9 ± 0.1 c | 80 ± 0.1 a | 60.7 ± 0.1 b |
| Crude lipid | 66.9 ± 0.1 a | 32.1 ± 0.1 e | 37.4 ± 0.1 d | 40.3 ± 0.1 c |
| Moisture | 77.4 ± 0.1 d | 89.5 ± 0.1 b | 89.6 ± 0.1 b | 90.3 ± 0.1 a |
| Ash | 0.8 ± 0.01 b | 0.6 ± 0.01 c | 0.6 ± 0.01 c | 0.8 ± 0.01 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Velásquez, C.; Atencio-Garcia, V.; Ayazo-Genes, J.E.; Espinosa-Araujo, J.; Prieto-Guevara, M. Management of the First Feeding of Dorada Brycon sinuensis with Two Species of Cladocerans. Appl. Sci. 2021, 11, 9379. https://doi.org/10.3390/app11209379
Jiménez-Velásquez C, Atencio-Garcia V, Ayazo-Genes JE, Espinosa-Araujo J, Prieto-Guevara M. Management of the First Feeding of Dorada Brycon sinuensis with Two Species of Cladocerans. Applied Sciences. 2021; 11(20):9379. https://doi.org/10.3390/app11209379
Chicago/Turabian StyleJiménez-Velásquez, César, Victor Atencio-Garcia, Julia Eva Ayazo-Genes, José Espinosa-Araujo, and Martha Prieto-Guevara. 2021. "Management of the First Feeding of Dorada Brycon sinuensis with Two Species of Cladocerans" Applied Sciences 11, no. 20: 9379. https://doi.org/10.3390/app11209379
APA StyleJiménez-Velásquez, C., Atencio-Garcia, V., Ayazo-Genes, J. E., Espinosa-Araujo, J., & Prieto-Guevara, M. (2021). Management of the First Feeding of Dorada Brycon sinuensis with Two Species of Cladocerans. Applied Sciences, 11(20), 9379. https://doi.org/10.3390/app11209379






