Antibacterial Fluorinated Diamond-like Carbon Coating Promotes Osteogenesis—Comparison with Titanium Alloy
Abstract
:Featured Application
Abstract
1. Introduction
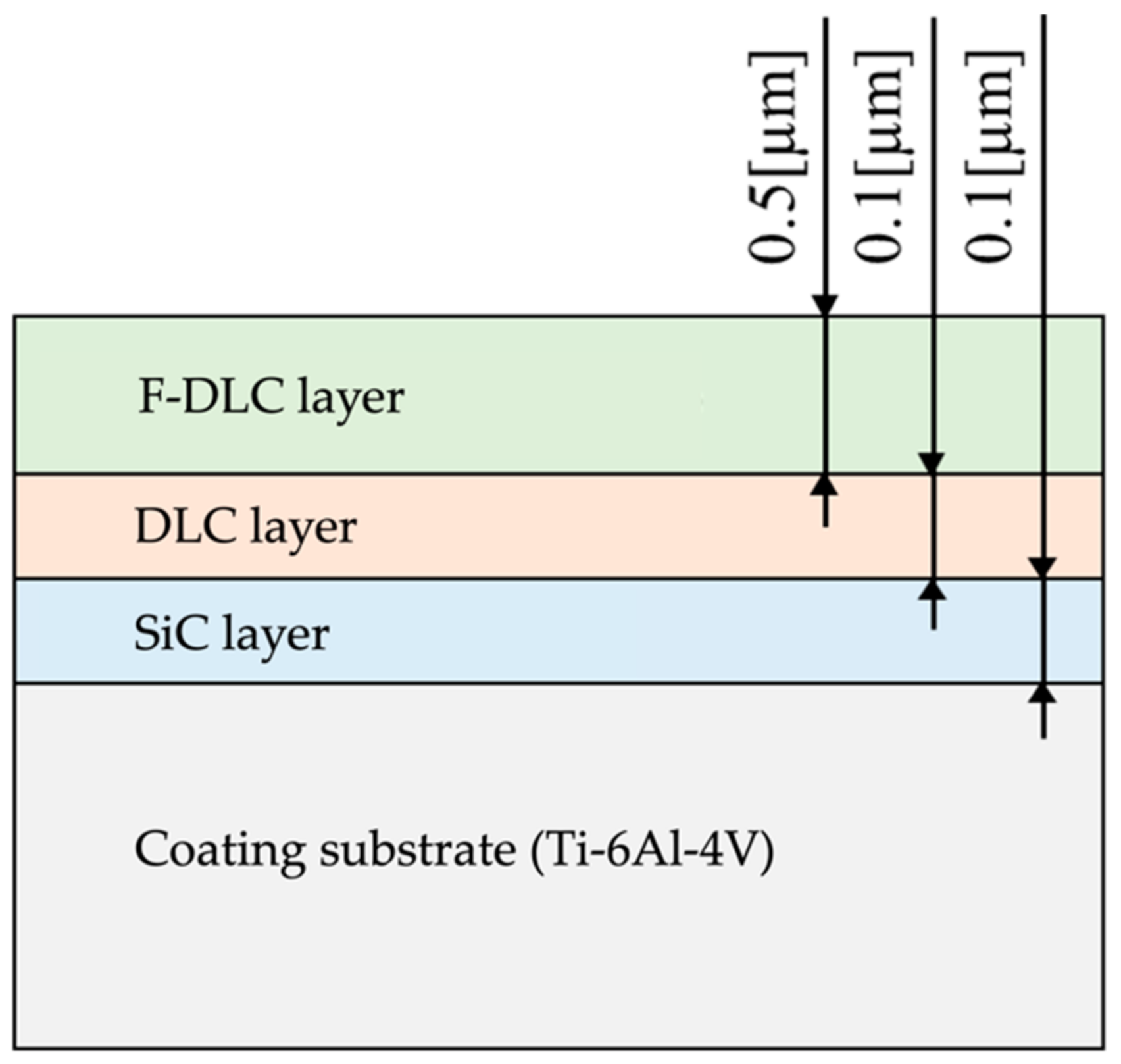
2. Materials and Methods
3. Results
3.1. Assessing the Maturity of Newly Formed Tissue under Natural Light, Polarized Light and Fluorescent Light
3.1.1. Observations under Natural Light
3.1.2. Observations under Polarized Light
3.1.3. Observations under Fluorescent Light
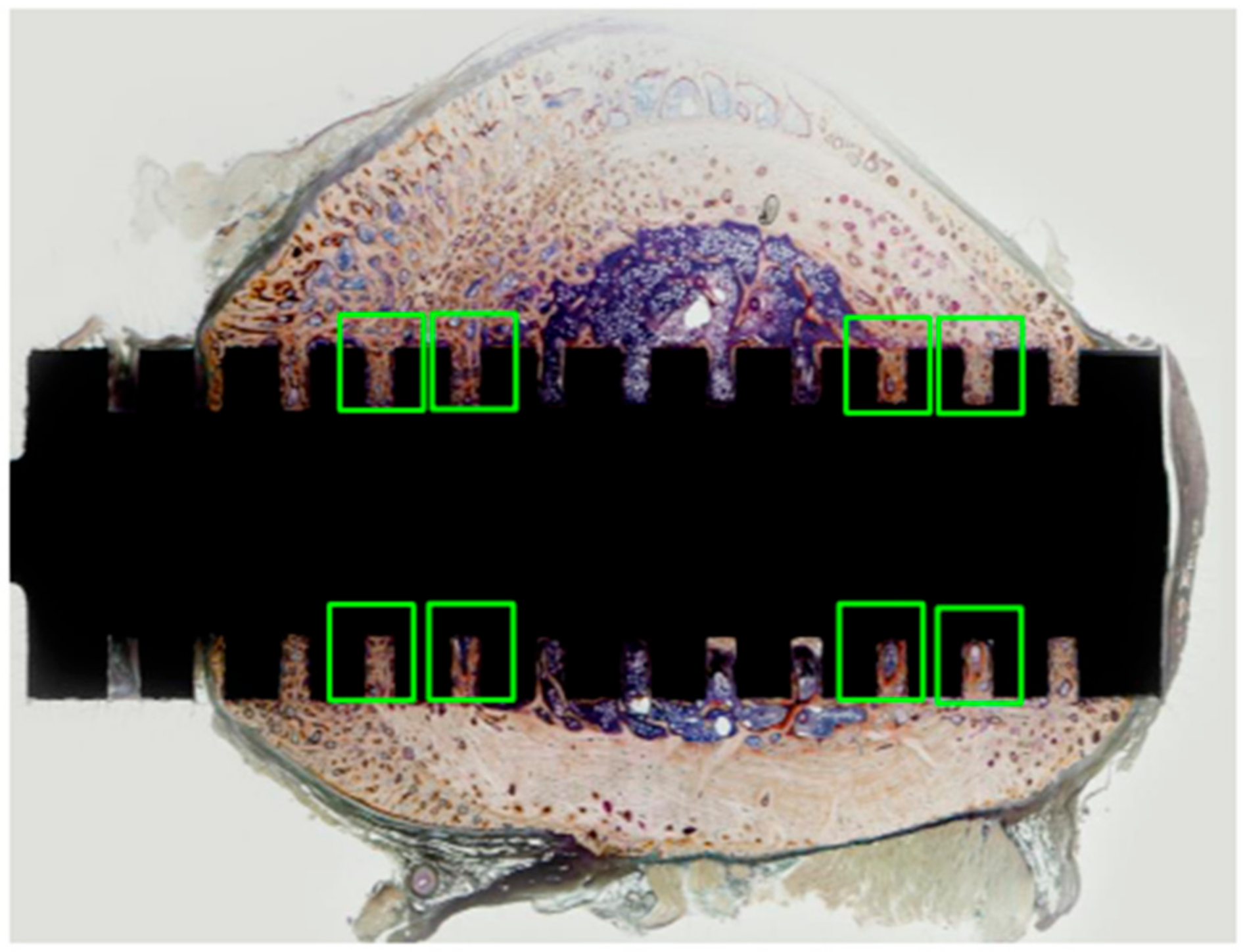
3.2. Results of Bone Histomorphometry
3.2.1. Bone Volume/Tissue Volume (%)
3.2.2. Trabecular Thickness (µm)
3.2.3. Number of Osteoclasts/Bone Surface Length (N/mm)
3.2.4. Eroded Surface/Bone Surface (%)
3.2.5. Number of Osteoblasts/Bone Surface Length (N/mm), Osteoid Surface/Bone Surface (%), Mineral Apposition Rate (µm/day), and Bone Formation Rate/Tissue Volume (%/year)
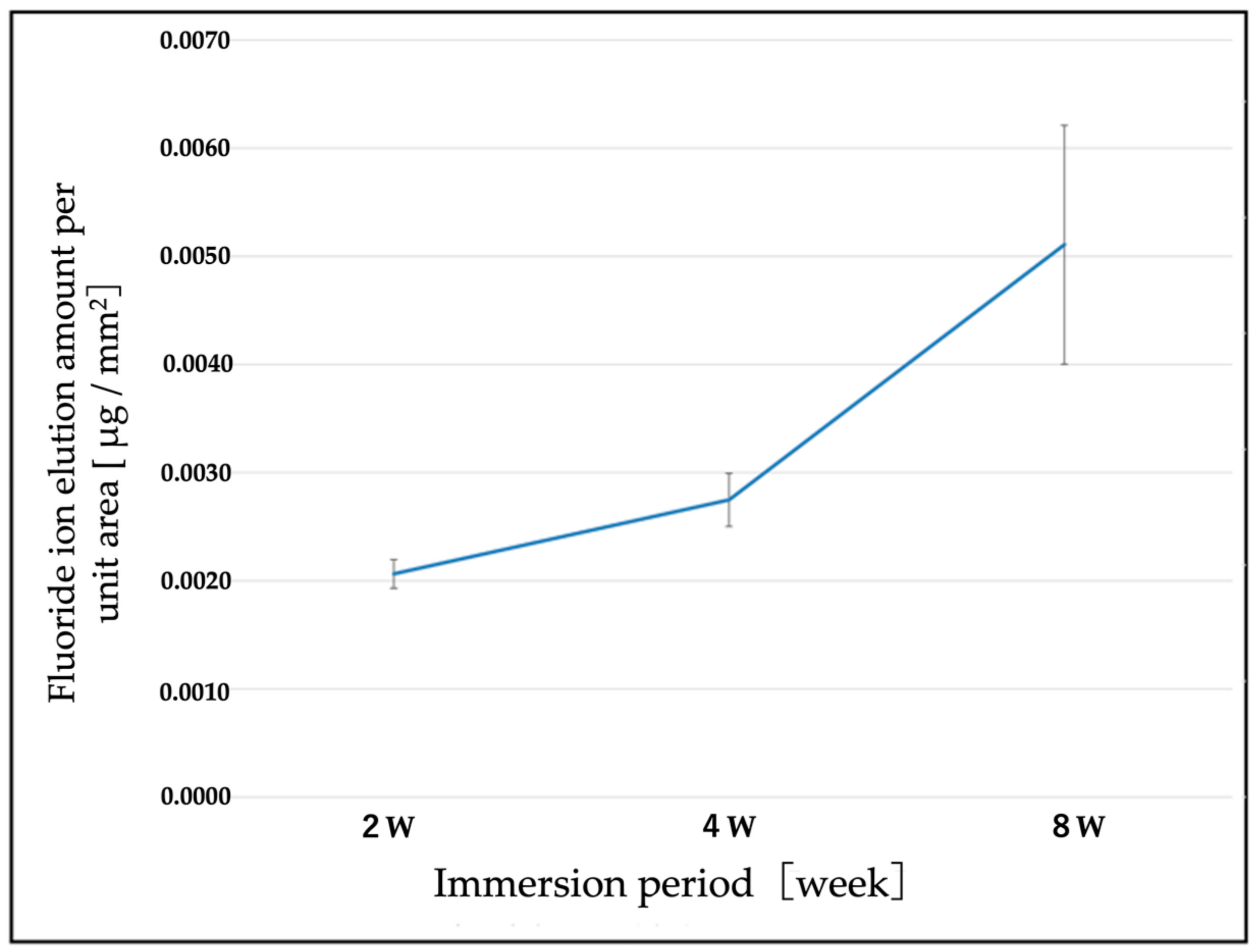
3.3. Elution of F Ions from the F-DLC Coated Titanium Alloy
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davne, S.H.; Myers, D.L. Complications of lumbar spinal fusion with transpedicular instrumentation. Spine 1992, 17 (Suppl. 6), S184–S189. [Google Scholar] [CrossRef] [PubMed]
- Sponseller, P.D.; LaPorte, D.M.; Hungerford, M.W.; Eck, K.; Bridwell, K.H.; Lenke, L.G. Deep wound infections after neuromuscular scoliosis surgery: A multicenter study of risk factors and treatment outcomes. Spine 2000, 25, 2461–2466. [Google Scholar] [CrossRef] [PubMed]
- Sperling, J.W.; Kozak, T.K.; Hanssen, A.D.; Cofield, R.H. Infection after shoulder arthroplasty. Clin. Orthop. Relat. Res. 2001, 382, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Minnema, B.; Vearncombe, M.; Augustin, A.; Gollish, J.; Simor, A.E. Risk factors for surgical-site infection following primary total knee arthroplasty. Infect. Control Hosp. Epidemiol. 2004, 25, 477–480. [Google Scholar] [CrossRef]
- Bengtson, S.; Knutson, K. The infected knee arthroplasty. A 6-year follow-up of 357 cases. Acta Orthop. Scand. 1991, 62, 301–311. [Google Scholar] [CrossRef] [Green Version]
- Hill, G.E.; Droller, D.G. Acute and subacute deep infection after uncemented total hip replacement using antibacterial prophylaxis. Orthop. Rev. 1989, 18, 617–623. [Google Scholar]
- Wymenga, A.B.; van Horn, J.R.; Theeuwes, A.; Muytjens, H.L.; Slooff, T.G. Perioperative factors associated with septic arthritis after arthroplasty. Prospective multicenter study of 362 knee and 2,651 hip operations. Acta Orthop. Scand. 1992, 63, 665–671. [Google Scholar]
- Phillips, C.B.; Barrett, J.A.; Losina, E.; Mahomed, N.N.; Lingard, E.A.; Gaudagnoli, E.; Baron, J.A.; Harris, W.H.; Poss, R.; Katz, J. Incidence rates of dislocation, pulmonary embolism, and deep infection during the first six months after elective total hip replacement. J. Bone Joint Surg. Am. 2003, 85, 20–26. [Google Scholar] [CrossRef]
- Lucke, M.; Schmidmaier, G.; Sadoni, S.; Wildemann, B.; Schiller, R.; Haas, N.P.; Raschke, M. Gentamicin coating of metallic implants reduces implant-related osteomyelitis in rats. Bone 2003, 32, 521–531. [Google Scholar] [CrossRef]
- Antoci, V., Jr.; Adams, C.S.; Hickok, N.J.; Shapiro, I.M.; Parvizi, J. Vancomycin bound to Ti rods reduces periprosthetic infection: Preliminary study. Clin. Orthop. Relat. Res. 2007, 461, 88–95. [Google Scholar] [CrossRef]
- Neut, D.A.; Dijkstra, R.J.; Thompson, J.I.; Kavanagh, C.; van der Mei, H.C.; Busscher, H.J. Biodegradable gentamicin-hydroxyapatite-coating for infection prophylaxis in cementless hip prostheses. Eur. Cell Mater. 2015, 2, 42–55. [Google Scholar] [CrossRef]
- Noda, I.; Miyaji, F.; Ando, Y.; Miyamoto, H.; Shimazaki, T.; Yonekura, Y.; Miyazaki, M.; Mawatari, M.; Hotokebuchi, T. Development of novel thermal sprayed antibacterial coating and evaluation of release properties of silver ions. J. Biomed. Mater. Res. Part B 2009, 89B, 456–465. [Google Scholar] [CrossRef]
- Funao, H.; Nagai, S.; Sasaki, A.; Hoshikawa, T.; Tsuji, T.; Okada, Y.; Koyasu, S.; Toyama, Y.; Nakamura, M.; Aizawa, M.; et al. A novel hydroxyapatite film coated with ionic silver via inositol hexaphosphate chelation prevents implant-associated infection. Sci. Rep. 2016, 6, 23238. [Google Scholar] [CrossRef] [Green Version]
- Ando, Y.; Miyamoto, H.; Noda, I.; Sakurai, N.; Akiyama, T.; Yonekura, Y.; Shimazaki, T.; Miyazaki, M.; Mawatari, M.; Hotokebuchi, T. Calcium phosphate coating containing silver shows high antibacterial activity and low cytotoxicity and inhibits bacterial adhesion. Mater Sci. Eng. C 2010, 30, 175–180. [Google Scholar] [CrossRef]
- Zainali, K.; Danscher, G.; Jakobsen, T.; Jakobsen, S.S.; Baas, J.; Møller, P.; Bechtold, J.E.; Soballe, K. Effects of gold coating on experimental implant fixation. J. Biomed. Mater. Res. A 2009, 88, 274–280. [Google Scholar] [CrossRef] [Green Version]
- Shirai, T.; Shimizu, T.; Ohtani, K.; Zen, Y.; Takaya, M.; Tsuchiya, H. Antibacterial iodine-supported titanium implants. Acta Biomater. 2011, 7, 1928–1933. [Google Scholar] [CrossRef] [Green Version]
- Stuart, B.W.; Stan, G.E.; Popa, A.C.; Carrington, M.J.; Zgura, I.; Necsulescu, M.; Grant, D.M. New solutions for combatting implant bacterial infection based on silver nano-dispersed and gallium incorporated phosphate bioactive glass sputtered films: A preliminary study. Bioact. Mater. 2021, 8, 325–340. [Google Scholar] [CrossRef]
- Bee, S.-L.; Bustami, Y.; Hamid, A.U.; Lim, K.; Hamid, Z.A.A. Synthesis of silver nanoparticle-decorated hydroxyapatite nanocomposite with combined bioactivity and antibacterial properties. J. Mater. Sci. Mater. Med. 2021, 32, 106. [Google Scholar] [CrossRef]
- Aisenberg, S.; Chabot, R. Ion-beam deposition of thin films of diamondlike carbon. J. Appl. Phys. 1971, 42, 2953. [Google Scholar] [CrossRef]
- Saito, T.; Hasebe, T.; Yohena, S.; Matsuoka, Y.; Kamijo, A.; Takahashi, K.; Suzuki, T. Antithrombogenicity of fluorinated diamond-like carbon films. Diam. Relat. Mat. 2005, 14, 1116–1119. [Google Scholar] [CrossRef]
- Yonezawa, K.; Kawaguchi, M.; Kawahara, N. Evaluation of antibacterial and cytotoxic properties of a fluorinated diamond-like carbon coating for the development of antibacterial medical implants. Antibiotics 2020, 9, 495. [Google Scholar] [CrossRef]
- Mine, Y.; Nakatani, T.; Okamoto, K.; Hara, S.; Takagi, K.; Nikawa, H. Impact of biomimetic diamond-like carbon coated titanium on osteoblast and osteoclast differentiation in vitro. J. Photopolym. Sci. Technol. 2014, 27, 373–378. [Google Scholar] [CrossRef] [Green Version]
- Mine, Y.; Nakatani, T.; Okamoto, K. Biomimetic diamond-like carbon coated titanium inhibits RANKL-dependent osteoclast differentiation in vitro. J. Photopolym. Sci. Technol. 2012, 25, 523–528. [Google Scholar] [CrossRef]
- Shuto, T.; Nakatani, T.; Okamoto, K.; Saizaki, N.; Mimura, S.; Kunitsugu, S.; Nikawa, H. Differentiation of osteoblast and osteoclast cells on hydrogenated-tetrahedral amorphous carbon coated titanium. J. Photopolym. Sci. Technol. 2016, 29, 413–418. [Google Scholar] [CrossRef] [Green Version]
- Japanese Industrial Standard: JIS K 0102:2016. Testing Methods for Industrial Wastewater; General Incorporated Association Industrial Environment Management Association; Japanese Standards Association: Tokyo, Japan, 2016; pp. 108–114. [Google Scholar]
- Kawaguchi, M.; Shintani, K.; Kawahara, N. Bone formation at Ti-6Al-7Nb scaffolds consisting of 3D honeycomb frame and diamond-like carbon coating implanted into the femur of beagles. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1283–1291. [Google Scholar] [CrossRef]
- Farley, J.R.; Wergedal, J.E.; Baylink, D.J. Fluoride directly stimulates proliferation and alkaline phosphatase activity of bone-forming cells. Science 1983, 222, 330–332. [Google Scholar] [CrossRef]
- Marie, P.J.; De Vernejoul, M.C.; Lomri, A. Stimulation of bone formation in osteoporosis patients treated with fluoride associated with increased DNA synthesis by osteoblastic cells in vitro. J. Bone Min. Res. 1992, 7, 103–113. [Google Scholar] [CrossRef]
- Khokher, M.A.; Dandona, P. Fluoride stimulates [3H]thymidine incorporation and alkaline phosphatase production by human osteoblasts. Metabolism 1990, 39, 1118–1121. [Google Scholar] [CrossRef]
- Wergedal, J.E.; Lau, K.H.W.; Baylink, D.J. Fluoride and bovine bone extract influence cell proliferation and phosphatase activities in human bone cell cultures. Clin. Orthop. 1988, 233, 274–282. [Google Scholar] [CrossRef]
- Taves, D.R. New approach to the treatment of bone disease with fluoride. Fed. Proc. 1970, 29, 1185–1187. [Google Scholar] [PubMed]
- Messer, H.H.; Armstrong, W.D.; Singer, L. Fluoride, parathyroid hormone and calcitonin: Effects on metabolic processes involved in bone resorption. Calc. Tiss. Res. 1973, 13, 227–233. [Google Scholar] [CrossRef]
- McCann, H.G. Reaction of fluoride ion with hydroxyapatite. J. Biol. Chem. 1953, 201, 247–259. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, H.; Liu, H.; Ji, H.; Fei, W.; Luo, E. Fluorine-contained hydroxyapatite suppresses bone resorption through inhibiting osteoclasts differentiation and function in vitro and in vivo. Cell Prolif. 2019, 52, e12613. [Google Scholar] [CrossRef]
- Rich, C.; Feist, E. The Action of Fluoride on Bone. In Fluoride in Medicine; Vischer, T., Ed.; Hans Huber Pub.: Bern, Switzerland, 1970; pp. 70–87. [Google Scholar]
- Hedlund, L.R.; Gallagher, J.C. Increased incidence of hip fracture in osteoporotic women treated with sodium fluoride. J. Bone Miner. Res. 1989, 4, 223–225. [Google Scholar] [CrossRef]
- Varughese, K.; Moreno, E.C. Crystal growth of calcium apatites in dilute solutions containing fluoride. Calif. Tiss. Int. 1981, 33, 431–439. [Google Scholar] [CrossRef]
- Eanes, E.D.; Reddi, A.H. The effect of fluoride on bone mineral apatite. Metab. Bone Dis. Relat. Res. 1979, 2, 3–10. [Google Scholar] [CrossRef]
- Moreno, E.C.; Kresak, M.; Zahradnik, R.T. Physicochemical aspects of fluoride-apatite systems relevant to the study of dental caries. Caries Res. 1977, 11, 142–171. [Google Scholar] [CrossRef]
- Bhawal, K.U.; Li, X.; Suzuki, M.; Taguchi, C.; Oka, S.; Arikawa, K.; Tewari, N.; Liu, Y. Treatment with low-level sodium fluoride on wound healing and the osteogenic differentiation of bone marrow mesenchymal stem cells. Dent. Traumatol. 2020, 36, 278–284. [Google Scholar] [CrossRef]
- Brown, J.H.; Pollock, S.H.; Taylor, J.L. Anti-inflammatory Activity of Sodium Fluoride. Arch. Int. Pharmacodyn. 1972, 195, 361–371. [Google Scholar]
- Lee, H.J.; Choi, C.H. Anti-inflammatory effects of bamboo salt and sodium fluoride in human gingival fibroblasts—An in vitro study. Kaohsiung J. Med. Sci. 2015, 31, 303–308. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academies Press: Washington, DC, USA, 1997; pp. 288–313. [Google Scholar]





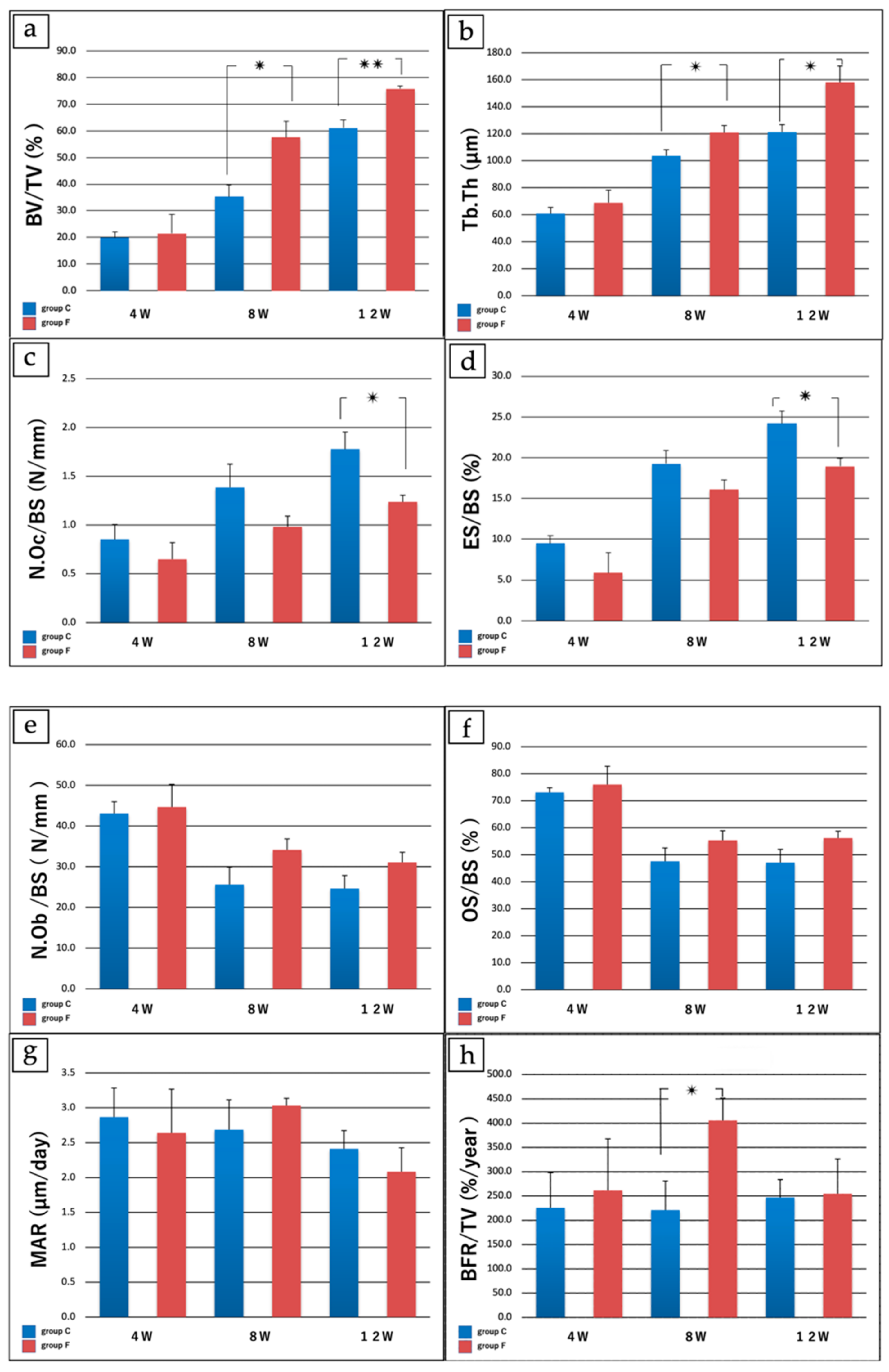
| 4 W (Groups C vs. F) | 8 W (Groups C vs. F) | 12 W (Groups C vs. F) | |
|---|---|---|---|
| a: Bone volume/Tissue volume (%) | 19.89 ± 4.51 vs. 21.51 ± 14.1 | 35.37 ± 8.70 vs. 57.53 ± 12.4 * | 61.17 ± 2.12 vs. 75.77 ± 5.88 ** |
| b: Trabecular thickness (μm) | 60.71 ± 9.10 vs. 68.92 ± 18.8 | 103.71 ± 8.8 vs. 120.87 ± 10.5 * | 121.19 ± 11.1 158.11 ± 24.2 * |
| c: No. osteoclasts/Bone surface (N/mm) | 0.85 ± 0.30 vs. 0.65 ± 0.34 | 1.39 ± 0.48 vs. 0.98 ± 0.22 | 1.78 ± 0.35 vs. 1.28 ± 0.13 * |
| d: Eroded surface/Bone surface (%) | 9.47 ± 1.87 vs. 5.88 ± 4.89 | 19.27 ± 3.23 vs. 16.08 ± 2.36 | 24.19 ± 3.03 vs. 18.94 ± 1.98 * |
| e: No. osteoblasts/Bone surface (N/mm) | 43.10 ± 5.78 vs. 44.68 ± 11.11 | 25.62 ± 8.51 vs. 34.15 ± 5.34 | 24.66 ± 6.35 vs. 31.08 ± 4.90 |
| f: Osteoid surface/bone surface (%) | 73.10 ± 3.40 vs. 76.00 ± 13.50 | 47.60 ± 9.84 vs. 55.31 ± 7.14 | 47.10 ± 9.90 vs. 56.17 ± 5.14 |
| g: Mineral apposition rate (μm/day) | 4.87 ± 0.83 vs. 2.64 ± 1.25 | 2.69 ± 0.86 vs. 3.03 ± 0.21 | 2.41 ± 0.52 vs. 2.08 ± 0.69 |
| h: Bone formation rate/bone volume (%/year) | 225.50 ± 145.50 vs. 261.67 ± 211.94 | 220.91 ± 119.08 vs. 405.47 ± 92.29 * | 247.09 ± 73.64 vs. 254.80 ± 142.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasamoto, T.; Kawaguchi, M.; Yonezawa, K.; Ichiseki, T.; Kaneuji, A.; Shintani, K.; Yoshida, A.; Kawahara, N. Antibacterial Fluorinated Diamond-like Carbon Coating Promotes Osteogenesis—Comparison with Titanium Alloy. Appl. Sci. 2021, 11, 9451. https://doi.org/10.3390/app11209451
Sasamoto T, Kawaguchi M, Yonezawa K, Ichiseki T, Kaneuji A, Shintani K, Yoshida A, Kawahara N. Antibacterial Fluorinated Diamond-like Carbon Coating Promotes Osteogenesis—Comparison with Titanium Alloy. Applied Sciences. 2021; 11(20):9451. https://doi.org/10.3390/app11209451
Chicago/Turabian StyleSasamoto, Takeshi, Masahito Kawaguchi, Katsutaka Yonezawa, Toru Ichiseki, Ayumi Kaneuji, Kazuhiro Shintani, Anzu Yoshida, and Norio Kawahara. 2021. "Antibacterial Fluorinated Diamond-like Carbon Coating Promotes Osteogenesis—Comparison with Titanium Alloy" Applied Sciences 11, no. 20: 9451. https://doi.org/10.3390/app11209451
APA StyleSasamoto, T., Kawaguchi, M., Yonezawa, K., Ichiseki, T., Kaneuji, A., Shintani, K., Yoshida, A., & Kawahara, N. (2021). Antibacterial Fluorinated Diamond-like Carbon Coating Promotes Osteogenesis—Comparison with Titanium Alloy. Applied Sciences, 11(20), 9451. https://doi.org/10.3390/app11209451






