Biodynamic Lighting and Functional Disability; a Single-Case Experimental Design in Three Community Dwelling People with Dementia
Abstract
:1. Introduction
2. Materials and Methods
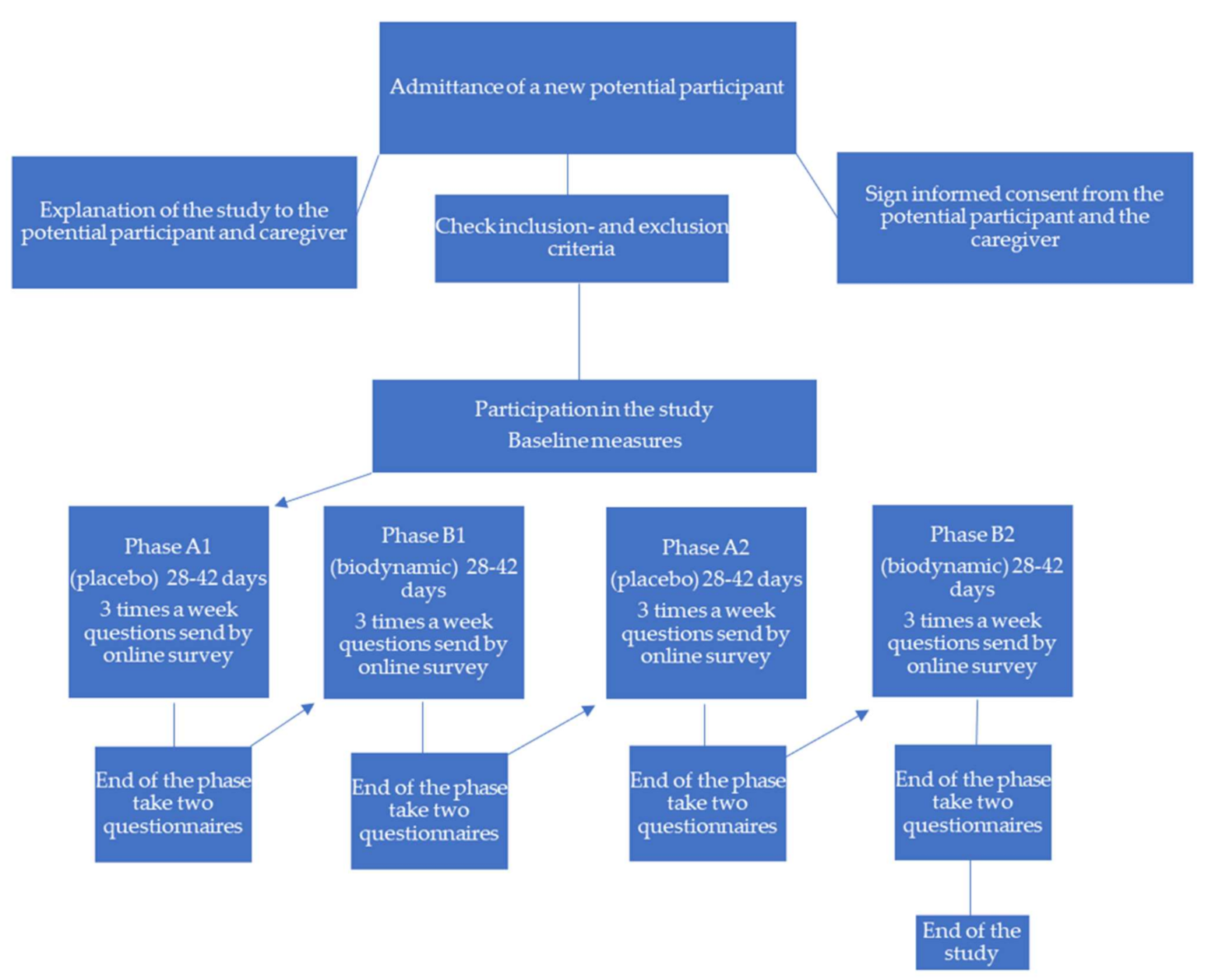
2.1. Design
2.2. Participants
- (i)
- Diagnosed with any type of dementia; in terms of DSM-5 [40] neurocognitive disorder
- (ii)
- Age > 65 years
- (iii)
- Living at home with an informal caregiver
- (iv)
- A score of 1 or higher on a minimum of 3 items of the Katz Index (‘limited help needed’)
- (v)
- A score of 1 or higher on a minimum of 3 items of the Lawton IADL (‘limited help needed’)
- (vi)
- Ocular/visual problems, e.g., ophthalmic abnormality that greatly impedes light perception, e.g., dense bilateral cataracts
- (vii)
- Pre-existing (severe) psychiatric problems (e.g., bipolar disorder, psychosis)
- (viii)
- Incapacitated according to an objective expert
- (ix)
- Bedridden
- (x)
- Palliative treatment or terminally ill
2.3. Setting
2.4. Measures and Materials
2.5. Intervention
2.6. Data Analysis
2.7. Institutional Review Board Statement
3. Results
3.1. Number of Light Minutes during the Day
3.2. Amount of Light Minutes during the Morning and Evening
3.3. Effect of BDL on ADL (Participant One)
3.4. ADL Course over Time (Participant One)
3.5. Effect of BDL on ADL (Participant Two)
3.6. ADL Course over Time (Participant Two)
3.7. Effect of BDL on ADL (Participant Three)
3.8. ADL Course over Time (Participant Three)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perry, R.J.; Hodges, J.R. Relationship between functional and neuropsychological performance in early Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2000, 14, 1–10. [Google Scholar] [CrossRef]
- Kamiya, M.; Osawa, A.; Kondo, I.; Sakurai, T. Factors associated with cognitive function that cause a decline in the level of activities of daily living in Alzheimer′s disease. Geriatr. Gerontol. Int. 2018, 18, 50–56. [Google Scholar] [CrossRef]
- Saari, T.; Hallikainen, I.; Hintsa, T.; Koivisto, A.M. Neuropsychiatric symptoms and activities of daily living in Alzheimer′s disease: ALSOVA 5-year follow-up study. Int. Psychogeriatr. 2019, 32, 741–751. [Google Scholar] [CrossRef]
- Desai, A.K.; Grossberg, G.T.; Sheth, D.N. Activities of daily living in patients with dementia: Clinical relevance, methods of assessment and effects of treatment. CNS Drugs 2004, 18, 853–875. [Google Scholar] [CrossRef] [PubMed]
- Royall, D.R.; Chiodo, L.K.; Polk, M.J. Correlates of disability among elderly retirees with “Subclinical” cognitive impairment. J. Gerontol. Med. Sci. 2000, 55, 541–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martyr, A.; Clare, L. Executive function and activities of daily living in Alzheimer′s disease: A correlation meta-analysis. Dement. Geriatr. Cognit. Disord. 2012, 33, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Ficarro, S.; Duberstein, P.; Chapman, B.P.; Dubovsky, S.; Paroski, M.; Szigeti, K.; Benedict, R.H.B. Executive function and personality predict instrumental activities of daily living in Alzheimer disease. Am. J. Geriatr. Psychiatry 2016, 24, 1074–1083. [Google Scholar] [CrossRef] [Green Version]
- Royall, D.R.; Mahurin, R.K. Neuroanatomy, measurement, and clinical significance of the executive cognitive functions. Am. Psychiatr. Press Rev. Psychiatry 1996, 15, 175–204. [Google Scholar]
- Riter, R.N.; Fries, B.E. Predictors of the placement of cognitively impaired residents on special care units. Gerontologist 1992, 32, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Boyle, P.A.; Cohen, R.A.; Paul, R.; Moser, D.; Gordon, N. Cognitive and motor impairments predict functional declines in patients with vascular dementia. Int. J. Geriatr. Psychiatry 2002, 17, 164–169. [Google Scholar] [CrossRef]
- Njegovan, V.; Man-Son-Hing, M.; Mitchell, S.L.; Molnar, F.J. The hierarchy of functional loss associated with cognitive decline in older people. J. Gerontol. 2001, 56, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Cotrell, V.; Schulz, R. The perspective of the patient with Alzheimer′s disease: A neglected dimension of dementia research. Gerontol. Soc. Am. 1993, 33, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Van den Kieboom, R.; Snaphaan, L.; Mark, R.; Bongers, I. The trajectory of caregiver burden and risk factors in dementia progression: A systematic review. J. Alzheimer Dis. 2020, 77, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Reed, C.; Belger, M.; Vellas, B.; Andrews, J.; Argimon, J.M.; Bruno, G.; Dodel, R.; Jones, R.W.; Wimo, A.; Haro, J.M. Identifying factors of activities of daily living important for cost and caregiver outcomes in Alzheimer′s disease. Int. Psychogeriatr. 2016, 28, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Giebel, C.M.; Sutcliffe, C.; Challis, D. Activities of daily living and quality of life across different stages of dementia: A UK study. Aging Ment. Health 2014, 19, 63–71. [Google Scholar] [CrossRef]
- Fauth, E.B.; Femia, E.E.; Zarit, S.H. Resistiveness to care during assistance with activities of daily living in non-institutionalized persons with dementia: Associations with informal caregivers′ stress and well-being. Aging Ment. Health 2016, 20, 888–898. [Google Scholar] [CrossRef] [Green Version]
- Van der Heide, I.; van den Buuse, S.; Francke, A.L. Dementiemonitor Mantelzorg 2018: Mantelzorgers Over Ondersteuning, Zorg, Belasting, en de Impact van Mantelzorg op Hun Leven; Nivel: Utrecht, The Netherlands; Amersfoort, The Netherlands, 2018. [Google Scholar]
- Azermai, M.; Petrovic, M.; Elseviers, M.M.; Bourgeois, J.; Van Bortel, L.M.; Vander Stichele, R.H. Systematic appraisal of dementia guidelines for the management of behavioural and psychological symptoms. Ageing Res. Rev. 2012, 11, 78–86. [Google Scholar] [CrossRef]
- Li, M.; Lye, J.-H.; Zhang, Y.; Gao, M.-L.; Li, R.; Mao, P.-X.; Li, W.-J.; Ma, X. Efficacy of group reminiscence therapy on cognition, depression, neuropsychiatric symptoms and activities of daily living for patients with Alzheimer disease. J. Geriatr. Psychiatry Neurol. 2020, 33, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-W.; Weng, S.-C.; Wu, H.-S.; Tsai, L.J.; Lin, Y.-L.; Yeh, S.-H. The effects of white noise on agitated behaviors, mental status, and activities of daily living in older adults with dementia. J. Nurs. Res. 2018, 26, 2–9. [Google Scholar] [CrossRef]
- Lyu, J.; Zhang, J.; Mu, H.; Li, W.; Champ, M.; Xiong, Q.; Gao, T.; Xie, L.; Jin, W.; Yang, W.; et al. The effects of music therapy on cognition, psychiatric symptoms and activities of daily living in patients with Alzheimer′s disease. J. Alzheimer Dis. 2018, 64, 1347–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bossers, W.J.R.; van der Woude, L.H.V.; Boersma, F.; Hortobágyi, T.; Scherder, E.J.A.; van Heuvelen, M.J.G. Comparison of effect of two exercise programs on activities of daily living in individuals with dementia: A 9-week randomized, controlled trial. J. Am. Geriatr. Soc. 2016, 64, 1258–1266. [Google Scholar] [CrossRef]
- Okabe, K.; Nagata, T.; Shinagawa, S.; Inamura, K.; Tagai, K.; Nukariya, K.; Shigeta, M. Effects of neuropsychiatric symptoms of dementia on reductions in activities of daily living in patients with Alzheimer′s disease. Geriatr. Gerontol. Int. 2020, 20. [Google Scholar] [CrossRef]
- Van Lieshout-van Dal, E.; Snaphaan, L.; Bongers, I. Biodynamic lighting effects on the sleep pattern of people with dementia. Build. Environ. 2019, 150, 245–253. [Google Scholar] [CrossRef]
- Van der Lek, R.F.; Swaab, D.F.; Twisk, J.; Hol, E.H.; Hoogendijk, W.J.G.; Van Someren, E.J.W. Effect of bright light and melatonin of cognitive and noncognitive function in elderly residents of group care facilities: A randomized controlled trial. JAMA 2008, 299, 2642–2655. [Google Scholar] [CrossRef]
- Haffmans, P.M.J.; Sival, R.C.; Lucius, S.A.P.; Cats, Q.; Van Gelder, L. Bright light therapy and melatonin in motor restless behaviour in dementia: A placebo-controlled study. Int. J. Geriatr. Psychiatry 2001, 16, 106–110. [Google Scholar] [CrossRef]
- Figueiro, M.G.; Plitnick, B.A.; Lok, A.; Jones, G.E.; Higgins, P.; Hornick, T.R.; Rea, M.S. Tailored lighting intervention improves measures of sleep, depression, and agitation in persons with Alzheimer′s disease and related dementia living in long-term care facilities. Clin. Interv. Aging 2014, 9, 1527–1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burns, A.; Allen, H.; Tomenson, B.; Duignan, D.; Byrne, J. Bright light therapy for agitation in dementia: A randomized controlled trial. Int. Psychogeriatr. 2009, 21, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Rubiño, J.A.; Nicolau, M.C.; Gamundí, A.; Akaarir, M.; Cañellas, F. Effect of bright light therapy on sleep and mood in elderly institutionalized subjects with mild to moderate cognitive impairment. Sleep Med. 2017, 40, e45. [Google Scholar] [CrossRef]
- Mitolo, M.; Tonon, C.; La Morgia, C.; Testa, C.; Carelli, V.; Lodi, R. Effects of light treatment on sleep, cognition, mood, and behavior in Alzheimer′s disease: A systematic review. Dement. Geriatr. Cogn. Disord. 2018, 46, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Cibeira, N.; Maseda, A.; Lorenzo-López, L.; Rodríguez-Villamil, J.L.; López-López, R.; Millán-Calenti, J.C. Application of light therapy in older adults with cognitive impairment: A systematic review. Geriatr. Nurs. 2020, 41, 970–983. [Google Scholar] [CrossRef] [PubMed]
- Forbes, D.; Blake, C.M.; Thiessen, E.J.; Peacock, S.; Hawranik, P. Light therapy for improving cognition, activities of daily living, sleep, challenging behaviour, and psychiatric disturbances in dementia. Cochrane Database Syst. Rev. 2014, 2, CD003946. [Google Scholar] [CrossRef] [Green Version]
- Videnovic, A.; Klerman, E.B.; Wang, W.; Marconi, A.; Kuhta, T.; Zee, P.C. Timed light therapy for sleep and daytime sleepiness associated with Parkinson disease. JAMA Neurol. 2017, 74, 411–418. [Google Scholar] [CrossRef]
- Middleton, B.; Stone, B.M.; Arendt, J. Human circadian phase in 12:12 h, 200: <8 lux and 1000: <8 lux light-dark cycles, without scheduled sleep or activity. Neurosci. Lett. 2002, 329, 41–44. [Google Scholar]
- Wulff, K.; Foster, R.G. Insight into the role of photoreception and light intervention for sleep and neuropsychiatric behaviour in the elderly. Curr. Alzheimer Res. 2017, 14, 1022–1029. [Google Scholar] [CrossRef]
- Dugard, P.; File, P.; Todman, J. Single-Case and Small-N Experimental Designs: A Practical Guide to Randomization Tests, 2nd ed.; Routledge: New York, NY, USA, 2012; pp. 1–10. [Google Scholar]
- Kratochwill, T.R.; Levin, J.R. Enhancing the scientific credibility of single-case intervention research: Randomization to the rescue. Psychol. Methods 2010, 15, 124–144. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, H.; Iritani, S.; Fujita, K. Bright light therapy for sleep disturbance in dementia is most effective for mild to moderate Alzheimer′s type dementia: A case series. Psychogeriatrics 2017, 5, 275–281. [Google Scholar] [CrossRef]
- Research Randomizer. Available online: http://randomizer.org (accessed on 8 October 2018).
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of illness and the aged. The index of ADL: A standardized measure of biological and psychosocial function. J. Am. Med. Assoc. 1963, 185, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Ferretti-Rebustini, R.E.; Balbinotti, M.A.; Jacob-Filho, W.; Rebustini, F.; Suemoto, C.K.; Pasqualucci, C.A.; Farfel, J.M.; Leite, R.E.; Grinberg, L.T.; Nitrini, R. Validity of the Katz index to assess activities of daily living by informants in neuropathological studies. Rev. Esc. Enferm. USP 2015, 49, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Reijneveld, S.A.; Spijker, J.; Dijkshoorn, H. Katz′ ADL index assessed functional performance of Turkish, Moroccan and Dutch elderly. J. Clin. Epidemiol. 2007, 60, 382–388. [Google Scholar] [CrossRef] [Green Version]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Graf, C. The lawton instrumental activities of daily living scale. AJN 2008, 108, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Positive Perception Program. Available online: www.ppp-zorg.nl (accessed on 20 August 2018).
- Olino Duurzame Energie. Measurement Report Vitaal Licht 2017. Available online: http://www.olino.org/private/129719/fb18f324120d03e4952d5dba8182fad0/ (accessed on 28 September 2021).
- Bulté, I.; Onghena, P. An R package for single-case randomization tests. Behav. Res. Methods 2008, 40, 467–478. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://tamalkd.shinyapps.io/scda/ (accessed on 1 October 2020).
- Bulté, I.; Onghena, P. The single-case data analysis package: Analysing single-case experiments with R software. J. Mod. Appl. Stat. Methods 2013, 12, 450–478. [Google Scholar] [CrossRef] [Green Version]
- Potkin, S.G.; Anand, R.; Hartman, R.; Veach, J.; Grossberg, G. Impact of Alzheimer′s disease and rivastigmine treatment on activities of daily living over the course of mild to moderately severe disease. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2002, 26, 713–720. [Google Scholar] [CrossRef]
- Østby, T.; Tyas, S.; McDowell, I.; Koval, J. Reported activities of daily living: Agreement between elderly subjects with and without dementia and their caregivers. Age Ageing 1997, 26, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Giebel, C.M.; Sutcliffe, C.; Stolt, M.; Karlsson, S.; Renom-Guiteras, A.; Soto, M.; Verbeek, H.; Zabalegui, A.; Challis, D.; on behalf of the Right Time Place Care Consortium. Deterioration of basic activities of daily living and their impact on quality of life across different cognitive stages of dementia: A European study. Int. Psychogeriatr. 2014, 26, 1283–1293. [Google Scholar] [CrossRef]
- Preto, S.; Gomes, C.C. Lighting in the workplace: Recommended illuminance (lux) at workplace environs. In Advances in Design for Inclusion. AHFE 2018. Advances in Intelligent Systems and Computing; Bucchianico, D., Ed.; Springer: Cham, Switzerland, 2019; pp. 180–191. [Google Scholar]
- Cajochen, C.; Zeitzer, J.M.; Czeiler, C.A.; Dijk, D.-J. Dose-response relationship for light intensity and ocular and electoencephalografic correlates of human alertness. Behav. Brain Res. 2000, 115, 75–83. [Google Scholar] [CrossRef]
- Zeitzer, J.M.; Dijk, D.-J.; Kronauer, R.; Brown, E.; Czeiler, C.A. Sensitivity of the human circadian pacemaker to noctural light: Melatonin phase resetting and supression. J. Physiol. 2000, 3, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.Y. Suprachiasmatic nucleus. International Encyclopedia of the Social & Behavioral Sciences; Smelser, N.J., Baltes, P.B., Eds.; Elsevier: Pergamon, Turkey, 2001; pp. 15290–15294. [Google Scholar] [CrossRef]
- Meguro, K.; Ueda, M.; Kobayashi, I.; Yamaguchi, S.; Yamazaki, H.; Oikawa, Y.; Kikuchi, Y.; Sasaki, H. Sleep disturbance in elderly patients with cognitive impairment, decreased daily activity and periventricular white matter lesions. Sleep 1995, 18, 109–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, Y.–E.S.; Lucey, B.P.; Holtzman, D.M. Sleep and Alzheimer disease pathology—A bidirectional relationship. Nat. Rev. Neurol. 2014, 10, 115–119. [Google Scholar] [CrossRef]
- Sakurai, T.; Iimuro, S.; Sakamaki, K.; Umegaki, H.; Araki, A.; Ohashi, Y.; Ito, H.; The Japanse Elderly Diabetes Intervention Trial Study Group. Risk factors for a 6-year decline in physical disability and functional limitations among elderly people with type 2 diabetes in the Japanese elderly diabetes intervention trial. Geriatr. Gerontol. Int. 2012, 12, 117–126. [Google Scholar] [CrossRef]
- Qaseem, A.; Snow, V.; Cross, T., Jr.; Forciea, M.A.; Hopkins, R., Jr.; Shekelle, P.; Adelman, A.; Mehr, D.; Schellhase, K.; Campos-Outcalt, D.; et al. Current pharmacologic treatment of dementia: A clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann. Intern. Med. 2008, 148, 370–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botanov, Y.; Ilardi, S.S. The acute side effects of bright light therapy: A placebo-controlled investigation. PLoS ONE 2013, 8, e75893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hjetland, G.J.; Pallesen, S.; Thun, E.; Kolberg, E.; Nordhus, I.H.; Flo, E. Light interventions and sleep, circadian, behavioral and psychological disturbances in dementia: A systematic review of methods and outcomes. Sleep Med. Rev. 2020, 52, 101310. [Google Scholar] [CrossRef] [PubMed]












| Participant One | Participant Two | Participant Three | |
|---|---|---|---|
| Gender | Male | Female | Female |
| Age | 66 | 67 | 84 |
| Diagnosis | Alzheimer’s disease | Vascular dementia | Alzheimer’s disease + Parkinson’s disease |
| Education | Primary technical school | Middle school | Domestic science school |
| Former work | Truck driver | Interpreter | Domestic help |
| Marital status/living situation | Married, living with his wife | Widow, living with her daughter and her family | Married, living with her husband |
| Medication | Asasantin Reminyl Rosuvastatine aurobindo | Mylan Acetylsalicylzuur Omeprazol | Lisinopril Madopar Quetiapine Omeprazol Simvastatine Mirtazepine Lormetazepam |
| Amount of lux in the living room (‘normal lighting’) | 86 lux in the living room; 190 lux near the window | 110 lux in the living room; 547 lux near the window | 121 lux in the living room; 410 lux near the window |
| Informal caregiver | Wife | Daughter | Husband |
| Mini-Mental State Examination (MMSE) Scores (impairment) | 19/30 (moderate) | 19/30 (moderate) | 20/30 (moderate) |
| ADL problems at start | Dressing (including preparations for dressing) Eating (including preparations for eating) Starting activities Perform activities | Washing/showering Dressing Cooking/ preparing food Medication intake Household | Washing/showering Dressing Eating Medication intake Visiting toilet |
| Parameter | Lamp Measurement | Remark |
|---|---|---|
| Colour temperature | 4847 K 4750 K | Direct light Indirect light |
| Light intensity | 1984.2 Cd | 1 m distance |
| Colour Rendering Index | 87 | CRI_Ra |
| S/P ratio | 2.0 | 1 m distance |
| Melanopic Effect Factor | 0.682 | According to standard DIN SPEC 5031-100:2015-08 |
| Light spectrum | 465–480 Nm | Melanopic lux |
| Luminous Flux | 6818 lm | 1 m distance |
| Blue light hazard risk | Group 0 | No risk |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aarden-van Delft, N.; Peeters, M.; Snaphaan, L. Biodynamic Lighting and Functional Disability; a Single-Case Experimental Design in Three Community Dwelling People with Dementia. Appl. Sci. 2021, 11, 9433. https://doi.org/10.3390/app11209433
Aarden-van Delft N, Peeters M, Snaphaan L. Biodynamic Lighting and Functional Disability; a Single-Case Experimental Design in Three Community Dwelling People with Dementia. Applied Sciences. 2021; 11(20):9433. https://doi.org/10.3390/app11209433
Chicago/Turabian StyleAarden-van Delft, Noortje, Manon Peeters, and Liselore Snaphaan. 2021. "Biodynamic Lighting and Functional Disability; a Single-Case Experimental Design in Three Community Dwelling People with Dementia" Applied Sciences 11, no. 20: 9433. https://doi.org/10.3390/app11209433
APA StyleAarden-van Delft, N., Peeters, M., & Snaphaan, L. (2021). Biodynamic Lighting and Functional Disability; a Single-Case Experimental Design in Three Community Dwelling People with Dementia. Applied Sciences, 11(20), 9433. https://doi.org/10.3390/app11209433





