Design of 3D Carbon Nanotube Monoliths for Potential-Controlled Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Particle Preparation and Characterization
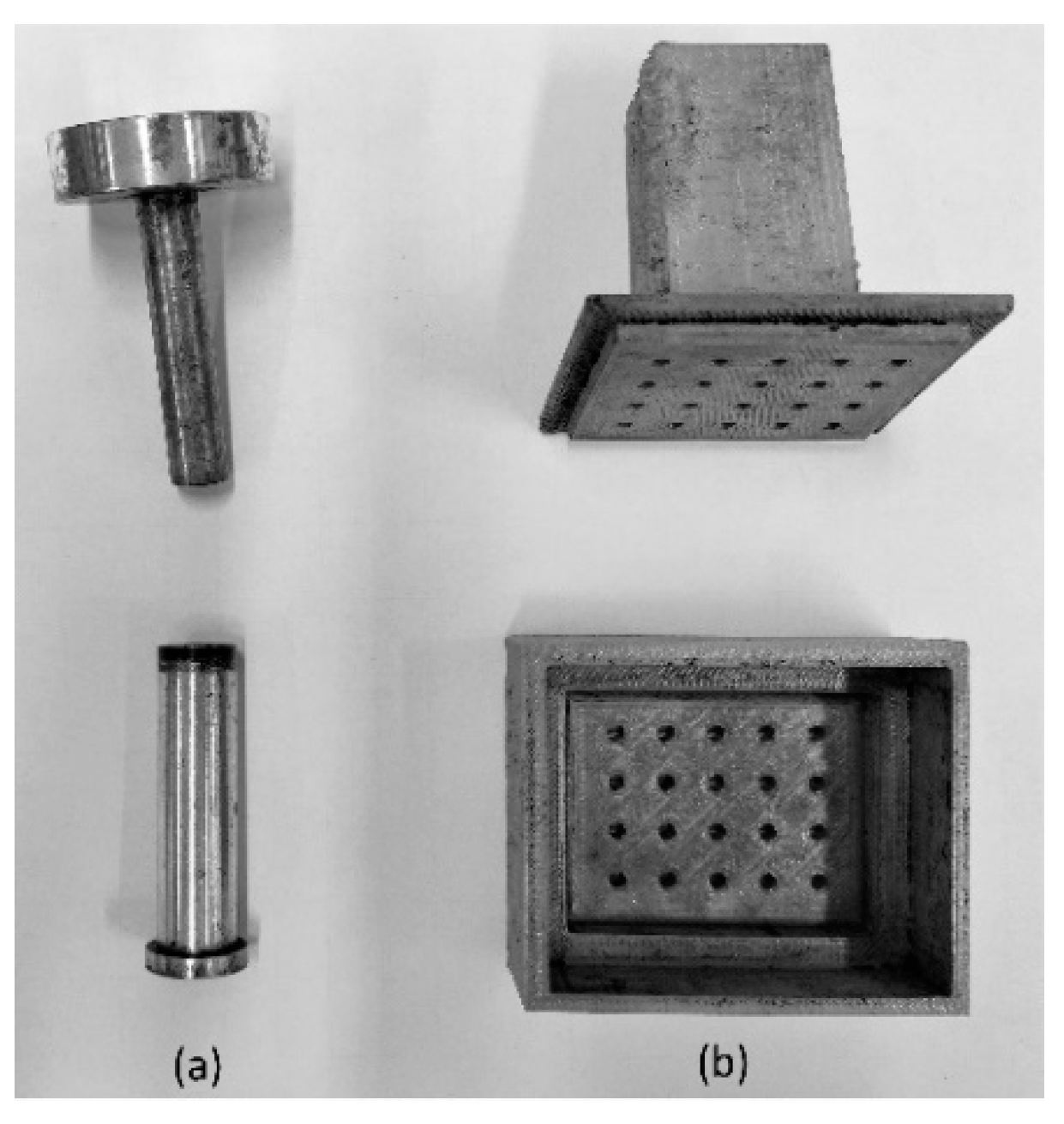
2.3. Monolith Synthesis
2.4. Monolith Characterization
2.5. Potential-Controlled Adsorption
3. Results
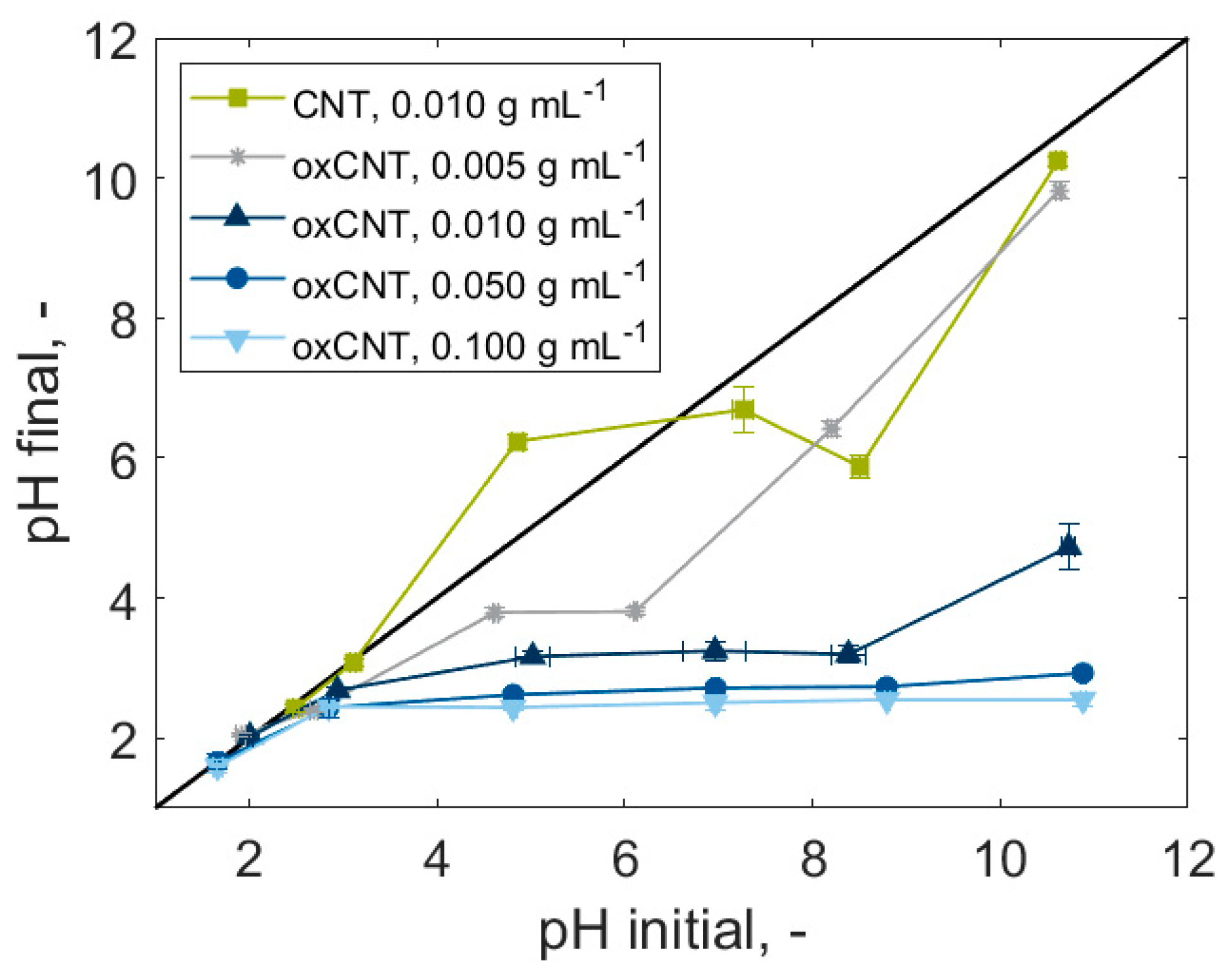
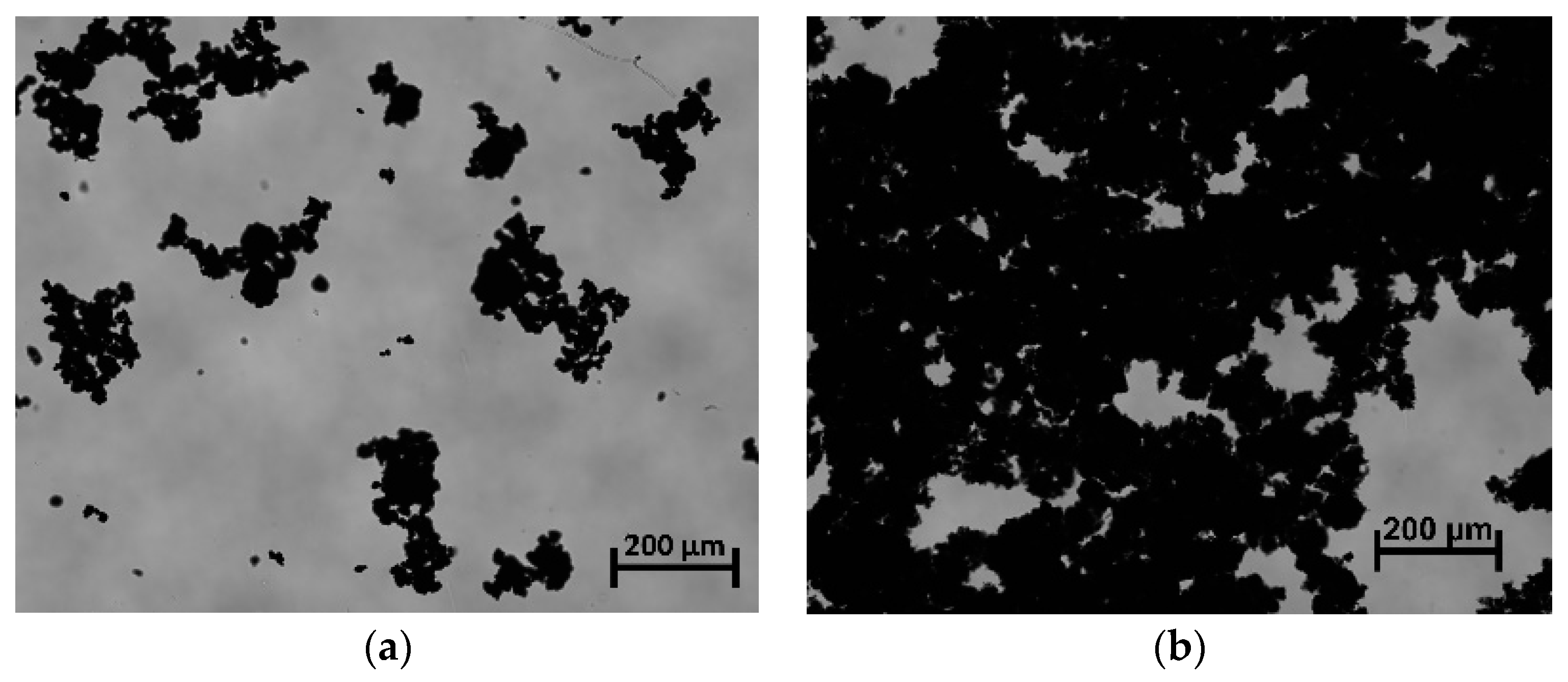
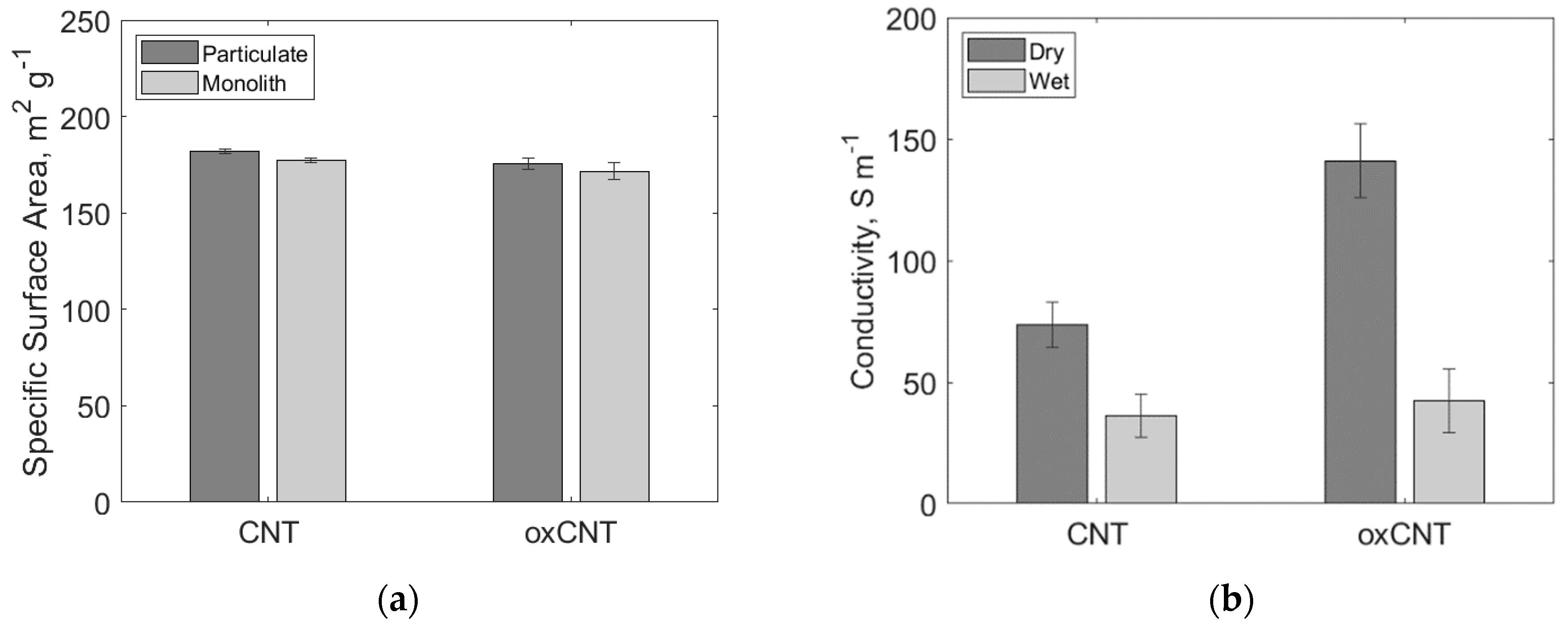
3.1. Particle Oxidation and Characterization
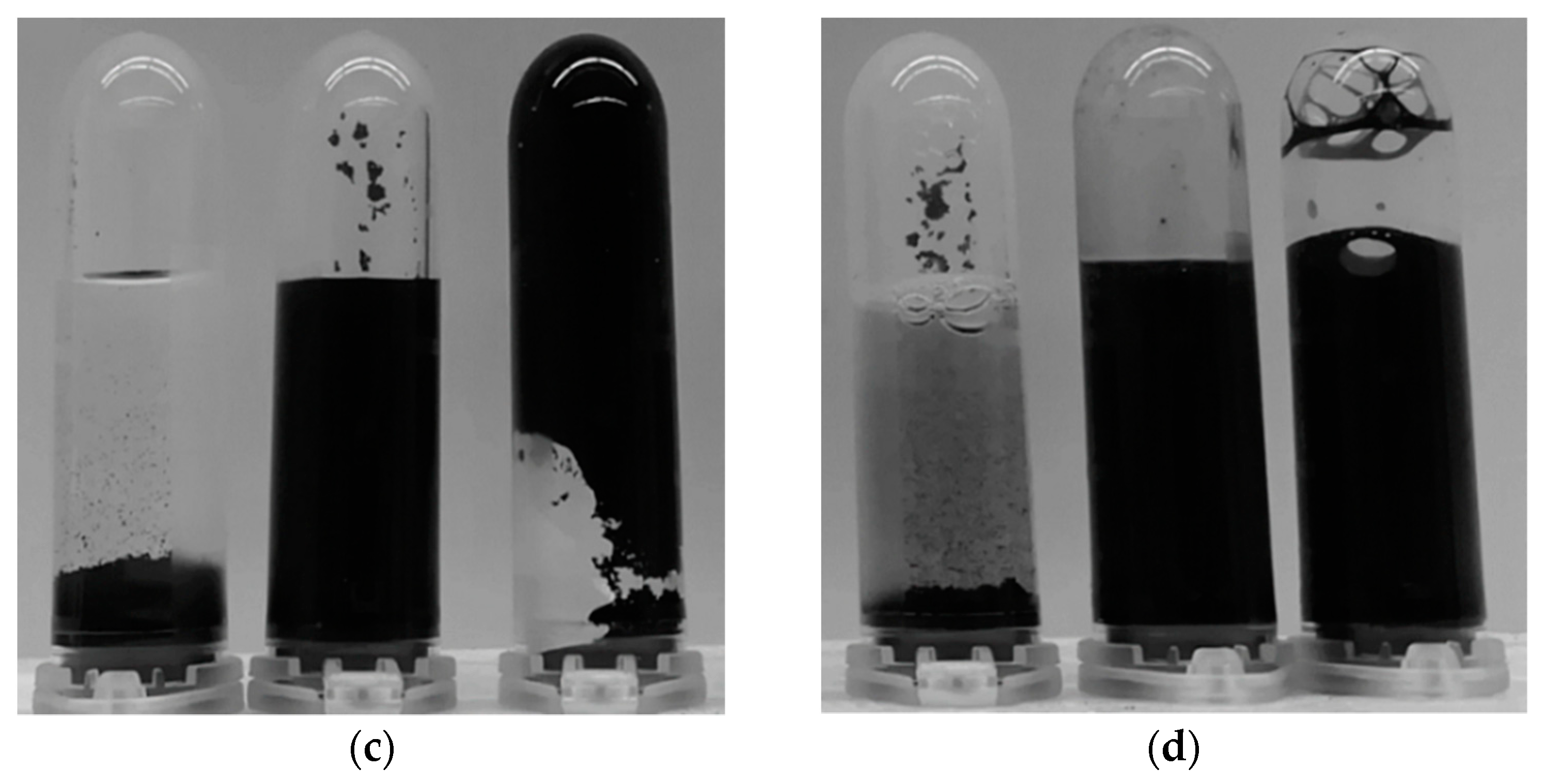
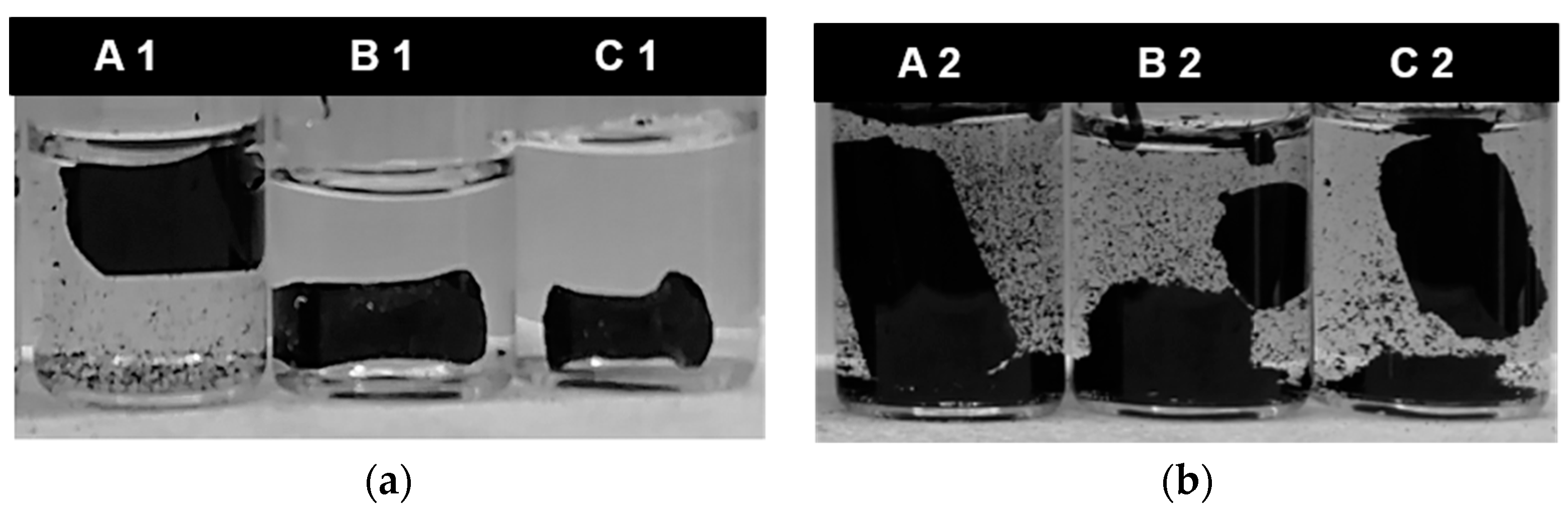
3.2. Monolith Stability Dependence on Ultrasonication and Drying
3.3. Monolith Structure and Conductivity
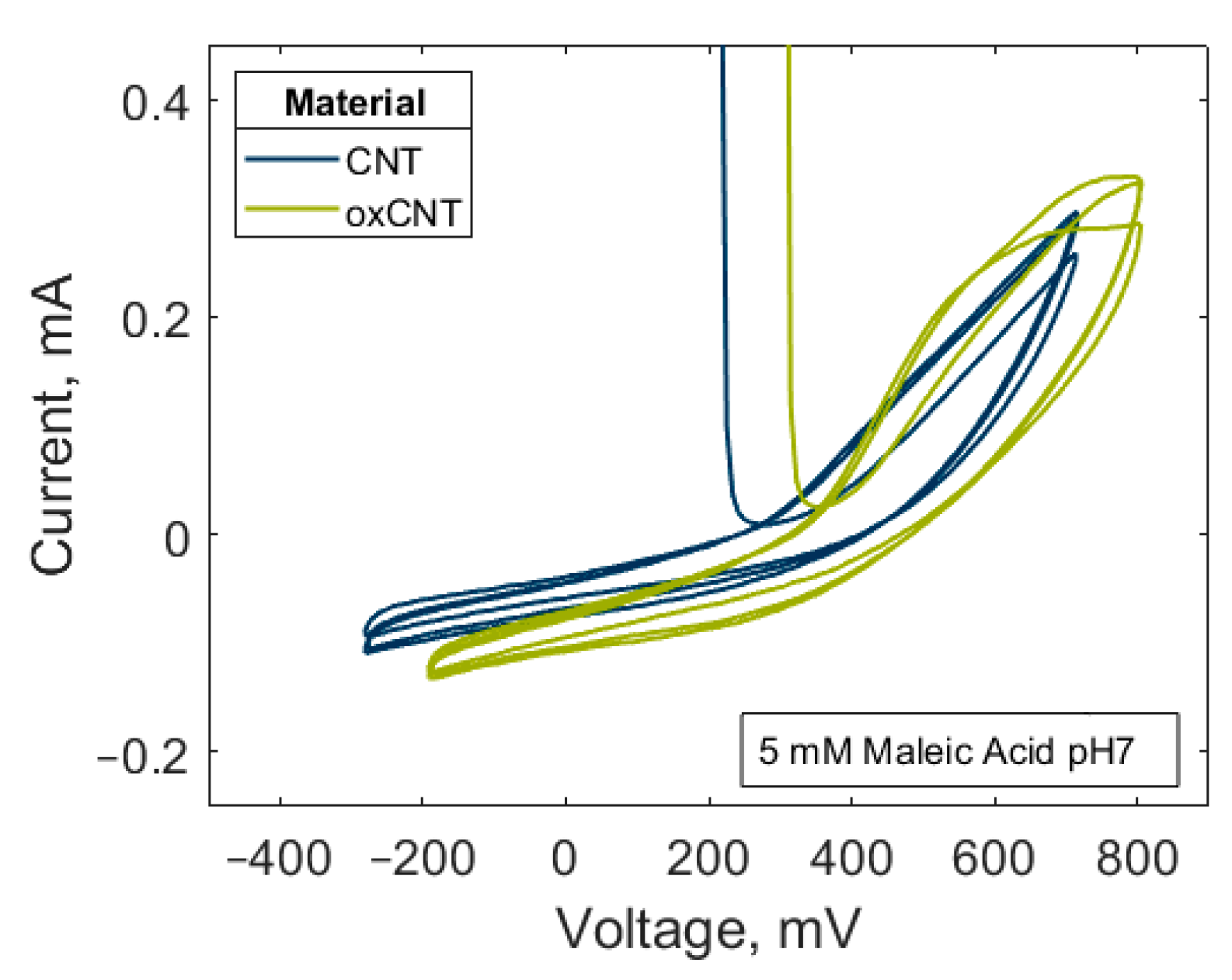
3.4. Electrochemical Characterization
3.5. Potential-Controlled Adsorption on CNT-Monoliths
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Ebbesen, T.W.; Lezec, H.J.; Hiura, H.; Bennett, J.W.; Ghaemi, H.F.; Thio, T. Electrical conductivity of individual carbon nanotubes. Nat. Cell Biol. 1996, 382, 54–56. [Google Scholar] [CrossRef]
- Pop, E.; Mann, D.; Wang, Q.; Goodson, K.; Dai, H. Thermal conductance of an individual single-wall carbon nanotube above room temperature. Nano Lett. 2006, 6, 96–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treacy, M.M.J.; Ebbesen, T.W.; Gibson, J.M. Exceptionally high Young’s modulus observed for individual carbon nanotubes. Nat. Cell Biol. 1996, 381, 678–680. [Google Scholar] [CrossRef]
- Evanoff, K.; Khan, J.; Balandin, A.A.; Magasinski, A.; Ready, W.J.; Fuller, T.F.; Yushin, G. Towards ultrathick battery electrodes: Aligned carbon nanotube-enabled architecture. Adv. Mater. 2012, 24, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Sotowa, C.; Origi, G.; Takeuchi, M.; Nishimura, Y.; Takeuchi, K.; Jang, I.Y.; Kim, Y.J.; Hayashi, T.; Kim, Y.A.; Endo, M.; et al. The reinforcing effect of combined carbon nanotubes and acetylene blacks on the positive electrode of lithium-ion batteries. ChemSusChem 2008, 1, 911–915. [Google Scholar] [CrossRef] [PubMed]
- De Volder, M.F.L.; Tawfick, S.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Park, M.-Y.; Kim, C.-G.; Kim, J.-H. CNT-coated quartz woven fabric electrodes for robust lithium-ion structural batteries. Appl. Sci. 2020, 10, 8622. [Google Scholar] [CrossRef]
- Comba, F.N.; Romero, M.R.; Garay, F.S.; Baruzzi, A.M. Mucin and carbon nanotube-based biosensor for detection of glucose in human plasma. Anal. Biochem. 2018, 550, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Cao, X.; Jiang, Y.; He, P.; Fang, Y. Carbon nanotube-enhanced electrochemical DNA biosensor for DNA hybridization detection. Anal. Bioanal. Chem. 2003, 375, 287–293. [Google Scholar] [CrossRef]
- Shen, Y.; Du, A.; Wu, X.-L.; Li, X.-G.; Shen, J.; Zhou, B. Low-cost carbon nanotube aerogels with varying and controllable density. J. Sol Gel Sci. Technol. 2016, 79, 76–82. [Google Scholar] [CrossRef]
- Kohlmeyer, R.R.; Lor, M.; Deng, J.; Liu, H.; Chen, J. Preparation of stable carbon nanotube aerogels with high electrical conductivity and porosity. Carbon 2011, 49, 2352–2361. [Google Scholar] [CrossRef]
- Zou, J.; Liu, J.; Karakoti, A.; Kumar, A.; Joung, D.; Li, Q.; Khondaker, S.I.; Seal, S.; Zhai, L. Ultralight multiwalled carbon nanotube aerogel. ACS Nano 2010, 4, 7293–7302. [Google Scholar] [CrossRef] [PubMed]
- You, B.; Wang, L.; Yao, L.; Yang, J. Threedimensional N-doped graphene–CNT networks for supercapacitor. Chem. Commun. 2013, 49, 5016–5018. [Google Scholar] [CrossRef]
- Haghgoo, M.; Yousefi, A.A.; Mehr, M.J.Z.; Celzard, A.; Fierro, V.; Léonard, A.; Job, N. Characterization of multi-walled carbon nanotube dispersion in resorcinol–formaldehyde aerogels. Microporous Mesoporous Mater. 2014, 184, 97–104. [Google Scholar] [CrossRef]
- Du, R.; Zhao, Q.; Zhang, N.; Zhang, J. Macroscopic carbon nanotube-based 3D monoliths. Small 2015, 11, 3263–3289. [Google Scholar] [CrossRef]
- Bryning, M.B.; Milkie, D.E.; Islam, M.; Hough, L.A.; Kikkawa, J.M.; Yodh, A.G. Carbon nanotube aerogels. Adv. Mater. 2007, 19, 661–664. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Xu, B.; Su, Y.; Luo, Y. Ultralight conducting polymer/carbon nanotube composite aerogels. Carbon 2011, 49, 1884–1893. [Google Scholar] [CrossRef]
- Du, R.; Zhang, N.; Xu, H.; Mao, N.; Duan, W.; Wang, J.; Zhao, Q.; Liu, Z.; Zhang, J. CMP aerogels: Ultrahigh-surface-area carbon-based monolithic materials with superb sorption performance. Adv. Mater. 2014, 26, 8053–8058. [Google Scholar] [CrossRef]
- Maghfirah, A.; Yudianti, R.; Sinuhaji, P.; Hutabarat, L.G. Preparation of poly(vinyl) alcohol—Multiwalled carbon nanotubes nanocomposite as conductive and transparent film using casting method. J. Phys. Conf. Ser. 2018, 1116, 032017. [Google Scholar] [CrossRef]
- Singh, R.; Singh, M.; Bhartiya, S.; Singh, A.; Kohli, D.; Ghosh, P.C.; Meenakshi, S.; Gupta, P. Facile synthesis of highly conducting and mesoporous carbon aerogel as platinum support for PEM fuel cells. Int. J. Hydrog. Energy 2017, 42, 11110–11117. [Google Scholar] [CrossRef]
- Chen, W.; Rakhi, R.B.; Alshareef, H.N. High energy density supercapacitors using macroporous kitchen sponges. J. Mater. Chem. 2012, 22, 14394–14402. [Google Scholar] [CrossRef]
- Li, Y.; Kang, Z.; Yan, X.; Cao, S.; Li, M.; Guo, Y.; Huan, Y.; Wen, X.; Zhang, Y. A three-dimensional reticulate CNT-aerogel for a high mechanical flexibility fiber supercapacitor. Nanoscale 2018, 10, 9360–9368. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, X.; Guo, S.; Yu, C.; Zhong, S.; Liu, X. Multi-functional graphene/carbon nanotube aerogels for its applications in supercapacitor and direct methanol fuel cell. Electrochim. Acta 2018, 264, 12–19. [Google Scholar] [CrossRef]
- Tesfaye, R.M.; Das, G.; Park, B.J.; Kim, J.; Yoon, H.H. Ni-Co bimetal decorated carbon nanotube aerogel as an efficient anode catalyst in urea fuel cells. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, R.; Zhang, N.; Zhu, J.; Wang, Y.; Xu, C.; Hu, Y.; Mao, N.; Xu, H.; Duan, W.; Zhuang, L.; et al. Nitrogen-doped carbon nanotube aerogels for high-performance ORR catalysts. Small 2015, 11, 3903–3908. [Google Scholar] [CrossRef] [PubMed]
- Biesheuvel, P.M.; Porada, S.; Levi, M.; Bazant, M. Attractive forces in microporous carbon electrodes for capacitive deionization. J. Solid State Electrochem. 2014, 18, 1365–1376. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, M.; Huang, Z.-H.; Cui, T.; Gui, X.; Kang, F.; Wang, K.; Wu, D. Capacitive deionization of NaCl solutions using carbon nanotube sponge electrodes. J. Mater. Chem. 2011, 21, 18295–18299. [Google Scholar] [CrossRef]
- Wang, X.Z.; Li, M.G.; Chen, Y.W.; Cheng, R.M.; Huang, S.M.; Pan, L.K.; Sun, Z. Electrosorption of NaCl solutions with carbon nanotubes and nanofibers composite film electrodes. Electrochem. Solid State Lett. 2006, 9, e23–e26. [Google Scholar] [CrossRef]
- Wagner, R.; Bag, S.; Trunzer, T.; Fraga-García, P.; Wenzel, W.; Berensmeier, S.; Franzreb, M. Adsorption of organic molecules on carbon surfaces: Experimental data and molecular dynamics simulation considering multiple protonation states. J. Colloid Interface Sci. 2021, 589, 424–437. [Google Scholar] [CrossRef]
- Trunzer, T.; Stummvoll, T.; Porzenheim, M.; Fraga-García, P.; Berensmeier, S. A carbon nanotube packed bed electrode for small molecule electrosorption: An electrochemical and chromatographic approach for process description. Appl. Sci. 2020, 10, 1133. [Google Scholar] [CrossRef] [Green Version]
- Trunzer, T.; Fraga-García, P.; Tschuschner, M.-P.A.; Voltmer, D.; Berensmeier, S. The electrosorptive response of a carbon nanotube flow-through electrode in aqueous systems. Chem. Eng. J. 2022, 428, 13100. [Google Scholar] [CrossRef]
- Moraes, R.A.; De Matos, C.F.; Castro, E.G.; Schreiner, W.H.; Oliveira, M.M.; Zarbin, A.J.G. The effect of different chemical treatments on the structure and stability of aqueous dispersion of iron- and iron oxide-filled multi-walled carbon nanotubes. J. Braz. Chem. Soc. 2011, 22, 2191–2201. [Google Scholar] [CrossRef] [Green Version]
- Shaffer, M.; Fan, X.; Windle, A. Dispersion and packing of carbon nanotubes. Carbon 1998, 36, 1603–1612. [Google Scholar] [CrossRef]
- Vigolo, B.; Pénicaud, A.; Coulon, C.; Sauder, C.; Pailler, R.; Journet, C.; Bernier, P.; Poulin, P. Macroscopic fibers and ribbons of oriented carbon nanotubes. Science 2000, 290, 1331–1334. [Google Scholar] [CrossRef] [PubMed]
- Brammen, M.; Fraga-García, P.; Berensmeier, S. Carbon nanotubes-A resin for electrochemically modulated liquid chromatography. J. Sep. Sci. 2017, 40, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Avilés, F.; Cauich-Rodríguez, J.; Moo-Tah, L.; May-Pat, A.; Vargas-Coronado, R.F. Evaluation of mild acid oxidation treatments for MWCNT functionalization. Carbon 2009, 47, 2970–2975. [Google Scholar] [CrossRef]
- Farbod, M.; Tadavani, S.K.; Kiasat, A. Surface oxidation and effect of electric field on dispersion and colloids stability of multiwalled carbon nanotubes. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 685–690. [Google Scholar] [CrossRef]
- Wepasnick, K.A.; Smith, B.A.; Schrote, K.E.; Wilson, H.K.; Diegelmann, S.R.; Fairbrother, D.H. Surface and structural characterization of multi-walled carbon nanotubes following different oxidative treatments. Carbon 2011, 49, 24–36. [Google Scholar] [CrossRef]
- Kim, Y.-T.; Mitani, T. Competitive effect of carbon nanotubes oxidation on aqueous EDLC performance: Balancing hydrophilicity and conductivity. J. Power Sour. 2006, 158, 1517–1522. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Brammen, M.W.; Zunhammer, F.; Däumler, N.; Fraga-García, P.; Berensmeier, S. Iron oxide nanoparticles: Multiwall carbon nanotube composite materials for batch or chromatographic biomolecule separation. Nanoscale Res. Lett. 2021, 16, 1–10. [Google Scholar] [CrossRef]
- Ţucureanu, V.; Matei, A.; Avram, M.A. FTIR spectroscopy for carbon family study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Kövér, L.; Tóth, J.; Biniak, S.; Trykowski, G.; Judek, J. Multiwall carbon nanotubes purification and oxidation by nitric acid studied by the FTIR and electron spectroscopy methods. J. Alloy. Compd. 2010, 501, 77–84. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Silva, T.L.S.; Pastrana-Martínez, L.M.; Brandão, A.T.S.C.; Figueiredo, J.L.; Silva, A.M.T. Modification of the surface chemistry of single- and multi-walled carbon nanotubes by HNO3 and H2SO4 hydrothermal oxidation for application in direct contact membrane distillation. Phys. Chem. Chem. Phys. 2014, 16, 12237–12250. [Google Scholar] [CrossRef]
- Gatabi, M.P.; Moghaddam, H.M.; Ghorbani, M. Point of zero charge of maghemite decorated multiwalled carbon nanotubes fabricated by chemical precipitation method. J. Mol. Liq. 2016, 216, 117–125. [Google Scholar] [CrossRef]
- Fu, R.; Zheng, B.; Liu, J.; Dresselhaus, M.S.; Dresselhaus, G.; Satcher, J.H.; Baumann, T.F. The fabrication and characterization of carbon aerogels by gelation and supercritical drying in isopropanol. Adv. Funct. Mater. 2003, 13, 558–562. [Google Scholar] [CrossRef]
- Pekala, R.W.; Farmer, J.C.; Alviso, C.T.; Tran, T.D.; Mayer, S.T.; Miller, J.M.; Dunn, B. Carbon aerogels for electrochemical applications. J. Non Cryst. Solids 1998, 225, 74–80. [Google Scholar] [CrossRef]
- Tamon, H.; Ishizaka, H.; Yamamoto, T.; Suzuki, T. Influence of freeze-drying conditions on the mesoporosity of organic gels as carbon precursors. Carbon 2000, 38, 1099–1105. [Google Scholar] [CrossRef]
- Thongprachan, N.; Nakagawa, K.; Sano, N.; Charinpanitkul, T.; Tanthapanichakoon, W. Preparation of macroporous solid foam from multi-walled carbon nanotubes by freeze-drying technique. Mater. Chem. Phys. 2008, 112, 262–269. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Knowles, T.P.J.; Terentjev, E.M. Strength of nanotubes, filaments, and nanowires from sonication-induced scission. Adv. Mater. 2009, 21, 3945–3948. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Miyauchi, Y.; Motoyanagi, J.; Fukushima, T.; Aida, T.; Kato, M.; Maruyama, S. Improved bath sonication method for dispersion of individual single-walled carbon nanotubes using new triphenylene-based surfactant. Jpn. J. Appl. Phys. 2008, 47, 2000–2004. [Google Scholar] [CrossRef] [Green Version]
- Park, H.J.; Park, M.; Chang, J.Y.; Lee, H. The effect of pre-treatment methods on morphology and size distribution of multi-walled carbon nanotubes. Nanotechnology 2008, 19, 335702. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Terentjev, E.M. Dispersion of carbon nanotubes: Mixing, sonication, stabilization, and composite properties. Polymers 2012, 4, 275–295. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wei, C.; Zhang, D.; Pan, B. Adsorption of bisphenol A on dispersed carbon nanotubes: Role of different dispersing agents. Sci. Total. Environ. 2019, 655, 807–813. [Google Scholar] [CrossRef]
- Niyogi, S.; Boukhalfa, S.; Chikkannanavar, S.B.; McDonald, T.J.; Heben, A.M.J.; Doorn, S.K. Selective aggregation of single-walled carbon nanotubes via salt addition. J. Am. Chem. Soc. 2007, 129, 1898–1899. [Google Scholar] [CrossRef]
- Clark, M.D.; Subramanian, S.; Krishnamoorti, R. Understanding surfactant aided aqueous dispersion of multi-walled carbon nanotubes. J. Colloid Interface Sci. 2011, 354, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Frackowiak, E.; Metenier, K.; Bertagna, V.; Beguin, F. Supercapacitor electrodes from multiwalled carbon nanotubes. Appl. Phys. Lett. 2000, 77, 2421–2423. [Google Scholar] [CrossRef]
- Wagner, R.; Winger, S.; Franzreb, M. Predicting the potential of capacitive deionization for the separation of pH-dependent organic molecules. Eng. Life Sci. 2021, 1–18. [Google Scholar] [CrossRef]
- Lee, S.W.; Gallant, B.M.; Lee, Y.; Yoshida, N.; Kim, D.Y.; Yamada, Y.; Noda, S.; Yamada, A.; Shao-Horn, Y. Self-standing positive electrodes of oxidized few-walled carbon nanotubes for light-weight and high-power lithium batteries. Energy Environ. Sci. 2012, 5, 5437–5444. [Google Scholar] [CrossRef]
- Lee, S.W.; Gallant, B.M.; Byon, H.R.; Hammond, P.T.; Shao-Horn, Y. Nanostructured carbon-based electrodes: Bridging the gap between thin-film lithium-ion batteries and electrochemical capacitors. Energy Environ. Sci. 2011, 4, 1972–1985. [Google Scholar] [CrossRef]
- Pan, B.; Xing, B. Adsorption mechanisms of organic chemicals on carbon nanotubes. Environ. Sci. Technol. 2008, 42, 9005–9013. [Google Scholar] [CrossRef] [PubMed]
- Suss, M.E.; Porada, S.; Sun, X.; Biesheuvel, P.M.; Yoon, J.; Presser, V. Water desalination via capacitive deionization: What is it and what can we expect from it? Energy Environ. Sci. 2015, 8, 2296–2319. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.; Zhang, D.; Yan, T.; Zhang, J.; Shi, L. Three-dimensional micro/mesoporous carbon composites with carbon nanotube networks for capacitive deionization. Appl. Surf. Sci. 2013, 282, 965–973. [Google Scholar] [CrossRef]
- Pan, L.; Wang, X.; Gao, Y.; Zhang, Y.; Chen, Y.; Sun, Z. Electrosorption of anions with carbon nanotube and nanofibre composite film electrodes. Desalination 2009, 244, 139–143. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Röcker, D.; Trunzer, T.; Heilingbrunner, J.; Rassloff, J.; Fraga-García, P.; Berensmeier, S. Design of 3D Carbon Nanotube Monoliths for Potential-Controlled Adsorption. Appl. Sci. 2021, 11, 9390. https://doi.org/10.3390/app11209390
Röcker D, Trunzer T, Heilingbrunner J, Rassloff J, Fraga-García P, Berensmeier S. Design of 3D Carbon Nanotube Monoliths for Potential-Controlled Adsorption. Applied Sciences. 2021; 11(20):9390. https://doi.org/10.3390/app11209390
Chicago/Turabian StyleRöcker, Dennis, Tatjana Trunzer, Jasmin Heilingbrunner, Janine Rassloff, Paula Fraga-García, and Sonja Berensmeier. 2021. "Design of 3D Carbon Nanotube Monoliths for Potential-Controlled Adsorption" Applied Sciences 11, no. 20: 9390. https://doi.org/10.3390/app11209390
APA StyleRöcker, D., Trunzer, T., Heilingbrunner, J., Rassloff, J., Fraga-García, P., & Berensmeier, S. (2021). Design of 3D Carbon Nanotube Monoliths for Potential-Controlled Adsorption. Applied Sciences, 11(20), 9390. https://doi.org/10.3390/app11209390






