Electrical Response of the Spinel ZnAl2O4 and Its Application in the Detection of Propane Gas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of ZnAl2O4 Powders
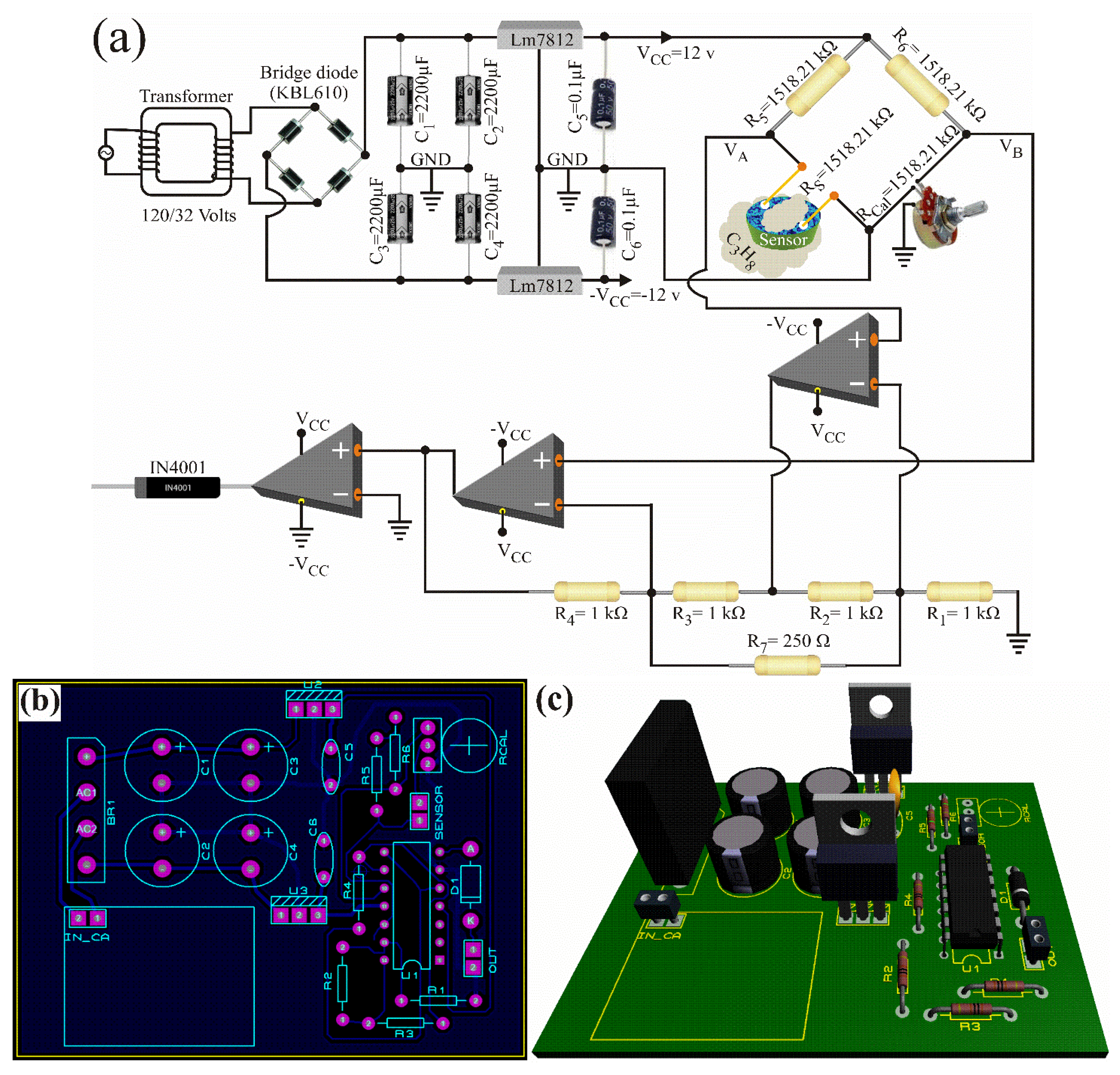
2.2. Gas Sensing Tests
3. Results
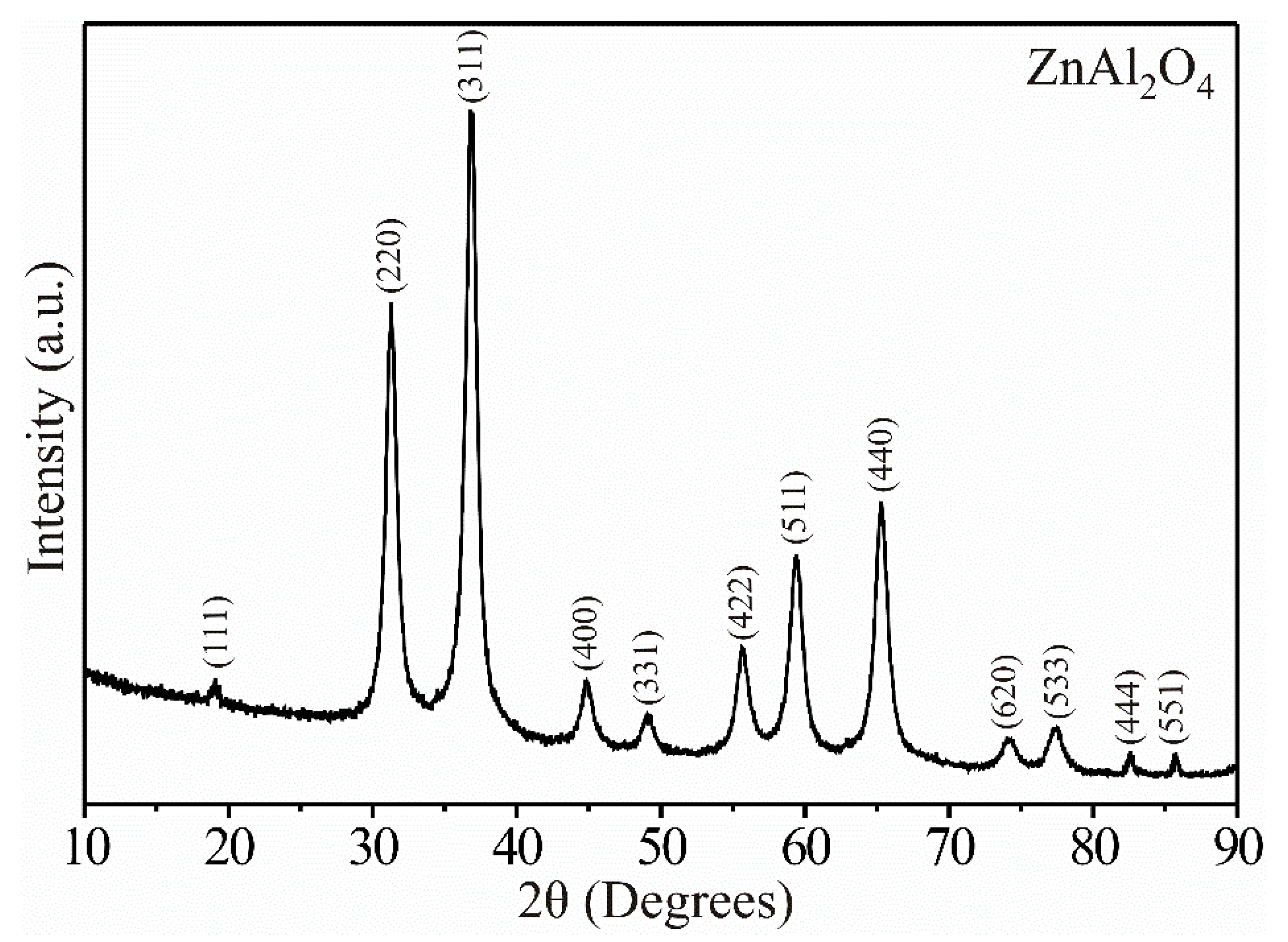
3.1. XRD Analysis
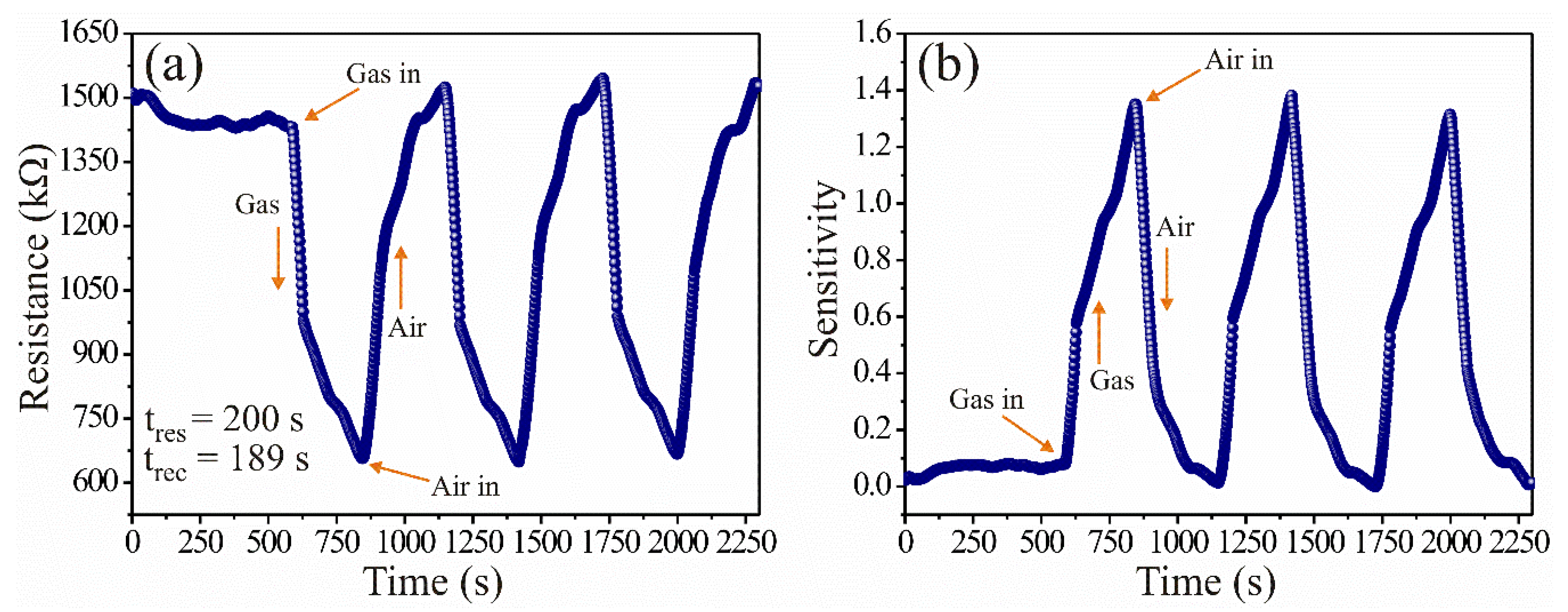
3.2. Analysis of the Dynamic Response of the ZnAl2O4
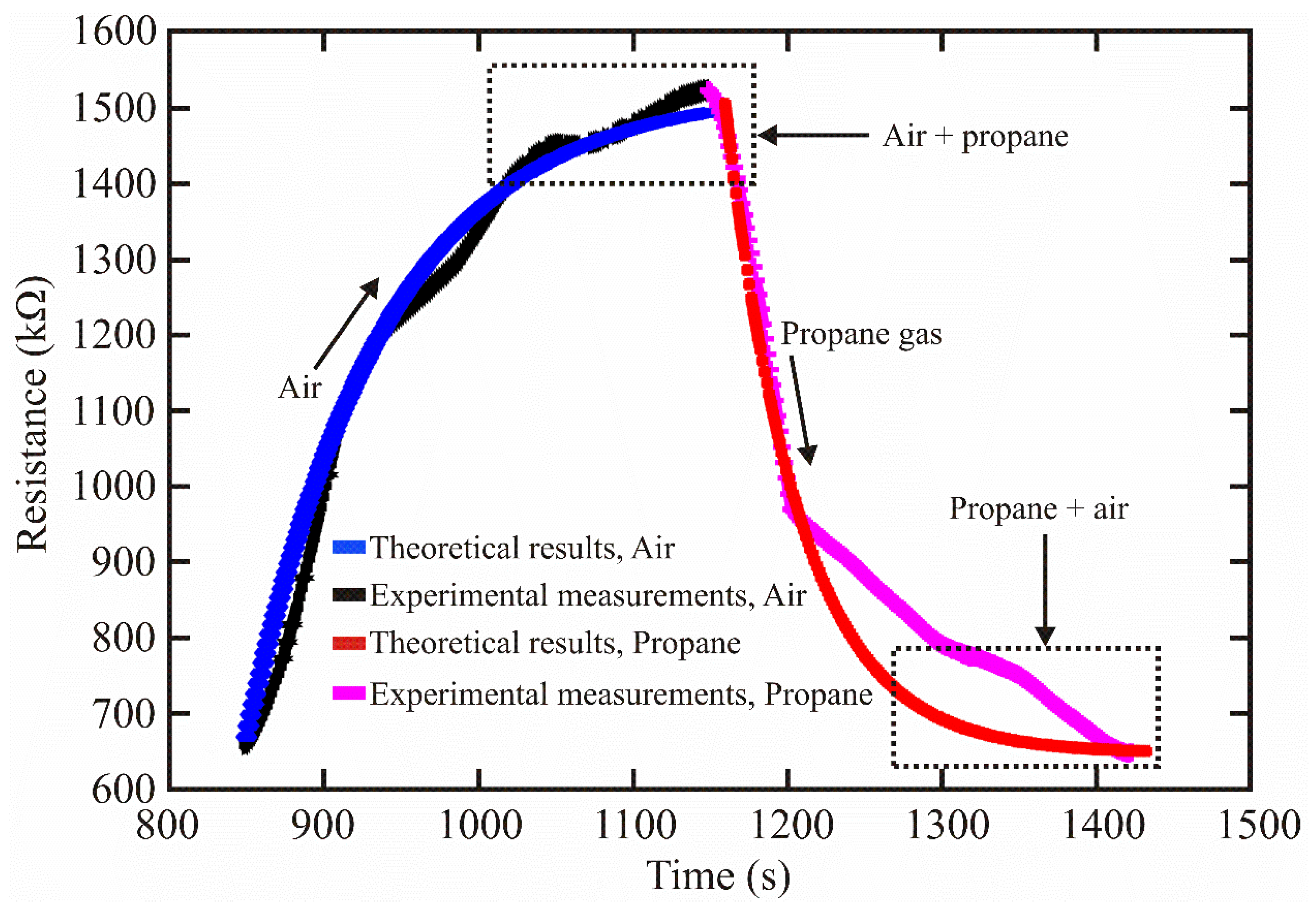
3.3. Theoretical Model
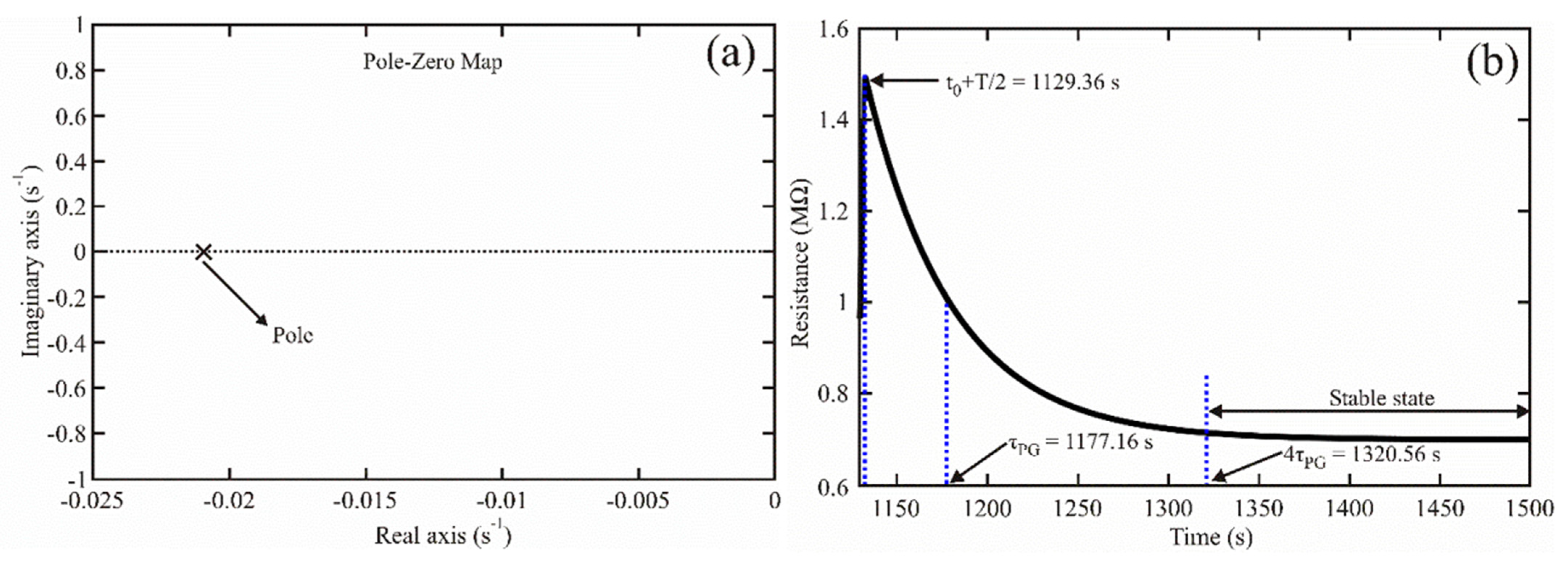
3.3.1. Transient Response
3.3.2. Establishment Time
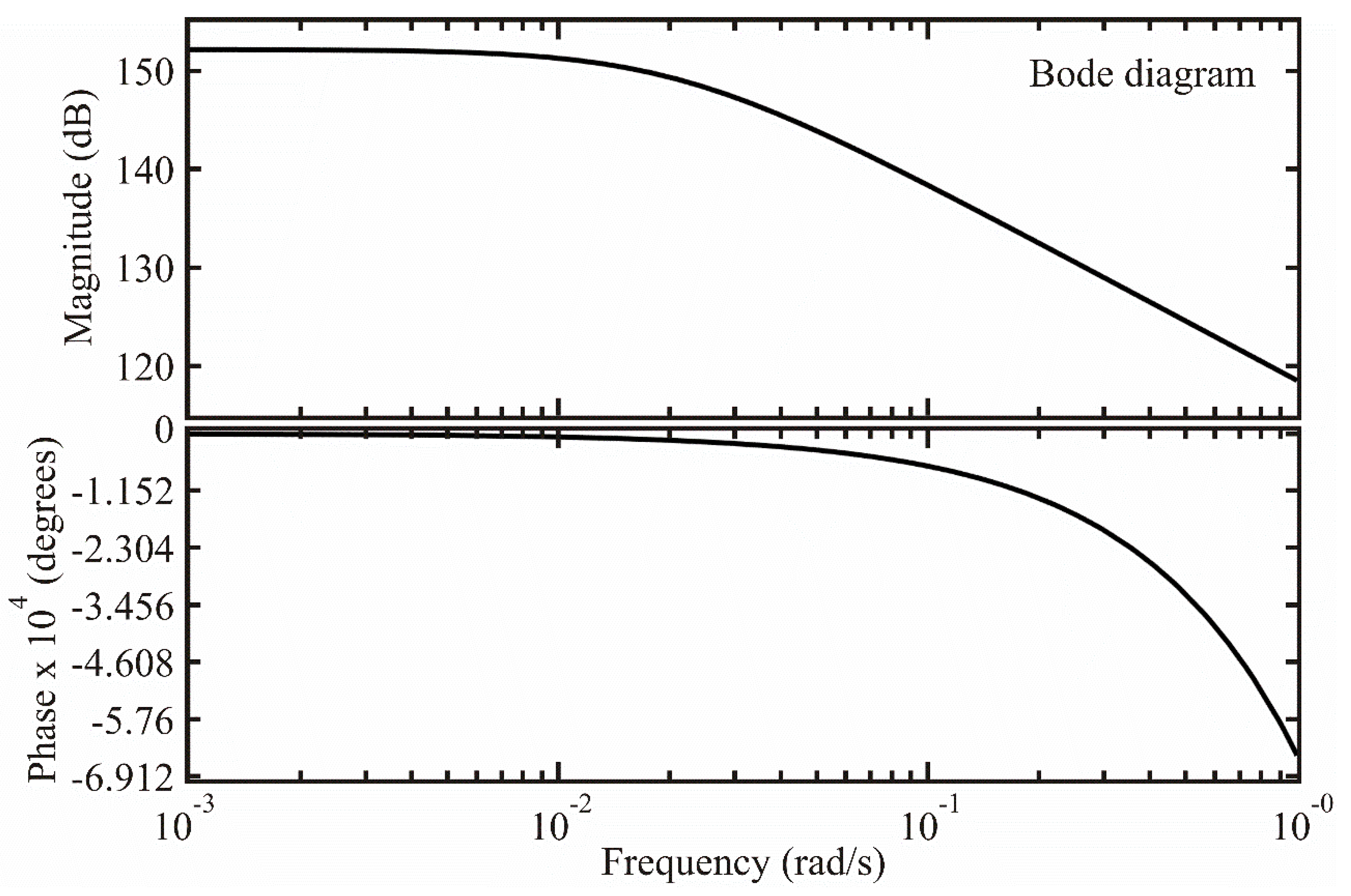
3.3.3. Frequency Response
3.3.4. Application to the Detection of Propane Gas
4. Discussion
- Operating temperature: 250 °C.
- Working concentration of 1000 ppm.
- 11.3 V alarm voltage due to the presence of propane gas.
- Short response times when detecting the presence of propane gas (e.g., three seconds).
- Applicable to high-temperature processes.
- Low fabrication cost.
- Easy repair.
- High sensitivity.
- Supply voltage of 120 Volts.
- The proposed device finds practical application in boiler safety systems where high temperatures and high concentrations are frequent.
- If the device is placed in an atmosphere with different conditions, the chemical sensor based on the oxide ZnAl2O6 will have a different electrical response, causing the Wheatstone bridge to decalibrate, and therefore, the device will send an erroneous alarm signal. To solve such a situation, the Wheatstone bridge must be calibrated for the new operating conditions.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huízar-Padilla, E.; Guillén-Bonilla, H.; Guillén-Bonilla, A.; Rodríguez-Betancourtt, V.-M.; Sánchez-Martínez, A.; Guillén-Bonilla, J.T.; Gildo-Ortiz, L.; Reyes-Gómez, J. Synthesis of ZnAl2O4 and evaluation of the response in propane atmospheres of pellets and thick films manufactured with powders of the oxide. Sensors 2021, 21, 2362. [Google Scholar] [CrossRef]
- Tielens, F.; Calatayud, M.; Franco, R.; Recio, J.M.; Pérez-Ramírez, J.; Minot, C. Periodic DFT study of the structural and electronic properties of bulk CoAl2O4 spinel. J. Phys. Chem. B 2006, 110, 988–995. [Google Scholar] [CrossRef]
- Confalonieri, G.; Rotiroti, N.; Bernasconi, A.; Dapiaggi, M. Structural study of nano-sized gahnite (ZnAl2O4): From the average to the local scale. Nanomaterials 2020, 10, 824. [Google Scholar] [CrossRef] [PubMed]
- Okal, J.; Zawadzki, M. Combustion of propane over novel zinc aluminate-supported ruthenium catalysts. Appl. Catal. B Environ. 2011, 105, 182–190. [Google Scholar] [CrossRef]
- Kapse, S.D.; Raghuwanshi, F.C.; Kapse, V.D.; Patil, D.R. Characteristics of high sensitivity ethanol gas sensors based on nanostructured spinel Zn1−xCoxAl2O4. Curr. Appl. Phys. 2012, 12, 307–312. [Google Scholar] [CrossRef]
- Dhak, D.; Pramanik, P. Particle size comparison of soft-chemically prepared transition metal (Co, Ni, Cu, Zn) aluminate spinels. J. Am. Ceram. Soc. 2006, 89, 1014–1021. [Google Scholar] [CrossRef]
- Quirino, M.R.; Oliveira, M.J.C.; Keyson, D.; Lucena, G.L.; Oliveira, J.B.L.; Gama, L. Synthesis of zinc aluminate with high surface area by microwave hydrothermal method applied in the transesterification of soybean oil (biodiesel). Mater. Res. Bull. 2016, 74, 124–128. [Google Scholar] [CrossRef]
- Ianoş, R.; Băbuţă, R.; Păcurariu, C.; Lazău, R.; Istratie, R.; Butaciu, C. Combustion synthesis of ZnAl2O4 powders with tuned surface area. Ceram. Int. 2017, 43, 8975–8981. [Google Scholar] [CrossRef]
- Cheng, B.; Ouyang, Z.; Tian, B.; Xiao, Y.; Lei, S. Porous ZnAl2O4 spinel nanorods: High sensitivity humidity sensors. Ceram. Int. 2013, 39, 7379–7386. [Google Scholar] [CrossRef]
- Fernández-Osorio, A.; Rivera, C.E.; Vazquez-Olmos, A.; Chávez, J. Luminescent ceramic nano-pigments based on terbium-doped zinc aluminate: Synthesis, properties and performance. Dyes Pigm. 2015, 119, 22–29. [Google Scholar] [CrossRef]
- El Habra, N.; Crociani, L.; Sada, C.; Zanella, P.; Casarin, M.; Rossetto, G.; Carta, G.; Paolucci, G. MOCVD of CoAl2O4 Thin films from {Co[Al(OiC3H7)4]2} as precursor. Chem. Mater. 2007, 19, 3381–3386. [Google Scholar] [CrossRef]
- Marconato Stringhini, F.; Foletto, E.L.; Sallet, D.; Assumpcao Bertuol, D.; Chiavone-Filho, O.; Oller do Nascimento, C.A. Synthesis of porous zinc aluminate spinel (ZnAl2O4) by metal-chitosan complexation method. J. Alloys Compd. 2014, 588, 305–309. [Google Scholar] [CrossRef]
- Ragupathi, C.; Judith Vijaya, J.; Narayanan, S.; John Kennedy, L.; Ramakrishna, S. Catalytic properties of nanosized zinc aluminates prepared by green process using opuntia dilenii haw plant extract. Chin. J. Catal. 2013, 34, 1951–1958. [Google Scholar] [CrossRef]
- Kumar, M.; Mohapatra, M. A case study of energy transfer mechanism from uranium to europium in ZnAl2O4 spinel host by photoluminescence spectroscopy. Spectrochim. Acta A 2016, 159, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Guillen Bonilla, J.T.; Guillen Bonilla, H.; Rodríguez-Betancourtt, V.M.; Guillen Bonilla, A.; Casillas Zamora, A.; Blanco Alonso, O.; Ramírez Ortega, J.A. A gas sensor for application as a propane leak detector. Sensors 2021, 2021, 8871166. [Google Scholar] [CrossRef]
- Basyooni, M.A.; Zaki, S.E.; Ertugrul, S.; Yilmaz, M.; Eker, Y.R. Fast response of CO2 room base donra gas sensor ase don mixed-valence phases in molybdenum and tungsten oxide nanostructured thin films. Ceram. Int. 2020, 46, 9839–9853. [Google Scholar] [CrossRef]
- Chang, J.; Meng, H.; Li, C.; Gao, J.; Chen, S.; Hu, Q.; Li, H.; Feng, L. Aweareble toxic gas-monitoring device based on triboelectric nanogenerator for self-powered aniline early warning. Adv. Mater. Technol. 2020, 5, 1901087. [Google Scholar] [CrossRef]
- Zuo, J.; Tavakoli, S.; Mathavakrishnan, D.; Ma, T.; Lim, M.; Rotondo, B.; Pauzauskie, P.; Pavanatto, F.; Mackenzie, D. Additive manufacturing of a flexible Carbon monoxide sensor based on a SnO2-graphene nanoink. Chemosensors 2020, 8, 36. [Google Scholar] [CrossRef]
- Steinhauer, S. Gas sensors based on copper oxide nanomaterials: A review. Chemosensors 2021, 9, 51. [Google Scholar] [CrossRef]
- Shellaiah, M.; Wen Sun, K. Review on sensing application of perovskite nanomaterials. Chemosensors 2020, 8, 55. [Google Scholar] [CrossRef]
- Delgado, E.; Michel, C.R. CO2 and O2 sensing behavior of nanostructured barium-doped SmCoO3. Mater. Lett. 2006, 60, 1613–1616. [Google Scholar] [CrossRef]
- Imaduddin, I.S.; Majid, S.R.; Aziz, S.B.; Brevik, I.; Yusuf, S.N.F.; Brza, M.A.; Saeed, S.R.; Kadir, M.F.Z.A. Fabrication of Co3O4 from Cobalt/2,6-Napthalenedicarboxylic acid metal-organic framework as electrode for supercapacitor application. Materials 2021, 14, 573. [Google Scholar] [CrossRef] [PubMed]
- Casillas-Zamora, A.; Guillén-Bonilla, J.T.; Guillén-Bonilla, A.; Rodríguez Betancourtt, M.; Casallas-Moreno, Y.L.; Gildo Ortiz, L.; Olvera-Amador, M.L.; Tomás, S.A.; Guillén-Bonilla, H. Synthesis of MnSb2O6 powders through a simple low-temperature method and their test as a gas sensor. J. Mater. Sci. Mater. Electron. 2019, 31, 7359–7372. [Google Scholar] [CrossRef]
- Chang, S.C. Oxygen chemisorption on tin oxide: Correlation between electrical conductivity and EPR measurements. J. Vac. Sci. Technol. 1979, 17, 366–369. [Google Scholar] [CrossRef]
- Alrammouz, R.; Podlecki, J.; Abboud, P.; Sorli, B.; Habchi, R. A review on flexible gas sensors: From materials to devices. Sens. Actuators A 2018, 284, 209–231. [Google Scholar] [CrossRef]
- Michel, C.R.; Guillén-Bonilla, H.; Martínez-Preciado, A.H.; Morán-Lázaro, J.P. Synthesis and gas sensing properties of nanostructured CoSb2O6 microspheres. Sens. Actuators B Chem. 2009, 143, 278–285. [Google Scholar] [CrossRef]
- Gómez-Pozos, H.; Karthik, T.V.K.; de la Olvera, M.L.; García Barrientos, A.; Pérez Cortés, O.; Vega-Pérez, J.; Maldonado, A.; Pérez-Hernández, R.; Rodríguez-Lugo, V. ZnO thin films as propane sensors: Band structure models to explicate the dependence between the structural and morphological properties on gas sensitivity. J. Phys. Chem. Solids. 2017, 106, 16–28. [Google Scholar] [CrossRef]
- Kita, J.; Engelbrecht, A.; Schubert, F.; Gro, A.; Rettig, F.; Moos, R. Some practical points to consider with respect to thermal conductivity and electrical resistivity of ceramic substrates for high-temperature gas sensors. Sens. Actuators B Chem. 2015, 213, 541–546. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, T. An overview: Facet-dependent metal oxide semiconductor gas sensors. Sens. Actuators B Chem. 2018, 277, 604–633. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088. [Google Scholar] [CrossRef] [Green Version]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors 2010, 10, 5469. [Google Scholar] [CrossRef] [Green Version]
- Campi, M.C.; Garatti, S.; Prandini, M. The scenario approach for systems and control design. Annu. Rev. Control 2009, 33, 149–157. [Google Scholar] [CrossRef]
- Khalil, H.K. Nonlinear Systems; Prentice-Hall: Upper Saddle River, NJ, USA, 2002. [Google Scholar]
- Guillen Bonilla, A.; Rodríguez Betancourtt, V.M.; Guillen Bonilla, H.; Gildo Ortíz, L.; Blanco Alonso, O.; Franco Rodríguez, N.E.; Reyes Gómez, J.; Guillen Bonilla, J.T. A new detection system based on the trirutile-type CoSb2O6 oxide. J. Mater. Sci. Mater. Electron. 2018, 29, 15741–15753. [Google Scholar] [CrossRef]
- Smith, C.A.; Corripio, A. Principles and Practice of Automatic Process Control, 2nd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1997. [Google Scholar]
- Bašić, M.; Vukadinović, D.; Petrović, G. Dynamic and pole-zero analysis of self-excited induction generator using a novel model with iron losses. Electr. Power Energy Syst. 2012, 42, 105. [Google Scholar] [CrossRef]
- Guillen Bonilla, J.T.; Guillen Bonilla, H.; Rodríguez Betancourtt, V.M.; Sánchez Morales, M.E.; Casillas Zamora, A.; Guillen Bonilla, A. Low-finesse Fabry-Pérot interferometers applied in the study of the relation between the optical path difference and the poles location. Sensors 2020, 20, 453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillen Bonilla, J.T.; Guillen Bonilla, H.; Rodríguez Betancourtt, V.M.; Casillas Zamora, A.; Ramírez Ortega, J.A.; Gildo Ortiz, L.; Sánchez Morales, M.E.; Blanco Alonso, O.; Guillen Bonilla, A. Carbon Monoxide (CO) detection device based on the nickel antimonate oxide and a DC electronic circuit. Appl. Sci. 2019, 9, 3799. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillén-Bonilla, H.; Guillén-Bonilla, J.T.; Rodríguez-Betancourtt, V.M.; Jiménez-Rodríguez, M.; Guillén-Bonilla, A.; Huízar-Padilla, E.; Sánchez-Morales, M.E.; Ramírez-Ortega, J.A.; Blanco-Alonso, O. Electrical Response of the Spinel ZnAl2O4 and Its Application in the Detection of Propane Gas. Appl. Sci. 2021, 11, 9488. https://doi.org/10.3390/app11209488
Guillén-Bonilla H, Guillén-Bonilla JT, Rodríguez-Betancourtt VM, Jiménez-Rodríguez M, Guillén-Bonilla A, Huízar-Padilla E, Sánchez-Morales ME, Ramírez-Ortega JA, Blanco-Alonso O. Electrical Response of the Spinel ZnAl2O4 and Its Application in the Detection of Propane Gas. Applied Sciences. 2021; 11(20):9488. https://doi.org/10.3390/app11209488
Chicago/Turabian StyleGuillén-Bonilla, Héctor, José Trinidad Guillén-Bonilla, Verónica María Rodríguez-Betancourtt, Maricela Jiménez-Rodríguez, Alex Guillén-Bonilla, Emilio Huízar-Padilla, María Eugenia Sánchez-Morales, Jorge Alberto Ramírez-Ortega, and Oscar Blanco-Alonso. 2021. "Electrical Response of the Spinel ZnAl2O4 and Its Application in the Detection of Propane Gas" Applied Sciences 11, no. 20: 9488. https://doi.org/10.3390/app11209488
APA StyleGuillén-Bonilla, H., Guillén-Bonilla, J. T., Rodríguez-Betancourtt, V. M., Jiménez-Rodríguez, M., Guillén-Bonilla, A., Huízar-Padilla, E., Sánchez-Morales, M. E., Ramírez-Ortega, J. A., & Blanco-Alonso, O. (2021). Electrical Response of the Spinel ZnAl2O4 and Its Application in the Detection of Propane Gas. Applied Sciences, 11(20), 9488. https://doi.org/10.3390/app11209488






