The Effect of an Ag Nanofilm on Low-Temperature Cu/Ag-Ag/Cu Chip Bonding in Air
Abstract
:1. Introduction
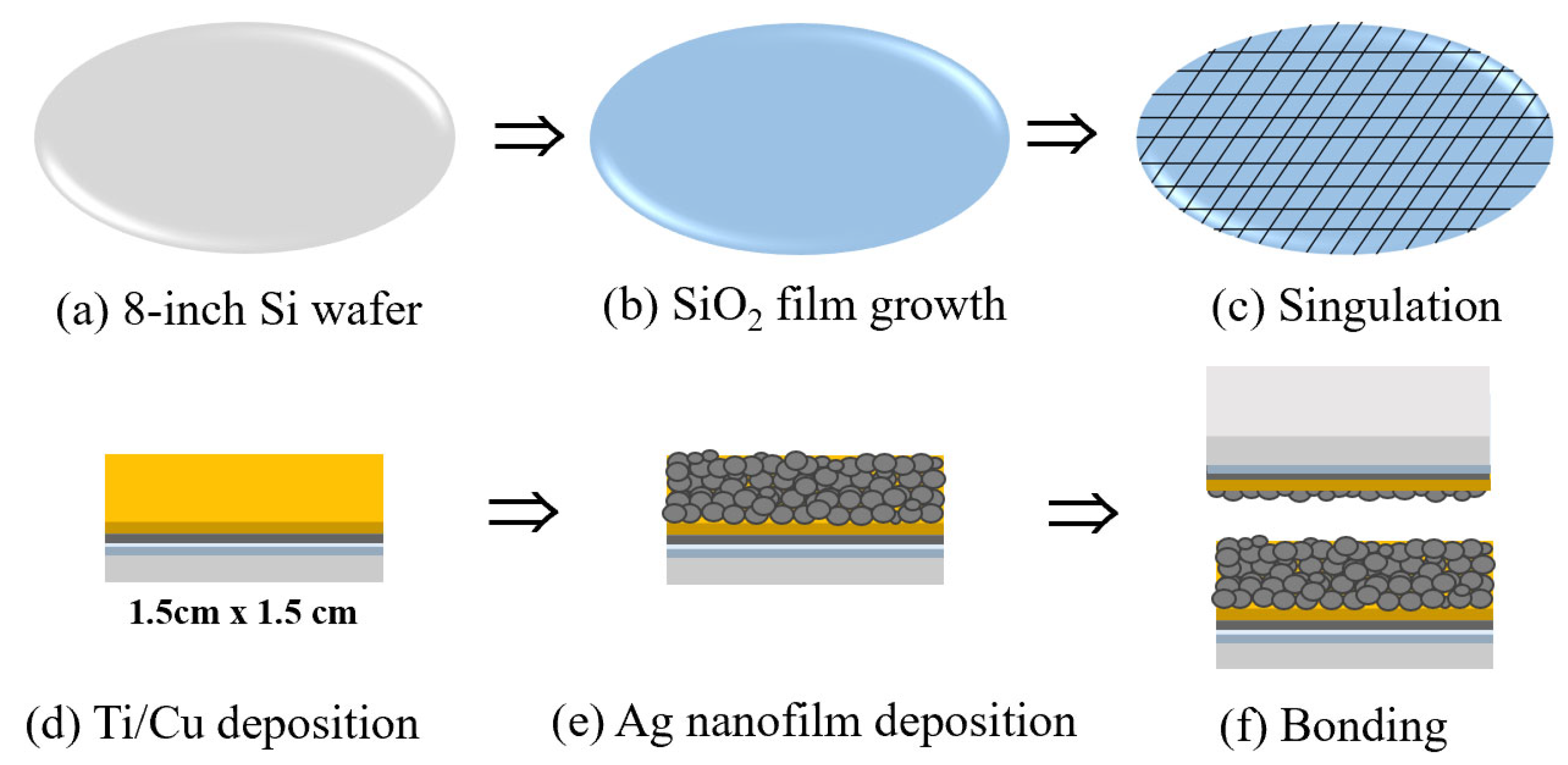
2. Materials and Methods
3. Results and Discussion
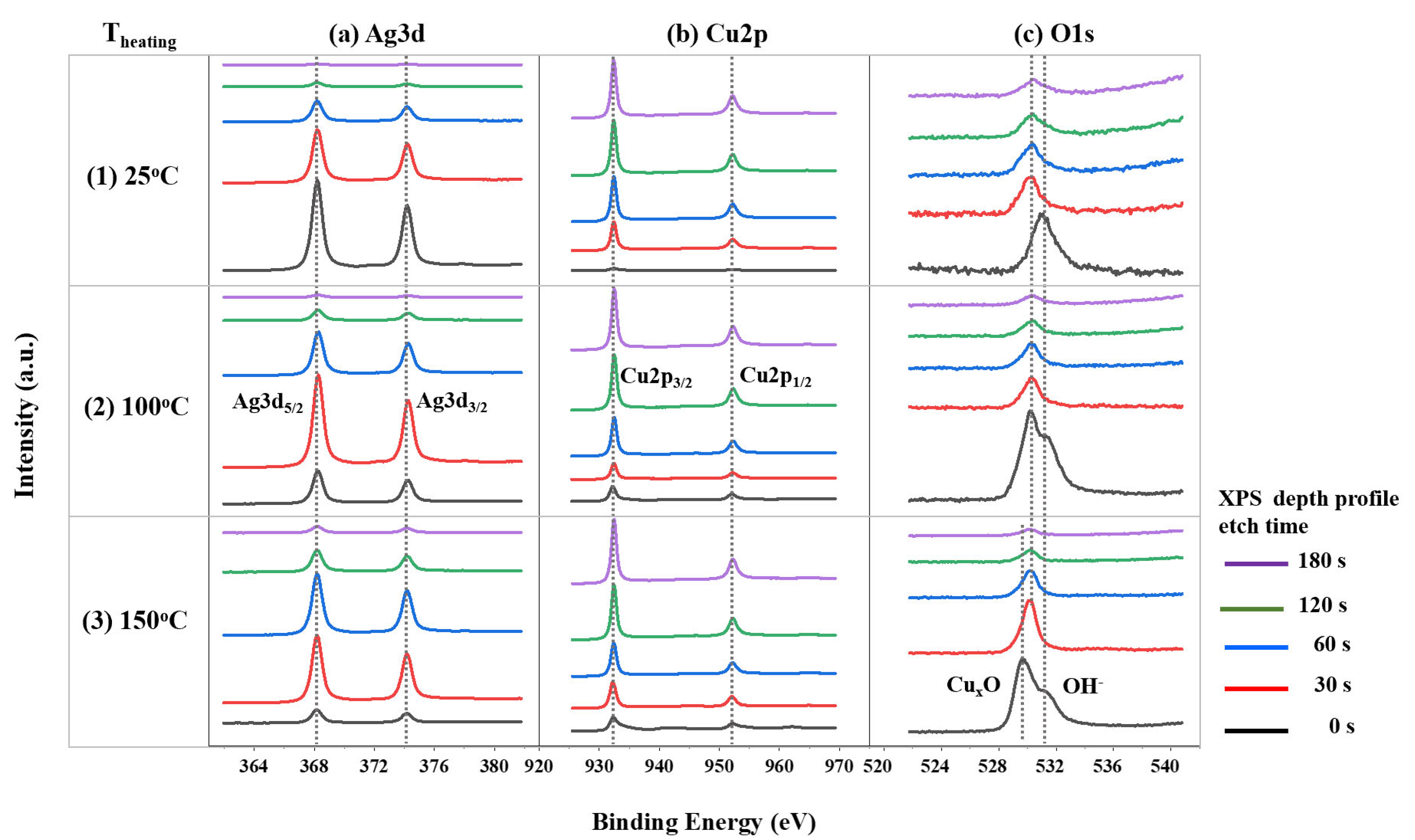
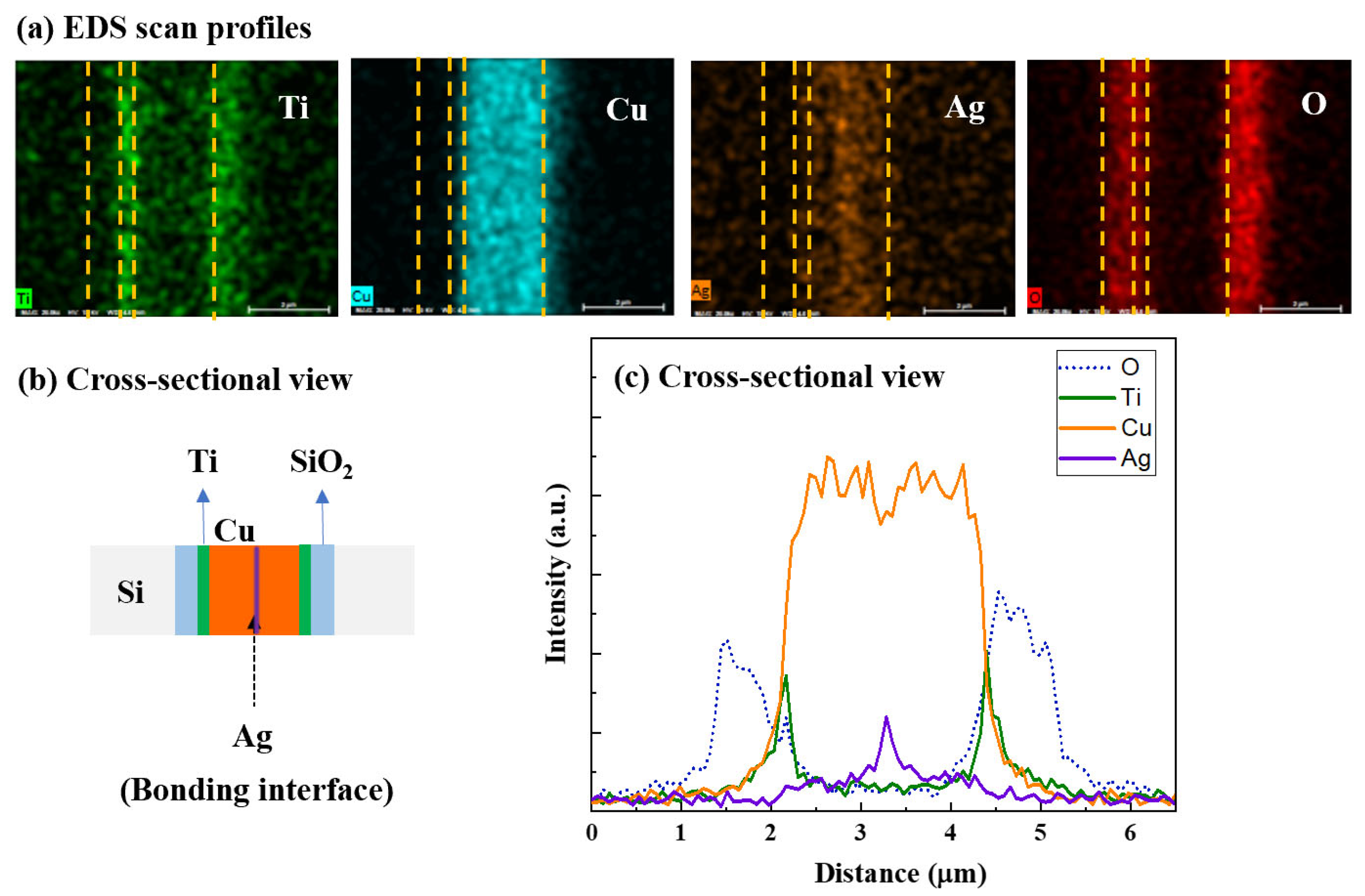
3.1. Ag Diffusion into Cu
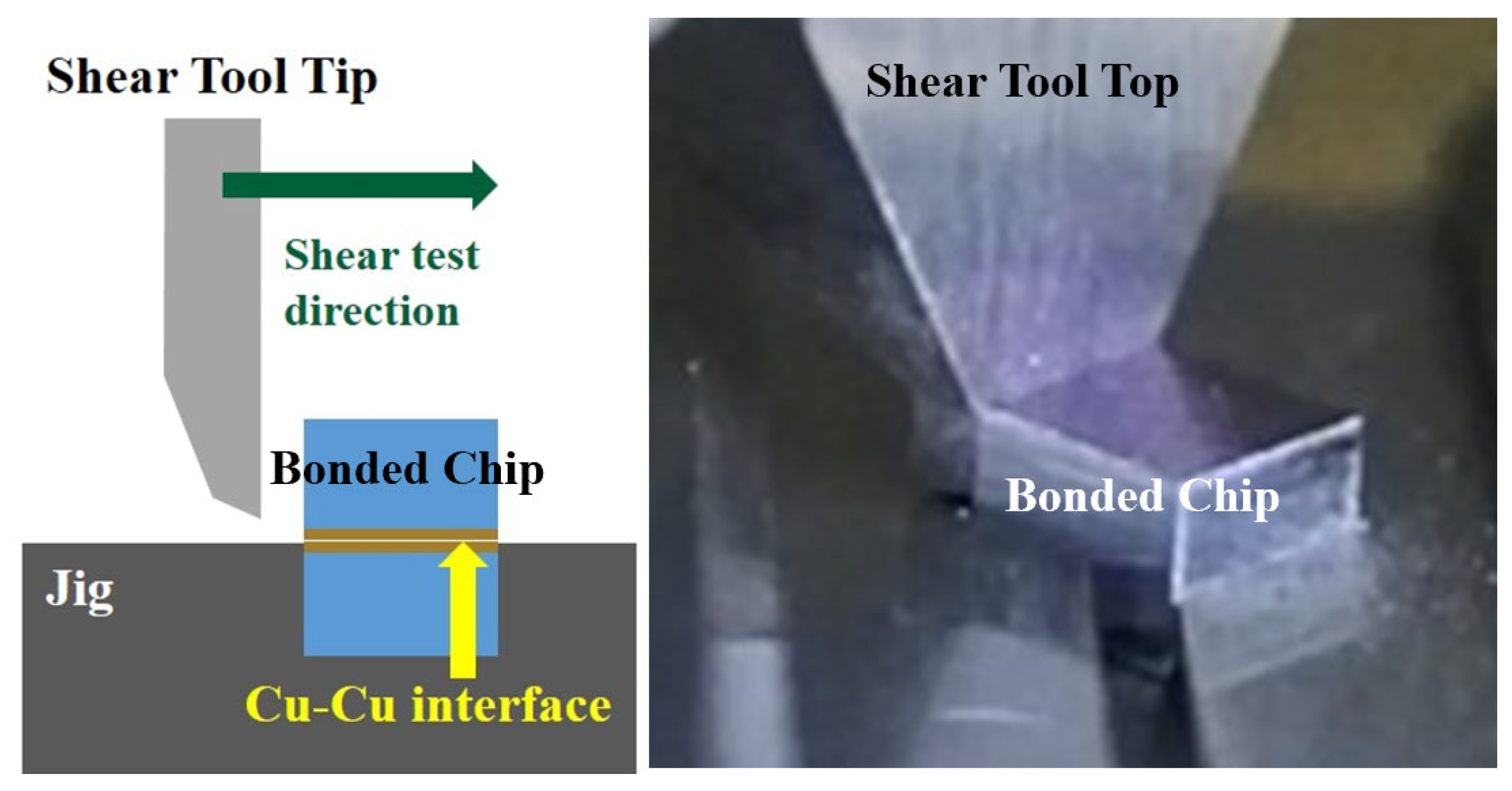
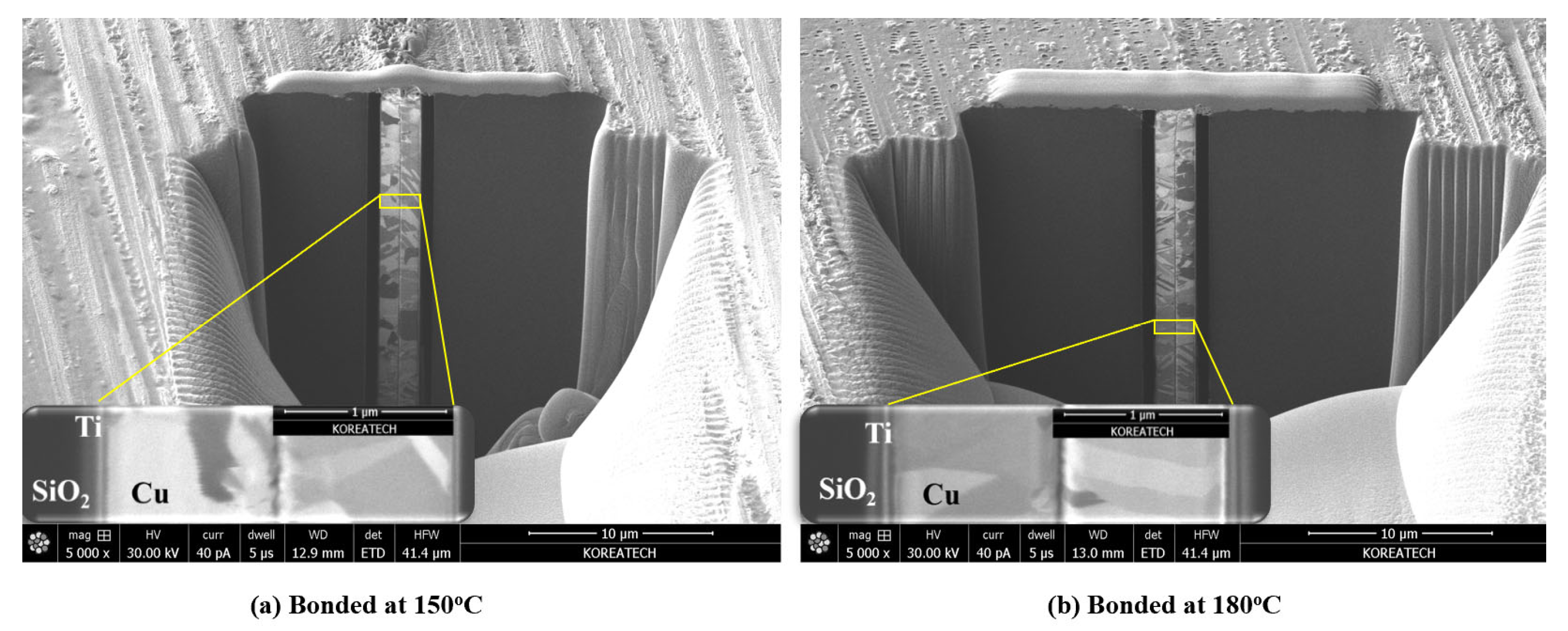
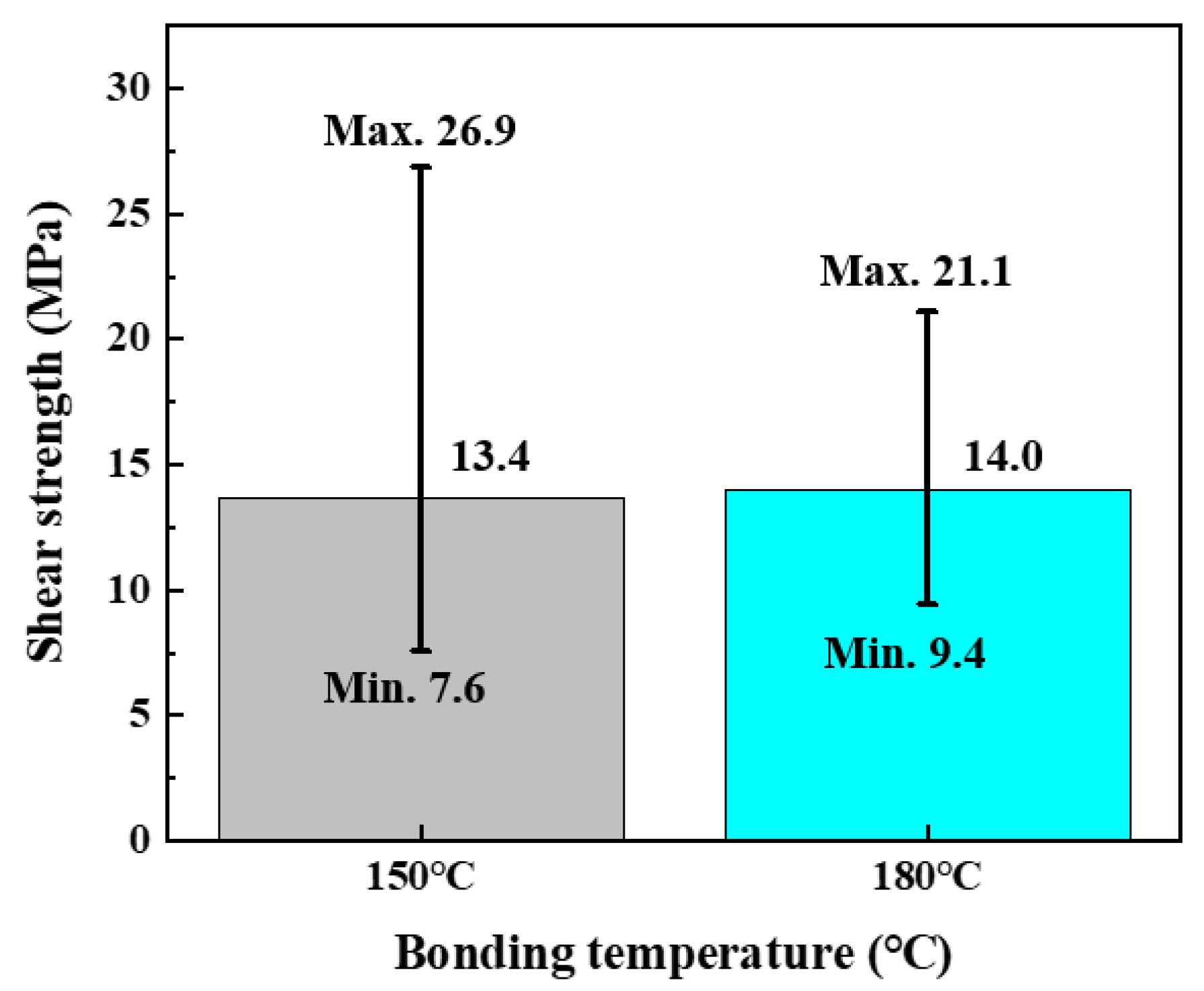
3.2. Cu/Ag-Ag/Cu Bonding
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Panigrahi, A.K.; Chen, K.N. Low Temperature Cu-Cu Bonding Technology in 3D Integration: An Extensive Review. J. Electron. Packag. 2017, 140, 010801. [Google Scholar] [CrossRef] [Green Version]
- IEEE Heterogeneous Integration Roadmap: Chapter 21 SiP and Module System Integration and Chapter 22 Interconnects for 3D and 3D Architectures. 2020. Available online: https://eps.ieee.org/images/files/HIR_2019/HIR1_ch21_sip-module.pdf (accessed on 3 September 2021).
- Park, H.; Seo, H.; Kim, S.E. Anti-oxidant copper layer by remote mode N2 plasma for low temperature copper–copper bonding. Sci. Rep. 2020, 10, 21720. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.S.; Lim, D.F.; Ang, X.F.; Wei, J.; Leong, K.C. Low temperature Cu-Cu thermo-compression bonding with temporary passivation of self-assembled monolayer and its bond strength enhancement. Microelectron. Reliab. 2012, 52, 321–324. [Google Scholar] [CrossRef]
- Hu, L.; Goh, S.C.K.; Tan, C.S. Ar/N2 Plasma Induced Metastable CuxNy for Cu-Cu Direct Bonding. ECS Trans. 2020, 98, 203–210. [Google Scholar] [CrossRef]
- Park, H.; Kim, S.E. Two-Step Plasma Treatment on Copper Surface for Low-Temperature Cu Thermo-Compression Bonding. IEEE Trans. Compon. Packag. Manuf. Technol. 2018, 10, 332–338. [Google Scholar] [CrossRef]
- Park, H.; Seo, H.; Kim, Y.; Park, S.; Kim, S.E. Low Temperature (260 °C) Solderless Cu-Cu Bonding for Fine Pitch 3D Packaging and Heterogeneous Integration. IEEE Trans. Compon. Packag. Manuf. Technol. 2021, 11, 565–572. [Google Scholar] [CrossRef]
- Huang, Y.; Chien, Y.; Tzeng, R.; Shy, M.; Lin, T.; Chen, K.; Chiu, C.; Chiou, J.; Chuang, C.T.; Hwang, W.; et al. Novel Cu-to-Cu Bonding with Ti Passivation at 180 °C in 3-D Integration. IEEE Electron Dev. Lett. 2013, 34, 1551–1553. [Google Scholar] [CrossRef]
- Panigrahi, A.K.; Bonam, S.; Ghosh, T.; Singh, S.G.; Vanjari, S.R.K. Ultra-thin Ti Passivation mediated breakthrough in high quality Cu-Cu bonding at low temperature and pressure. Mater. Lett. 2016, 169, 269–272. [Google Scholar] [CrossRef]
- Bonam, S.; Panigrahi, A.K.; Kumar, C.H.; Vanjari, S.R.K. Interface and Reliability Analysis of Au-Passivated Cu–Cu Fine-Pitch Thermocompression Bonding for 3-D IC Applications. IEEE Trans. Compon. Packag. Manuf. Technol. 2019, 9, 1227–1234. [Google Scholar] [CrossRef]
- Chou, T.; Huang, S.; Chen, P.; Hu, H.; Liu, D.; Chang, C.; Ni, T.; Chen, C.; Lin, Y.; Chang, T.; et al. Electrical and Reliability Investigation of Cu-to-Cu Bonding with Silver Passivation Layer in 3D Integration. IEEE Trans. Compon. Packag. Manuf. Technol. 2020, 11, 36–42. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, J.; Wang, Q.; Wang, Z.; Liu, L.; Zou, G. Thermal-stable void-free interface morphology and bonding mechanism of low-temperature Cu-Cu bonding using Ag nanostructure as intermediate. J. Alloys Compd. 2018, 767, 575–582. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Cai, J.; Wang, Q.; Tan, L.; Hu, Y. Low temperature Cu-Cu bonding using Ag nanostructure for 3D integration. ECS Solid State Lett. 2015, 4, 75–76. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Park, S.; Kim, S.E. Effect of Ag Nanolayer in Low Temperature Cu/Ag-Ag/Cu Bonding. J. Microelectron. Packag. Soc. 2021, 28, 59–64. (In Korean) [Google Scholar]
- Wang, M.Y.; Mei, Y.H.; Burgos, R.; Boroyevich, D.; Lu, G.Q. Effect of Substrate Surface Finish on Bonding Strength of Pressure-less Sintered Silver Die-attach. In Proceedings of the 2018 International Conference on Electronics Packaging and iMAPS All Asia Conference (ICEP-IAAC), Mie, Japan, 17–21 April 2018. [Google Scholar]
- Butrymowicz, D.B.; Manning, J.R.; Read, M.B. Diffusion in Copper and Copper Alloys Part ΙΙ. Copper-Silver and Copper-Colde Systems. J. Phys. Chem. 1974, 3, 533. [Google Scholar]
- Bokstein, B.S.; Vunkov, V.I.E.; Golosov, V.; Karpov, M.I.; Kolobov, Y.R.; Kolesnikov, D.A.; Korzhov, V.P.; Rodin, A.O. Structure and diffusion processes in laminated composites of a Cu–Ti system. Russ. Phys. J. 2009, 52, 811–815. [Google Scholar] [CrossRef] [Green Version]

| Temperature (°C) | Pressure (MPa) | Time (min) | Annealing |
|---|---|---|---|
| 150 | 30 | 30 | No |
| 180 | 30 | 30 | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Park, S.; Kim, S.E. The Effect of an Ag Nanofilm on Low-Temperature Cu/Ag-Ag/Cu Chip Bonding in Air. Appl. Sci. 2021, 11, 9444. https://doi.org/10.3390/app11209444
Kim Y, Park S, Kim SE. The Effect of an Ag Nanofilm on Low-Temperature Cu/Ag-Ag/Cu Chip Bonding in Air. Applied Sciences. 2021; 11(20):9444. https://doi.org/10.3390/app11209444
Chicago/Turabian StyleKim, Yoonho, Seungmin Park, and Sarah Eunkyung Kim. 2021. "The Effect of an Ag Nanofilm on Low-Temperature Cu/Ag-Ag/Cu Chip Bonding in Air" Applied Sciences 11, no. 20: 9444. https://doi.org/10.3390/app11209444







