Physicochemical and Microbial Quality of Prepackaged Shrimp Processed by a Scaled-Up Microwave-Assisted Induction Heating Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Treatment
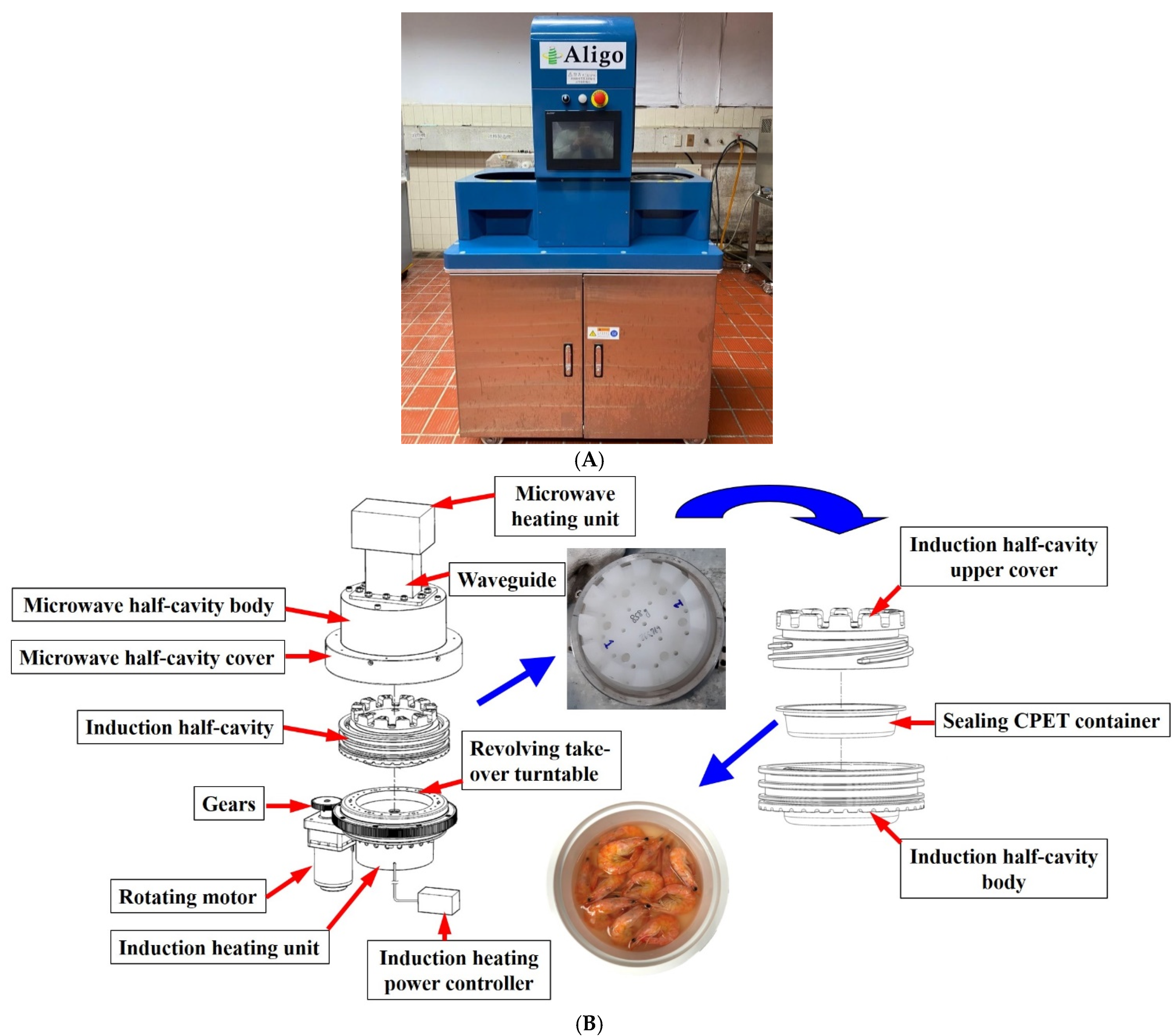
2.2. Scaled-Up MAIH Processing
2.3. Cooking Loss Measurement
2.4. Microbiological Measurement
2.5. Appearance and Color Analysis
2.6. Texture Determination
2.7. Sensory Analysis
2.8. Statistical Analysis
3. Results and Discussion
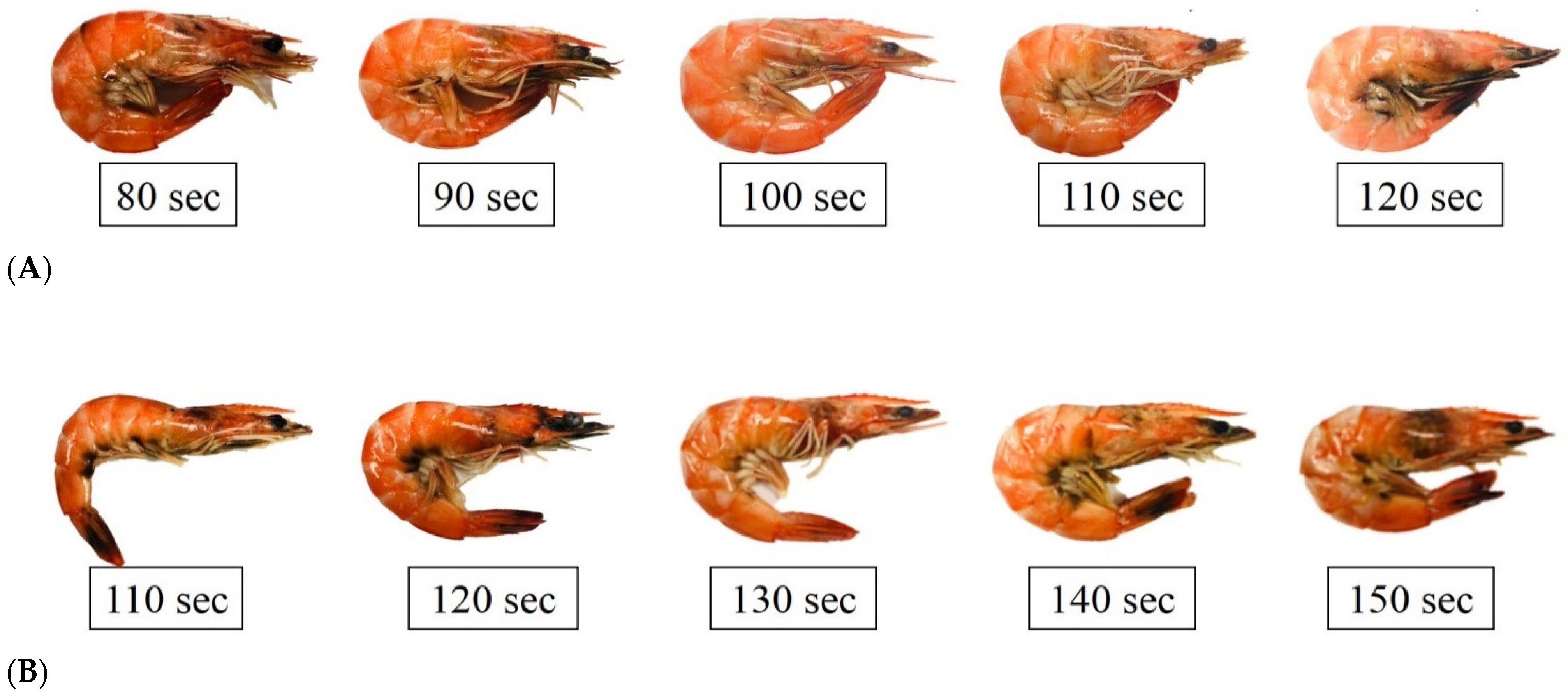
3.1. Appearance of Shrimp
3.2. Cooking Loss and Microbial Counts of Shrimp
3.3. Color Values of Shrimp
3.4. Texture Properties of Shrimp
3.5. Sensory Attributes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alfaro-Montoya, J.; Braga, A.; Umaña-Castro, R. Research frontiers in penaeid shrimp reproduction: Future trends to improve commercial production. Aquaculture 2019, 503, 70–87. [Google Scholar] [CrossRef]
- Liao, I.C.; Chien, Y.H. The pacific white shrimp, Litopenaeus vannamei, in Asia: The World’s Most Widely Cultured Alien Crustacean. In The Wrong Place-Alien Marine Crustaceans: Distribution, Biology and Impacts; Galil, B.S., Clark, P.F., Carlton, J.T., Eds.; Springer: New York, NY, USA, 2011; pp. 489–520. [Google Scholar]
- Sae-leaw, T.; Benjakul, S.; Vongkamjam, K. Retardation of melanosis and quality loss of pre-cooked Pacific white shrimp using epigallocatechin gallate with the aid of ultrasound. Food Control 2018, 84, 75–82. [Google Scholar] [CrossRef]
- Kim, J.; Marshall, M.R.; Wei, C. Polyphenoloxidase. In Seafood Enzyme: Utilization and Influence on Post Harvest Seafood Quality; Haard, N.F., Simpson, B.K., Eds.; Marcel Dekker: New York, NY, USA, 2000; pp. 271–315. [Google Scholar]
- Guo, Q.; Sun, D.W.; Cheng, J.H.; Han, Z. Microwave processing techniques and their recent applications in the food industry. Trends Food Sci. Technol. 2017, 67, 236–247. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Ramanathan, S.; Basak, T. Microwave food processing—A review. Food Res. Int. 2013, 52, 243–261. [Google Scholar] [CrossRef]
- Tang, Z.; Mikhaylenko, G.; Liu, F.; Mah, J.H.; Pandit, R.; Younce, F.; Tang, J. Microwave sterilization of sliced beef in gravy in 7-oz trays. J. Food Eng. 2008, 89, 375–383. [Google Scholar] [CrossRef]
- Vadivambal, R.; Jayas, D.S. Non-uniform temperature distribution during microwave heating of food materials—A review. Food Bioprocess Technol. 2010, 3, 61–71. [Google Scholar] [CrossRef]
- Chizoba Ekezie, F.G.; Sun, D.W.; Han, Z.; Cheng, J.H. Microwave-assisted food processing technologies for enhancing product quality and process efficiency: A review of recent developments. Trends Food Sci. Technol. 2017, 67, 58–69. [Google Scholar] [CrossRef]
- Tang, J. Unlocking potentials of microwaves for food safety and quality. J. Food Sci. 2015, 80, E1776–E1793. [Google Scholar] [CrossRef] [Green Version]
- Barbosa-Cánovas, G.V.; Medina-Meza, I.; Candoğan, K.; Bermúdez-Aguirre, D. Advanced retorting, microwave assisted thermal sterilization (MATS), and pressure assisted thermal sterilization (PATS) to process meat products. Meat Sci. 2014, 98, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.I.; Chin, K.T.; Yu, Y.C.; Hsieh, J.K.; Lin, C.H. Cavity detachable modular composite microwave heating system. Taiwan Patent I614457, 2 November 2018. [Google Scholar]
- Lee, Y.C.; Lin, C.Y.; Wei, C.I.; Tung, H.N.; Chiu, K.; Tsai, Y.H. Preliminary evaluation of a novel microwave-assisted induction heating (MAIH) system on white shrimp cooking. Foods 2021, 10, 545. [Google Scholar] [CrossRef] [PubMed]
- Ovissipour, M.; Rasco, B.; Tang, J.; Sablani, S.S. Kinetics of quality changes in whole blue mussel (Mytilus edulis) during pasteurization. Food Res. Int. 2013, 53, 141–148. [Google Scholar] [CrossRef]
- Cousin, M.A.; Jay, J.M.; Vasavada, P.C. Psychrotrophic microorganism. In Compendium of Methods for the Microbiological Examination of Foods; Vanderzand, C., Splittstoesser, D.F., Eds.; American Public Health Association: Washington, DC, USA, 1992; pp. 153–168. [Google Scholar]
- Meilgaard, M.C.; Civille, G.V.; Carr, B.T. Sensory Evaluation Techniques; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Erdogdu, F.; Balaban, M.O.; Otwell, W.S.; Garrido, L. Cook-related yield loss for pacific white (Penaeus vannamei) shrimp previously treated with phosphates: Effects of shrimp size and internal temperature distribution. J. Food Eng. 2004, 64, 297–300. [Google Scholar] [CrossRef]
- Kong, F.; Tang, J.; Rasco, B.; Crapo, C. Kinetics of salmon quality changes during thermal processing. J. Food Eng. 2007, 83, 510–520. [Google Scholar] [CrossRef]
- Kruk, Z.A.; Yun, H.; Rutley, D.L.; Lee, E.J.; Kim, Y.J.; Jo, C. The effect of high pressure on microbial population, meat quality and sensory characteristics of chicken breast fillet. Food Control 2011, 22, 6–12. [Google Scholar] [CrossRef]
- Niamnuy, C.; Devahastin, S.; Soponronnarit, S. Quality changes of shrimp during boiling in salt solution. J. Food Sci. 2007, 72, S289–S297. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Choudhury, G.S.; Studebaker, S. Hydrothermal processing of pacific Chum Salmon: Effects on texture and in-vitro digestibility. J. Food Qual. 1993, 16, 243–251. [Google Scholar] [CrossRef]
- Tsai, Y.H.; Hwang, C.C.; Lin, C.S.; Lin, C.Y.; Ou, T.Y.; Chang, T.H.; Lee, Y.C. Comparison of microwave-assisted induction heating system (MAIH) and individual heating methods on the quality of pre-packaged white shrimp. Innov. Food Sci. Emerg. Technol. 2021, 73, 102787. [Google Scholar] [CrossRef]
| Temperature | First Stage Heating (s) | Second Stage Heating (s) | Total Heating Time (s) |
|---|---|---|---|
| MW + IH | IH | ||
| 130 °C | 80 | 0 | 80 |
| 80 | 10 | 90 | |
| 80 | 20 | 100 | |
| 80 | 30 | 110 | |
| 80 | 40 | 120 | |
| 90 °C | 100 | 10 | 110 |
| 100 | 20 | 120 | |
| 100 | 30 | 130 | |
| 100 | 40 | 140 | |
| 100 | 50 | 150 |
| Temperatures | Heating Time (s) | Cook Loss (%) | TPC (log CFU/g) | PBC (log CFU/g) | Coliform (log CFU/g) | E. coli (log CFU/g) |
|---|---|---|---|---|---|---|
| Raw shrimp | 0 | 4.93 ± 0.23 a | 3.27 ± 0.11 a | 2.21 ± 0.14 a | <1.0 | |
| 130 °C | 80 | 1.16 ± 0.12 e | 3.45 ± 0.52 b | 2.88 ± 0.32 b | 1.68 ± 0.19 b | <1.0 |
| 90 | 2.41 ± 0.29 d | 3.26 ± 0.39 b | 2.17 ± 0.27 c | <1.0 c | <1.0 | |
| 100 | 3.45 ± 0.11 c | <2.0 d | <2.0 d | <1.0 c | <1.0 | |
| 110 | 3.50 ± 0.15 c | <2.0 d | <2.0 d | <1.0 c | <1.0 | |
| 120 | 3.66 ± 0.18 b | <2.0 d | <2.0 d | <1.0 c | <1.0 | |
| 90 °C | 110 | 1.50 ± 0.28 e | 2.81 ± 0.38 c | 2.54 ± 0.29 bc | 1.26 ± 0.42 b | <1.0 |
| 120 | 2.05 ± 0.33 d | 2.45 ± 0.05 c | 2.05 ± 0.25 c | <1.0 c | <1.0 | |
| 130 | 2.73 ± 0.19 cd | <2.0 d | <2.0 d | <1.0 c | <1.0 | |
| 140 | 3.46 ± 0.24 c | <2.0 d | <2.0 d | <1.0 c | <1.0 | |
| 150 | 4.39 ± 0.11 a | <2.0 d | <2.0 d | <1.0 c | <1.0 |
| Treatments | Heating Time (s) | L* | a* | b* | W |
|---|---|---|---|---|---|
| Peeled raw shrimp | 25.23 ± 0.60 e | 1.90 ± 0.12 d | −1.06 ± 0.04 a | 25.20 ± 0.58 d | |
| 130 °C | 80 | 51.78 ± 0.52 d | 6.79 ± 0.22 c | −1.86 ± 0.17 b | 51.51 ± 0.38 c |
| 90 | 52.63 ± 0.83 d | 7.96 ± 0.32 b | −1.77 ± 0.16 b | 52.22 ± 0.63 c | |
| 100 | 52.65 ± 0.77 d | 8.53 ± 0.25 a | −1.87 ± 0.28 b | 52.17 ± 0.52 c | |
| 110 | 56.04 ± 0.65 b | 8.62 ± 0.40 a | −2.03 ± 0.52 bc | 55.50 ± 0.55 b | |
| 120 | 58.09 ± 0.91 a | 8.82 ± 0.39 a | −2.29 ± 0.37 cd | 57.48 ± 0.50 a | |
| 90 °C | 110 | 52.79 ± 0.66 d | 6.86 ± 0.18 c | −1.92 ± 0.61 bc | 51.66 ± 0.54 c |
| 120 | 52.39 ± 1.01 d | 8.12 ± 0.09 b | −1.99 ± 0.25 bc | 52.26 ± 0.65 c | |
| 130 | 54.96 ± 0.76 c | 8.18 ± 0.13 b | −2.02 ± 0.26 bc | 54.18 ± 0.40 bc | |
| 140 | 54.06 ± 0.96 c | 8.57 ± 0.30 a | −2.12 ± 0.17 cd | 53.22 ± 0.71 bc | |
| 150 | 55.66 ± 0.75 b | 8.69 ± 0.23 a | −2.30 ± 0.22 cd | 55.24 ± 0.53 b |
| Temperatures | Heating Time (s) | Hardness (g) | Cohesiveness | Springiness (mm) | Chewiness (mJ) |
|---|---|---|---|---|---|
| Peeled raw shrimp | 101.2 ± 2.60 e | 0.22 ± 0.01 e | 5.08 ± 0.04 a | 5.90 ± 0.61 c | |
| 130 °C | 80 | 250.7 ± 20.2 c | 0.54 ± 0.02 c | 5.00 ± 0.21 a | 8.01 ± 0.83 b |
| 90 | 275.6 ± 23.8 bc | 0.58 ± 0.04 b | 5.02 ± 0.16 a | 8.80 ± 0.93 b | |
| 100 | 271.5 ± 11.7 bc | 0.63 ± 0.03 b | 5.01 ± 0.26 a | 9.79 ± 0.95 ab | |
| 110 | 289.4 ± 20.5 ab | 0.65 ± 0.04 ab | 5.23 ± 0.29 a | 10.21 ± 0.88 a | |
| 120 | 295.9 ± 15.9 a | 0.70 ± 0.03 a | 5.20 ± 0.19 a | 11.05 ± 0.90 a | |
| 90 °C | 110 | 210.9 ± 17.6 d | 0.38 ± 0.03 d | 4.95 ± 0.21 a | 8.06 ± 1.04 b |
| 120 | 249.3 ± 19.1 c | 0.56 ± 0.01 bc | 5.01 ± 0.25 a | 9.76 ± 0.69 ab | |
| 130 | 260.6 ± 25.1 bc | 0.60 ± 0.05 b | 5.02 ± 0.26 a | 10.01 ± 1.00 ab | |
| 140 | 274.6 ± 21.6 ab | 0.67 ± 0.02 ab | 5.10 ± 0.17 a | 10.95 ± 0.78 a | |
| 150 | 299.6 ± 30.7 a | 0.69 ± 0.04 a | 5.15 ± 0.12 a | 11.50 ± 0.99 a |
| Treatments | Color | Flavor | Odor | Taste | Texture | Overall Acceptance |
|---|---|---|---|---|---|---|
| Boiling water | 7.24 ± 1.24 a | 6.68 ± 1.20 a | 7.10 ± 0.69 a | 6.85 ± 1.50 a | 6.94 ± 1.37 a | 7.02 ± 1.16 a |
| MAIH 130 °C | 7.50 ± 1.11 a | 6.84 ± 1.35 a | 7.31 ± 0.99 a | 6.78 ± 1.22 a | 6.93 ± 1.06 a | 7.06 ± 1.04 a |
| MAIH 90 °C | 7.38 ± 1.31 a | 6.58 ± 1.24 a | 7.26 ± 0.74 a | 6.94 ± 1.01 a | 6.96 ± 0.87 a | 7.03 ± 1.35 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, C.-C.; Lin, C.-S.; Lee, Y.-C.; Wei, C.-I.; Tung, H.-N.; Ou, T.-Y.; Chen, T.-Y.; Tsai, Y.-H. Physicochemical and Microbial Quality of Prepackaged Shrimp Processed by a Scaled-Up Microwave-Assisted Induction Heating Technology. Appl. Sci. 2021, 11, 9514. https://doi.org/10.3390/app11209514
Hwang C-C, Lin C-S, Lee Y-C, Wei C-I, Tung H-N, Ou T-Y, Chen T-Y, Tsai Y-H. Physicochemical and Microbial Quality of Prepackaged Shrimp Processed by a Scaled-Up Microwave-Assisted Induction Heating Technology. Applied Sciences. 2021; 11(20):9514. https://doi.org/10.3390/app11209514
Chicago/Turabian StyleHwang, Chiu-Chu, Chung-Saint Lin, Yi-Chen Lee, Cheng-I Wei, Hung-Nan Tung, Tsung-Yin Ou, Tai-Yuan Chen, and Yung-Hsiang Tsai. 2021. "Physicochemical and Microbial Quality of Prepackaged Shrimp Processed by a Scaled-Up Microwave-Assisted Induction Heating Technology" Applied Sciences 11, no. 20: 9514. https://doi.org/10.3390/app11209514
APA StyleHwang, C.-C., Lin, C.-S., Lee, Y.-C., Wei, C.-I., Tung, H.-N., Ou, T.-Y., Chen, T.-Y., & Tsai, Y.-H. (2021). Physicochemical and Microbial Quality of Prepackaged Shrimp Processed by a Scaled-Up Microwave-Assisted Induction Heating Technology. Applied Sciences, 11(20), 9514. https://doi.org/10.3390/app11209514







