Endothelial Cell Morphogenesis and Capillary-like Network Induced by Soluble and Bound VEGF in a Definite Biogel Composed of Collagen and Fibronectin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Materials
2.2. HUVECs Subculture
2.3. Biogel Preparation
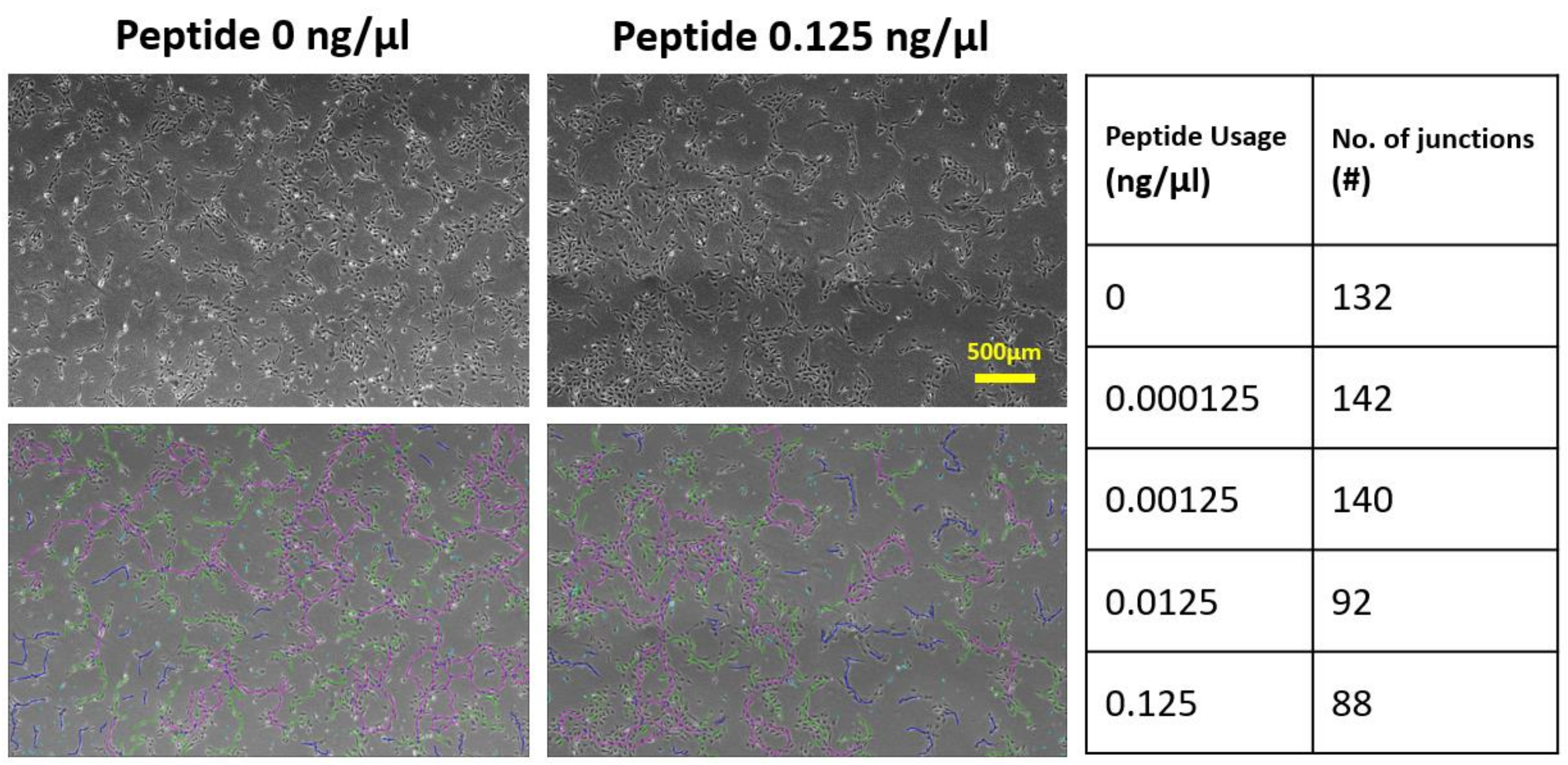
2.4. VEGF-Blocking-Peptide (VBP) Design
2.5. Immunofluorescence
2.6. Images and Statistical Analysis
3. Results
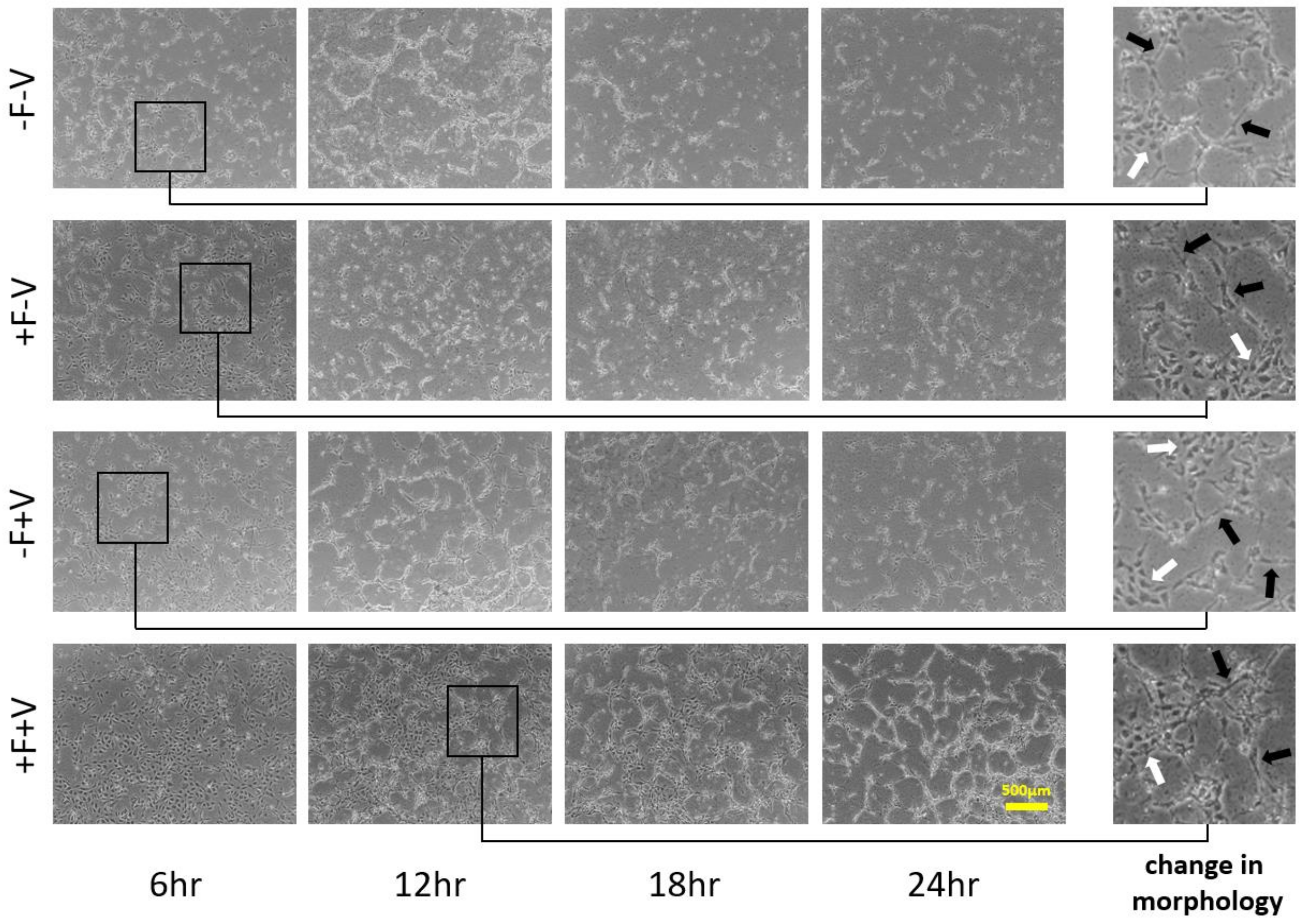
3.1. HUVECs Cultivation on Type I Collagen and Fibronectin
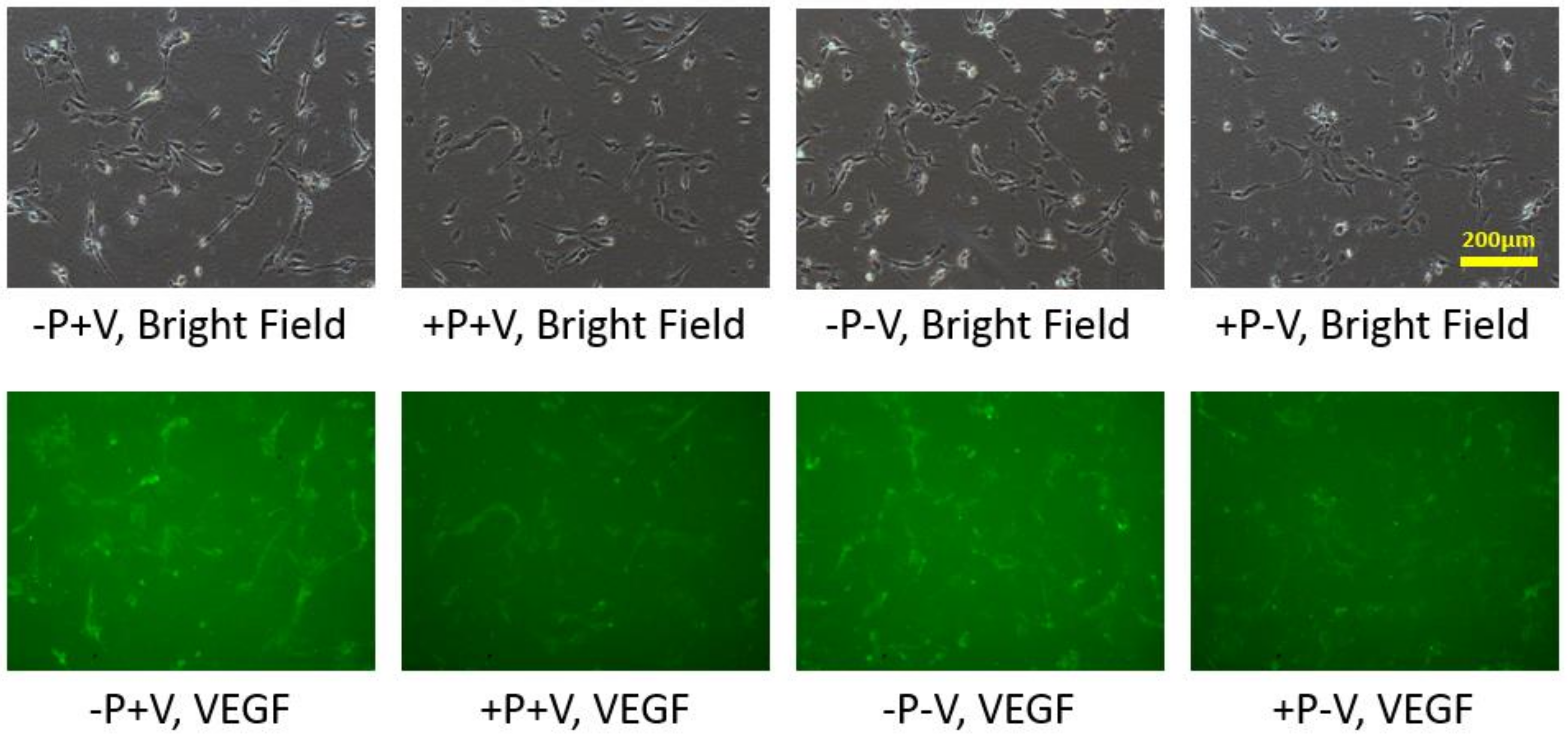
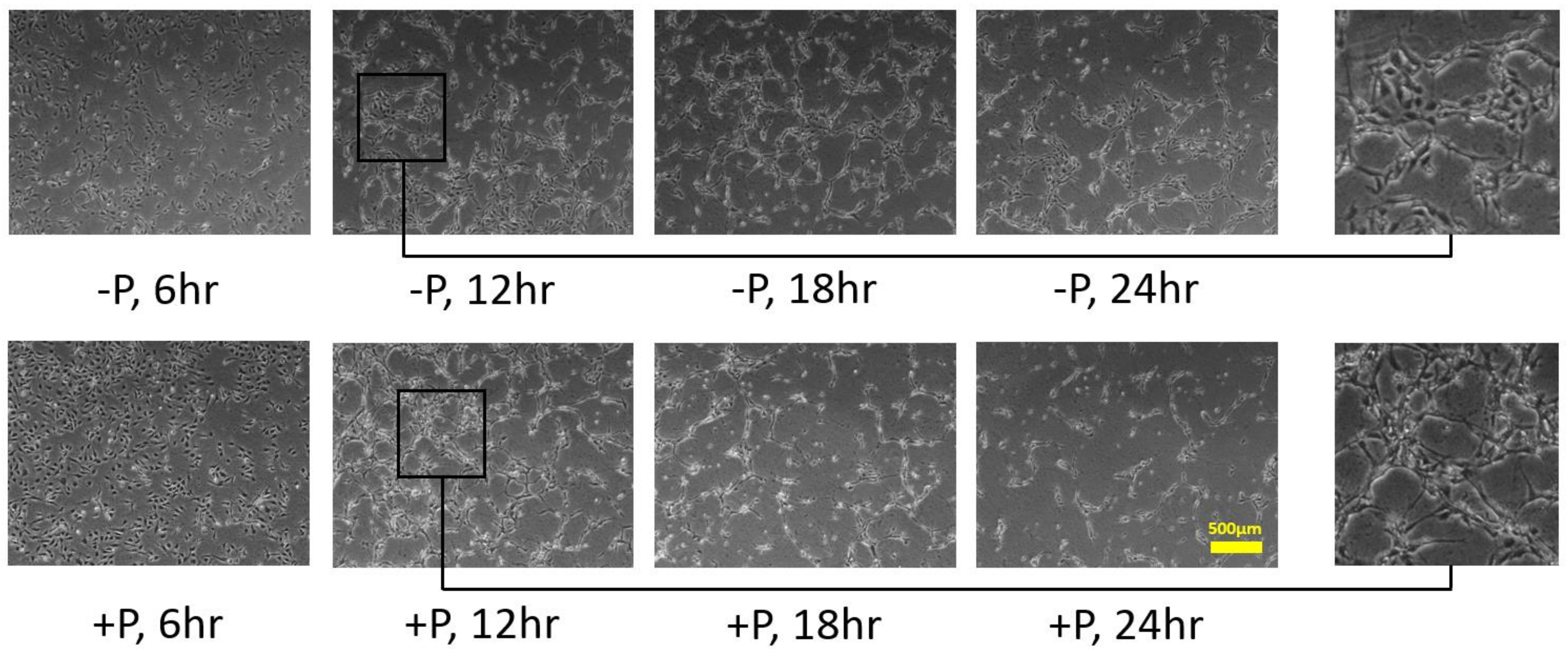
3.2. Effects of Soluble and Bound VEGF
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Griffith, L.G.; Naughton, G. Tissue engineering—Current challenges and expanding opportunities. Science 2002, 295, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.S.; Vacanti, J.P. Tissue engineering: The challenges ahead. Sci. Am. 1999, 280, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, A.M.; James, P.F.; Akbarzadeh, R.; Subramanian, A.; Flavin, C.; Oudadesse, H. Prospect of stem cells in bone tissue engineering: A review. Stem Cells Int. 2016, 2016, 6180487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubarsky, B.; Krasnow, M.A. Tube morphogenesis: Making and shaping biological tubes. Cell 2003, 112, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249. [Google Scholar] [CrossRef]
- Arnaoutova, I.; Kleinman, H.K. In vitro angiogenesis: Endothelial cell tube formation on gelled basement membrane extract. Nat. Protoc. 2010, 5, 628–635. [Google Scholar] [CrossRef]
- Cross, V.L.; Zheng, Y.; Choi, N.W.; Verbridge, S.S.; Sutermaster, B.A.; Bonassar, L.J.; Fischbach, C.; Stroock, A.D. Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials 2010, 31, 8596–8607. [Google Scholar] [CrossRef] [Green Version]
- Kubota, Y.; Kleinman, H.K.; Martin, G.R.; Lawley, T.J. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J. Cell Biol. 1988, 107, 1589–1598. [Google Scholar] [CrossRef]
- Vernon, R.B.; Lara, S.L.; Drake, C.J.; Iruela-Arispe, M.L.; Angello, J.C.; Little, C.D.; Wight, T.N.; Sage, E.H. Organized type I collagen influences endothelial patterns during “spontaneous angiogenesis in vitro”: Planar cultures as models of vascular development. Vitr. Cell. Dev. Biol. Anim. 1995, 31, 120–131. [Google Scholar] [CrossRef] [Green Version]
- Chan, T.R.; Stahl, P.J.; Li, Y.; Yu, S.M. Collagen–gelatin mixtures as wound model and substrates for VEGF-mimetic peptide binding and endothelial cell activation. Acta Biomater. 2015, 15, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Mitsi, M.; Hong, Z.; Costello, C.E.; Nugent, M.A. Heparin-mediated conformational changes in fibronectin expose vascular endothelial growth factor binding sites. Biochemistry 2006, 45, 10319–10328. [Google Scholar] [CrossRef] [PubMed]
- Moulisová, V.; Gonzalez-García, C.; Cantini, M.; Rodrigo-Navarro, A.; Weaver, J.; Costell, M.; Serra, R.S.; Dalby, M.J.; García, A.J.; Salmerón-Sánchez, M. Engineered microenvironments for synergistic VEGF–Integrin signaling during vascularization. Biomaterials 2017, 126, 61–74. [Google Scholar] [CrossRef]
- Sechler, J.L.; Corbett, S.A.; Schwarzbauer, J.E. Modulatory roles for integrin activation and the synergy site of fibronectin during matrix assembly. Mol. Biol. Cell 1997, 8, 2563–2573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martino, M.M.; Hubbell, J.A. The 12th–14th type III repeats of fibronectin function as a highly promiscuous growth factor-binding domain. FASEB J. 2010, 24, 4711–4721. [Google Scholar] [PubMed]
- Sawicka, K.M.; Seeliger, M.; Musaev, T.; Macri, L.K.; Clark, R.A. Fibronectin interaction and enhancement of growth factors: Importance for wound healing. Adv. Wound Care 2015, 4, 469–478. [Google Scholar] [CrossRef] [Green Version]
- Martino, M.M.; Mochizuki, M.; Rothenfluh, D.A.; Rempel, S.A.; Hubbell, J.A.; Barker, T.H. Controlling integrin specificity and stem cell differentiation in 2D and 3D environments through regulation of fibronectin domain stability. Biomaterials 2009, 30, 1089–1097. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Battig, M.R.; Chen, N.; Gaddes, E.R.; Duncan, K.L.; Wang, Y. Chimeric aptamer–gelatin hydrogels as an extracellular matrix mimic for loading cells and growth factors. Biomacromolecules 2016, 17, 778–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, S.; Tugues, S.; Li, X.; Gualandi, L.; Claesson-Welsh, L. Signal transduction by vascular endothelial growth factor receptors. Biochem. J. 2011, 437, 169–183. [Google Scholar] [CrossRef] [Green Version]
- Gabhann, F.M.; Popel, A.S. Systems biology of vascular endothelial growth factors. Microcirculation 2008, 15, 715–738. [Google Scholar] [CrossRef] [Green Version]
- Karkkainen, M.J.; Petrova, T.V. Vascular endothelial growth factor receptors in the regulation of angiogenesis and lymphangiogenesis. Oncogene 2000, 19, 5598–5605. [Google Scholar] [CrossRef] [Green Version]
- Holmes, K.; Roberts, O.L.; Thomas, A.M.; Cross, M.J. Vascular endothelial growth factor receptor-2: Structure, function, intracellular signalling and therapeutic inhibition. Cell. Signal. 2007, 19, 2003–2012. [Google Scholar] [CrossRef] [PubMed]
- Vempati, P.; Popel, A.S.; Mac Gabhann, F. Extracellular regulation of VEGF: Isoforms, proteolysis, and vascular patterning. Cytokine Growth Factor Rev. 2014, 25, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krilleke, D.; DeErkenez, A.; Schubert, W.; Giri, I.; Robinson, G.S.; Ng, Y.S.; Shima, D.T. Molecular mapping and functional characterization of the VEGF164 heparin-binding domain. J. Biol. Chem. 2007, 282, 28045–28056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houck, K.A.; Leung, D.W.; Rowland, A.M.; Winer, J.; Ferrara, N.J. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J. Biol. Chem. 1992, 267, 26031–26037. [Google Scholar] [CrossRef]
- Houck, K.A.; Ferrara, N.; Winer, J.; Cachianes, G.; Li, B.; Leung, D.W. The vascular endothelial growth factor family: Identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol. Endocrinol. 1991, 5, 1806–1814. [Google Scholar] [CrossRef]
- Panoilia, E.; Schindler, E.; Samantas, E.; Aravantinos, G.; Kalofonos, H.P.; Christodoulou, C.; Patrinos, G.P.; Friberg, L.E.; Sivolapenko, G. A pharmacokinetic binding model for bevacizumab and VEGF 165 in colorectal cancer patients. Cancer Chemother. Pharmacol. 2015, 75, 791–803. [Google Scholar] [CrossRef]
- Park, J.E.; Keller, G.A.; Ferrara, N. The vascular endothelial growth factor (VEGF) isoforms: Differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol. Biol. Cell 1993, 4, 1317–1326. [Google Scholar] [CrossRef] [Green Version]
- Grunstein, J.; Masbad, J.J.; Hickey, R.; Giordano, F.; Johnson, R.S. Isoforms of vascular endothelial growth factor act in a coordinate fashion to recruit and expand tumor vasculature. Mol. Cell. Biol. 2000, 20, 7282–7291. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.T.; Luque, A.; Lee, S.; Anderson, S.M.; Segura, T.; Iruela-Arispe, M.L. Anchorage of VEGF to the extracellular matrix conveys differential signaling responses to endothelial cells. J. Cell Biol. 2010, 188, 595–609. [Google Scholar] [CrossRef] [Green Version]
- Ruhrberg, C.; Gerhardt, H.; Golding, M.; Watson, R.; Ioannidou, S.; Fujisawa, H.; Betsholtz, C.; Shima, D.T. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002, 16, 2684–2698. [Google Scholar] [CrossRef] [Green Version]
- Wijelath, E.S.; Rahman, S.; Namekata, M.; Murray, J.; Nishimura, T.; Mostafavi-Pour, Z.; Patel, Y.; Suda, Y.; Humphries, M.J.; Sobel, M. Heparin-II domain of fibronectin is a vascular endothelial growth factor-binding domain: Enhancement of VEGF biological activity by a singular growth factor/matrix protein synergism. Circ. Res. 2006, 99, 853–860. [Google Scholar] [CrossRef]
- Martino, M.M.; Tortelli, F.; Mochizuki, M.; Traub, S.; Ben-David, D.; Kuhn, G.A.; Müller, R.; Livne, E.; Eming, S.A.; Hubbell, J.A. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci. Transl. Med. 2011, 3, 100ra89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, J.D.; Headen, D.M.; Aquart, J.; Johnson, C.T.; Shea, L.D.; Shirwan, H.; García, A.J. Vasculogenic hydrogel enhances islet survival, engraftment, and function in leading extrahepatic sites. Sci. Adv. 2017, 3, e1700184. [Google Scholar] [CrossRef] [Green Version]
- Stamati, K.; Priestley, J.V.; Mudera, V.; Cheema, U. Laminin promotes vascular network formation in 3D in vitro collagen scaffolds by regulating VEGF uptake. Exp. Cell Res. 2014, 327, 68–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köhn-Luque, A.; De Back, W.; Starruß, J.; Mattiotti, A.; Deutsch, A.; Pérez-Pomares, J.M.; Herrero, M.A. Early embryonic vascular patterning by matrix-mediated paracrine signaling: A mathematical model study. PLoS ONE 2011, 6, e24175. [Google Scholar] [CrossRef]
- Köhn-Luque, A.; De Back, W.; Yamaguchi, Y.; Yoshimura, K.; Herrero, M.A.; Miura, T. Dynamics of VEGF matrix-retention in vascular network patterning. Phys. Biol. 2013, 10, 066007. [Google Scholar] [CrossRef] [PubMed]
- Merks, R.M.; Perryn, E.D.; Shirinifard, A.; Glazier, J.A. Contact-inhibited chemotaxis in de novo and sprouting blood-vessel growth. PLoS Comput. Biol. 2008, 4, e1000163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namy, P.; Ohayon, J.; Tracqui, P. Critical conditions for pattern formation and in vitro tubulogenesis driven by cellular traction fields. J. Theor. Biol. 2004, 227, 103–120. [Google Scholar] [CrossRef]
- Patterson, J.; Martino, M.M.; Hubbell, J.A. Biomimetic materials in tissue engineering. Materials Today 2010, 13, 14–22. [Google Scholar] [CrossRef]
- Silva, A.K.A.; Richard, C.; Bessodes, M.; Scherman, D.; Merten, O.W. Growth factor delivery approaches in hydrogels. Biomacromolecules 2008, 10, 9–18. [Google Scholar] [CrossRef]
- Plate, K.H.; Breier, G.; Weich, H.A.; Risau, W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature 1992, 359, 845–848. [Google Scholar] [CrossRef]
- Dye, J.F.; Lawrence, L.; Linge, C.; Leach, L.; Firth, J.A.; Clark, P. Distinct patterns of microvascular endothelial cell morphology are determined by extracellular matrix composition. J. Endothel. Cell Res. 2004, 11, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Cleaver, O. Tubulogenesis during blood vessel formation. Semin. Cell Dev. Biol. 2011, 22, 993–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in signaling and disease: Beyond discovery and development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merks, R.M.; Glazier, J.A. Dynamic mechanisms of blood vessel growth. Nonlinearity 2005, 19, C1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serini, G.; Ambrosi, D.; Giraudo, E.; Gamba, A.; Preziosi, L.; Bussolino, F. Modeling the early stages of vascular network assembly. EMBO J. 2003, 22, 1771–1779. [Google Scholar] [CrossRef] [Green Version]
- Nashimoto, Y.; Hayashi, T.; Kunita, I.; Nakamasu, A.; Torisawa, Y.S.; Nakayama, M.; Takigawa-Imamura, H.; Kotera, H.; Nishiyama, K.; Miura, T.; et al. Integrating perfusable vascular networks with a three-dimensional tissue in a microfluidic device. Integr. Biol. 2017, 9, 506–518. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, H.; Cheng, Y.-C.; Chung, C.-A. Endothelial Cell Morphogenesis and Capillary-like Network Induced by Soluble and Bound VEGF in a Definite Biogel Composed of Collagen and Fibronectin. Appl. Sci. 2021, 11, 9501. https://doi.org/10.3390/app11209501
Chiang H, Cheng Y-C, Chung C-A. Endothelial Cell Morphogenesis and Capillary-like Network Induced by Soluble and Bound VEGF in a Definite Biogel Composed of Collagen and Fibronectin. Applied Sciences. 2021; 11(20):9501. https://doi.org/10.3390/app11209501
Chicago/Turabian StyleChiang, Hsun, Yu-Che Cheng, and Chih-Ang Chung. 2021. "Endothelial Cell Morphogenesis and Capillary-like Network Induced by Soluble and Bound VEGF in a Definite Biogel Composed of Collagen and Fibronectin" Applied Sciences 11, no. 20: 9501. https://doi.org/10.3390/app11209501
APA StyleChiang, H., Cheng, Y.-C., & Chung, C.-A. (2021). Endothelial Cell Morphogenesis and Capillary-like Network Induced by Soluble and Bound VEGF in a Definite Biogel Composed of Collagen and Fibronectin. Applied Sciences, 11(20), 9501. https://doi.org/10.3390/app11209501




