Apoptotic Effect of Brassinin via Inhibition of CNOT2 and Activation of p53 and Its Combination Effect with Doxorubicin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. RNA Interference
2.3. Cytotoxicity Assay
2.4. Colony Formation Assay
2.5. Western Blotting
2.6. Flow Cytometry Analysis
2.7. Statistical Analysis
3. Results
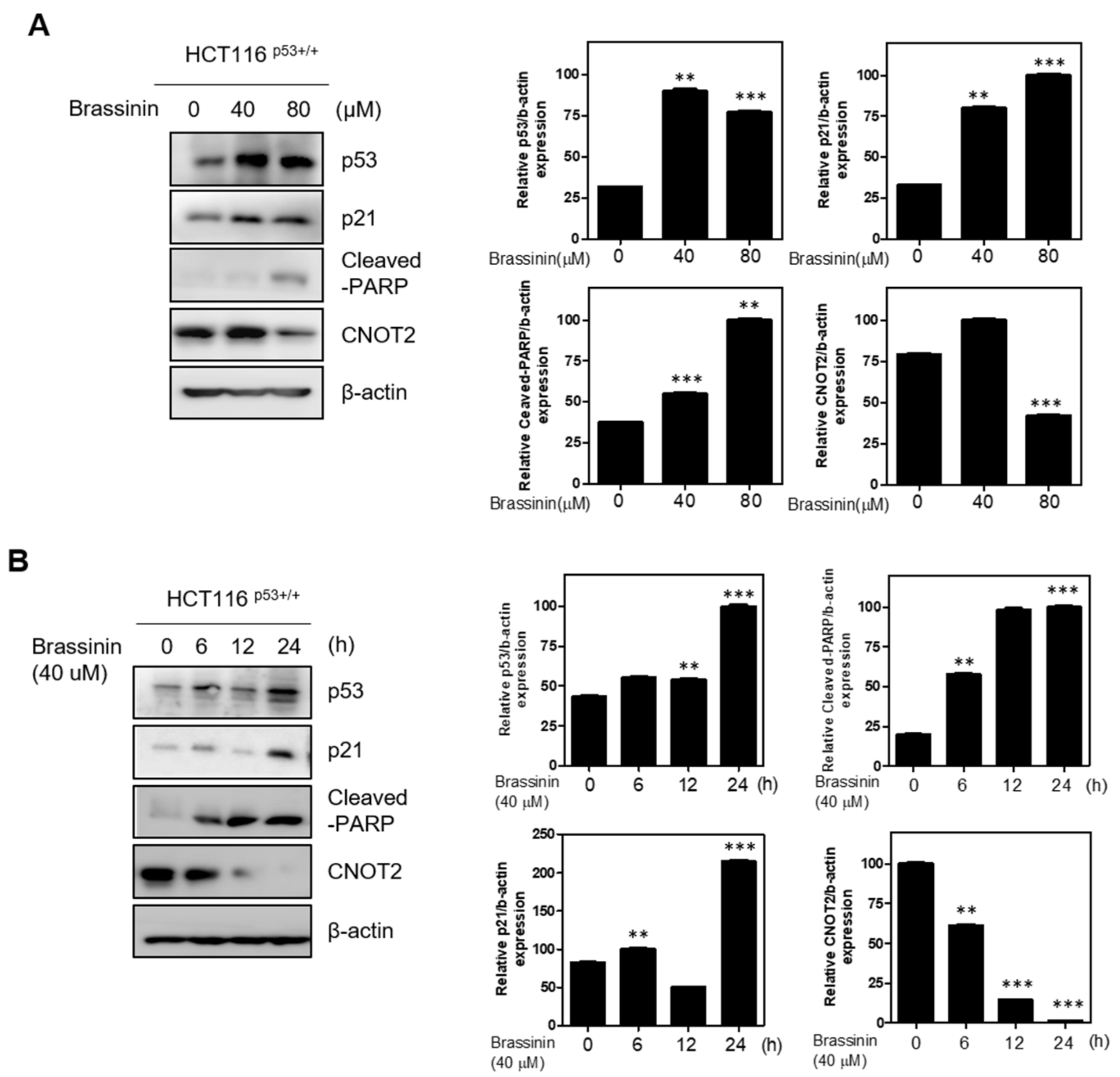
3.1. Brassinin Induces p53 and Its Target Gene p21
3.2. Brassinin Induces Apoptosis by Activating p53
3.3. Effect of RPL5 Depletion with Brassinin on the Apoptosis in Colon Cancer Cells
3.4. Brassinin Is Cytotoxic and Preservative to Colorectal Cancer Cells
3.5. Brassinin Regulates Apoptosis with a Growing Sub-G1 Population
3.6. Combination Effect of Brassinin and Doxorubicin in HCT116p53+/+ Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jung, J.H.; Lee, H.; Cao, B.; Liao, P.; Zeng, S.X.; Lu, H. RNA-binding motif protein 10 induces apoptosis and suppresses proliferation by activating p53. Oncogene 2020, 39, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Vousden, K.H.; Lu, X. Live or let die: The cell’s response to p53. Nat. Rev. Cancer 2002, 2, 594–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.H.; Lee, H.; Zeng, S.X.; Lu, H. RBM10, a New Regulator of p53. Cells 2020, 9, 2107. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.; Zhou, X.; Cao, B.; Liao, P.; Liu, H.; Chen, Y.; Park, H.W.; Zeng, S.X.; Lu, H. Pleckstrin homology domain-containing protein PHLDB3 supports cancer growth via a negative feedback loop involving p53. Nat. Commun. 2016, 7, 13755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horn, H.F.; Vousden, K.H. Cooperation between the ribosomal proteins L5 and L11 in the p53 pathway. Oncogene 2008, 27, 5774–5784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Jung, J.H.; Hwang, J.; Park, J.E.; Kim, J.H.; Park, W.Y.; Suh, J.Y.; Kim, S.H. CNOT2 Is Critically Involved in Atorvastatin Induced Apoptotic and Autophagic Cell Death in Non-Small Cell Lung Cancers. Cancers 2019, 11, 1470. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.X.; Wang, Y.G.; Xirodimas, D.P.; Dai, M.S. Perturbation of 60 S ribosomal biogenesis results in ribosomal protein L5- and L11-dependent p53 activation. J. Biol. Chem. 2010, 285, 25812–25821. [Google Scholar] [CrossRef] [Green Version]
- Hao, Q.; Chen, Y.; Zhou, X. The Janus Face of p53-Targeting Ubiquitin Ligases. Cells 2020, 9, 1656. [Google Scholar] [CrossRef]
- Dai, M.S.; Shi, D.; Jin, Y.; Sun, X.X.; Zhang, Y.; Grossman, S.R.; Lu, H. Regulation of the MDM2-p53 pathway by ribosomal protein L11 involves a post-ubiquitination mechanism. J. Biol. Chem. 2006, 281, 24304–24313. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Hao, Q.; Zhang, Q.; Liao, J.M.; Ke, J.W.; Liao, P.; Cao, B.; Lu, H. Ribosomal proteins L11 and L5 activate TAp73 by overcoming MDM2 inhibition. Cell Death Differ. 2015, 22, 755–766. [Google Scholar] [CrossRef]
- Dai, M.S.; Lu, H. Inhibition of MDM2-mediated p53 ubiquitination and degradation by ribosomal protein L5. J. Biol. Chem. 2004, 279, 44475–44482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, M.S.; Zeng, S.X.; Jin, Y.; Sun, X.X.; David, L.; Lu, H. Ribosomal protein L23 activates p53 by inhibiting MDM2 function in response to ribosomal perturbation but not to translation inhibition. Mol. Cell Biol. 2004, 24, 7654–7668. [Google Scholar] [CrossRef] [Green Version]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [Green Version]
- Polk, A.; Vistisen, K.; Vaage-Nilsen, M.; Nielsen, D.L. A systematic review of the pathophysiology of 5-fluorouracil-induced cardiotoxicity. BMC Pharm. Toxicol 2014, 15, 47. [Google Scholar] [CrossRef] [Green Version]
- Kurokawa, Y.; Hasuike, Y.; Hattori, T.; Hayashi, S.; Fujitani, K.; Shin, E.; Mishima, H.; Sawamura, T.; Nishisho, I.; Kobayashi, K.; et al. Efficacy and side effect of continuous intra-arterial infusion of high-dose 5-FU for liver metastases of colorectal cancer. Gan Kagaku Ryoho 1999, 26, 1737–1740. [Google Scholar]
- Hijri, F.Z.; Arifi, S.; Ouattassi, N.; Mellas, N.; El Mesbahi, O. Oxaliplatin-induced ototoxicity in adjuvant setting for colorectal cancer: Unusual side effect. J. Gastrointest. Cancer 2014, 45, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Ham, J.; Song, J.; Song, G.; Lim, W. Brassinin Inhibits Proliferation in Human Liver Cancer Cells via Mitochondrial Dysfunction. Cells 2021, 10, 332. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.H.; Baek, S.H.; Ha, I.J.; Um, J.Y.; Ahn, K.S. Brassinin enhances the anticancer actions of paclitaxel by targeting multiple signaling pathways in colorectal cancer cells. Phytother. Res. 2021, 35, 3875–3885. [Google Scholar] [CrossRef]
- Yang, M.H.; Lee, J.H.; Ko, J.H.; Jung, S.H.; Sethi, G.; Ahn, K.S. Brassinin Represses Invasive Potential of Lung Carcinoma Cells through Deactivation of PI3K/Akt/mTOR Signaling Cascade. Molecules 2019, 24, 1584. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.H.; Kim, M.J.; Lee, H.; Lee, J.; Kim, J.; Lee, H.J.; Shin, E.A.; Kim, Y.H.; Kim, B.; Shim, B.S.; et al. Farnesiferol c induces apoptosis via regulation of L11 and c-Myc with combinational potential with anticancer drugs in non-small-cell lung cancers. Sci. Rep. 2016, 6, 26844. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.H.; Lee, H.; Kim, J.H.; Sim, D.Y.; Ahn, H.; Kim, B.; Chang, S.; Kim, S.H. p53-Dependent Apoptotic Effect of Puromycin via Binding of Ribosomal Protein L5 and L11 to MDM2 and its Combination Effect with RITA or Doxorubicin. Cancers 2019, 11, 582. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Deisenroth, C.; Zhang, Y. RP-MDM2-p53 Pathway: Linking Ribosomal Biogenesis and Tumor Surveillance. Trends Cancer 2016, 2, 191–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fregoso, O.I.; Das, S.; Akerman, M.; Krainer, A.R. Splicing-factor oncoprotein SRSF1 stabilizes p53 via RPL5 and induces cellular senescence. Mol. Cell 2013, 50, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Xiao, H.; Chai, S.C.; Hoang, Q.Q.; Lu, H. Hydrophilic residues are crucial for ribosomal protein L11 (RPL11) interaction with zinc finger domain of MDM2 and p53 protein activation. J. Biol. Chem. 2011, 286, 38264–38274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayama, K.; Watanabe, S.; Takafuji, T.; Tsuji, T.; Hironaka, K.; Matsumoto, M.; Nakayama, K.I.; Enari, M.; Kohno, T.; Shiraishi, K.; et al. GRWD1 negatively regulates p53 via the RPL11-MDM2 pathway and promotes tumorigenesis. EMBO Rep. 2017, 18, 123–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Lang, Y.; Zhang, Q.; Cui, D.; Sun, H.; Jiang, L.; Chen, Z.; Zhang, R.; Gao, Y.; Tian, W.; et al. Structure of human MDM2 complexed with RPL11 reveals the molecular basis of p53 activation. Genes Dev. 2015, 29, 1524–1534. [Google Scholar] [CrossRef] [Green Version]
- Boultwood, J. The role of haploinsufficiency of RPS14 and p53 activation in the molecular pathogenesis of the 5q- syndrome. Pediatric Rep. 2011, 3, e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhang, Q.; Zeng, S.X.; Hao, Q.; Lu, H. Inauhzin sensitizes p53-dependent cytotoxicity and tumor suppression of chemotherapeutic agents. Neoplasia 2013, 15, 523–534. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Zeng, S.X.; Zhang, Y.; Zhang, Y.; Ding, D.; Ye, Q.; Meroueh, S.O.; Lu, H. A small molecule Inauhzin inhibits SIRT1 activity and suppresses tumour growth through activation of p53. EMBO Mol. Med. 2012, 4, 298–312. [Google Scholar] [CrossRef]
- Zhou, X.; Hao, Q.; Liao, P.; Luo, S.; Zhang, M.; Hu, G.; Liu, H.; Zhang, Y.; Cao, B.; Baddoo, M.; et al. Nerve growth factor receptor negates the tumor suppressor p53 as a feedback regulator. Elife 2016, 5, e15099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgado-Palacin, L.; Llanos, S.; Urbano-Cuadrado, M.; Blanco-Aparicio, C.; Megias, D.; Pastor, J.; Serrano, M. Non-genotoxic activation of p53 through the RPL11-dependent ribosomal stress pathway. Carcinogenesis 2014, 35, 2822–2830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohn, E.J.; Jung, D.B.; Lee, H.; Han, I.; Lee, J.; Lee, H.; Kim, S.H. CNOT2 promotes proliferation and angiogenesis via VEGF signaling in MDA-MB-231 breast cancer cells. Cancer Lett. 2018, 412, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Lee, H.J.; Kim, J.H.; Sim, D.Y.; Im, E.; Kim, S.; Chang, S.; Kim, S.H. Colocalization of MID1IP1 and c-Myc is Critically Involved in Liver Cancer Growth via Regulation of Ribosomal Protein L5 and L11 and CNOT2. Cells 2020, 9, 985. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, W.Y.; Park, J.E.; Jung, J.H. Apoptotic Effect of Brassinin via Inhibition of CNOT2 and Activation of p53 and Its Combination Effect with Doxorubicin. Appl. Sci. 2021, 11, 10036. https://doi.org/10.3390/app112110036
Park WY, Park JE, Jung JH. Apoptotic Effect of Brassinin via Inhibition of CNOT2 and Activation of p53 and Its Combination Effect with Doxorubicin. Applied Sciences. 2021; 11(21):10036. https://doi.org/10.3390/app112110036
Chicago/Turabian StylePark, Woon Yi, Ji Eon Park, and Ji Hoon Jung. 2021. "Apoptotic Effect of Brassinin via Inhibition of CNOT2 and Activation of p53 and Its Combination Effect with Doxorubicin" Applied Sciences 11, no. 21: 10036. https://doi.org/10.3390/app112110036






