The analysis of the far-infrared (THz) absorption spectra of phospholipid membranes allows us to gain insight into both their intermolecular interactions and the torsional modes of the acyl chains and skeleton vibrations. By comparing the two investigated systems, DMPC and DPPE phospholipid membranes, we can expect that differences in their spectral signatures will mainly reflect different intermolecular interaction principles.
3.1. DMPC
In
Figure 2, the far-IR spectra of DMPC/H
2O samples at five distinct hydration degrees (dry, RH = 11%, 43%, 64%, 75%) are shown with the corresponding fitting curve and the Gaussian components for the dry and mostly hydrated samples.
We clearly observed that absorbance increases as the hydration level increases, as hypothesized (
Figure 2a). We performed the curve fitting of the spectra as a linear combination of individual Gaussian component bands following the procedure described in detail in
Section 2.3. We found that eight components are needed to reproduce each spectrum independently of the hydration level and the phase state of the lipid multilayers. The frequencies of the vibrations in the DMPC sample with the highest hydration (75%) are: 50, 70, 90, 119, 154, 186, 219, and 259 cm
−1 (named in the following A, B, C, D, E, F, G, and H). The frequencies in the dry DMPC sample are reported in
Table 1. They coincide with those of the wet sample, except for bands D and E, which are shifted to lower frequencies in the wet sample (110 and 145 cm
−1). Thus, the frequencies of the vibrational modes were almost unaffected by the hydration, while significant changes amongst the DMPC samples at different hydration degrees were observed in the width and the area of all of the bands, as will be detailed in the following.
The experimental data and the data analysis reproduced our previous findings [
6], with slight differences attributable to the different solvent (H
2O was used in this work; D
2O was used in the previous one). Additionally, while in the current work we carried out measurements under controlled hydration and in an equilibrated condition, in our previous work data were acquired in an out of equilibrium phase—i.e., as a function of the dehydration time by dehydrating under vacuum. Thus, it was not possible to obtain precise information on the hydration status of the samples. Differences between the two sets of measurements were also attributable to the different experimental modalities used. In
Table 1, the number of water molecules per lipid, n
W, are reported (see
Section 2.1 for details concerning the determination of n
W). Therefore, we fully confirm the results of [
6] and the assignment previously proposed for the revealed bands. The A, B, C, and E bands were assigned to vibrations of different lipids interacting through their intermolecular van der Waals forces. The D band was assigned to an unspecified motion of the terminal methyl carbons of the phospholipid chains C−CH
3 [
14]. The F and H bands were attributed to the torsion modes of the hydrocarbon chains and the terminal methyl groups, respectively. The assignments of the observed excitations are reported in
Table 2.
Returning to the observed frequency shift to lower values observed for the D and E bands in the wet DMPC sample, this signifies that the corresponding vibrational motions of the lipid molecular groups are influenced, even indirectly, by the presence of the solvent. We interpret the blue shift in the dry sample as a strengthening of the interactions within the compact structure in the absence of a solvent.
For all of the components, we observed a decrease in the full-width half-maximum (fwhm) as the hydration level decreased (data not shown). This can be interpreted as being due to both decreased motional rates and a smaller number of explored conformations going towards the more ordered solid gel phase at extreme or complete dehydration.
Concerning the contribution of each band to the spectrum (
Figure 3), the area of all the bands increased with the hydration level, thus reflecting the high absorbance of the hydration water, which could be due to both water–water and water–lipid coupled vibrations.
By calculating the fractional variation of the area of each band in the wet state (75%) with respect to the dry state (
Figure 3b), we found that the highest frequency bands (D, E, F, G, H) showed very high variation (3 ÷ 8%), and the H band (259 cm
−1), attributed to the torsional modes of the CH
3 groups, was the one that shows the most significant percentage variation.
Notably, the frequency range of the bands between 110 and 260 cm
−1, i.e., from D to H, almost matches that of the so-called “connectivity band” of bulk liquid water [
15]. The connectivity band, a pronounced and broad peak centered at 200 cm
−1 as seen in the absorption spectrum of pure water, is described in terms of the collective intermolecular stretching of the hydrogen bond network [
5,
15]. This shows a composite structure based on four components (88, 130, 195, 251 cm
−1) corresponding to different states of connectivity [
15], with the component at the highest frequency reflecting water molecules having established a maximum number of hydrogen bonds [
15]. Concerning the minimal variation in the lowest energy bands (A, B, C) as a function of increasing relative humidity and thus of the number of water molecules per lipid, it is worth mentioning that in pure water, the hydrogen bond bending vibrations and the umbrella-like motion of two hydrogen bonded tetrahedra along the connecting hydrogen bond axis that both contribute to the 50–70 cm
−1 region are clearly visible in the Raman and optical Kerr effect spectra but are not very pronounced in the far-IR absorption spectra [
5].
The main features of the vibrational excitations of the DMPC/H2O samples at THz frequencies can be summarized in the following statements: (i) eight vibrational modes have been detected independently from the hydration level; (ii) all models show a sensitivity to water, as seen in the area and in the fwhm; (iii) in particular, the highest frequency bands (from D to H, and to a greater extent the H band) that match the connectivity band of water are the ones that show the largest percentage variation upon hydration; (iv) two vibrations, namely D and E, undergo a shift to higher frequencies upon dehydration. This can be attributed to a strengthening in the corresponding intermolecular lipid–lipid interactions.
3.2. DPPE
In
Figure 4, the far-IR spectra of DPPE/H
2O samples at four distinct hydration degrees (dry, RH = 11%, 53%, 75%) with the corresponding fitting curve and the Gaussian components for the dry and mostly hydrated samples are reported.
The acquired spectra at the different hydrations (
Figure 4a) showed complex profiles, with the absorbance increasing as the hydration increased. Analogously to the procedure followed for the DMPC samples and described above, we performed the curve fitting of the spectra as a linear combination of individual Gaussian component bands. While in the DMPC samples eight components were needed to properly analyze the data, in the case of the DPPE samples we found that ten components must be considered for an appropriate description of the absorbance profiles. However, similarly to the DMPC samples, the same number of band components (ten) were needed for the data analysis of the samples, independent of the relative humidity. Most interestingly, eight out of ten frequencies were almost coincident with those of the DMPC samples. We thus labelled the corresponding eight bands with the same letters used for the DMPC samples, and we used the Greek letters, α and β, for the bands that are characteristic only of the DPPE samples. Furthermore, no drift in frequency was observed, independent of the relative humidity of the samples. Thus, the frequencies of all the DPPE samples are: 46, 61, 89, 118, 144, 162, 189, 218, 236, and 263 cm
−1 (named in the following as A, B, C, D, E, α, F, G, β, and H (see
Table 2)). The α and β bands have a frequency of 162 and 236 cm
−1, respectively. Although the frequency of the vibrational modes was not influenced by the hydration degrees, notable differences among the DPPE samples emerged when comparing the areas and widths of the excitations. In other words, the same vibrational modes characterized the DPPE samples at different hydration degrees, including the dry state, by their vibrational areas, and widths are affected by the level of hydration.
The almost total coincidence of the frequencies of the DMPC bands with those of the DPPE, together with the very similar molecular structures of the two phospholipids (apart from the polar head structure), leads us to assign the vibrations observed in the DPPE as previously conducted for the DMPC.
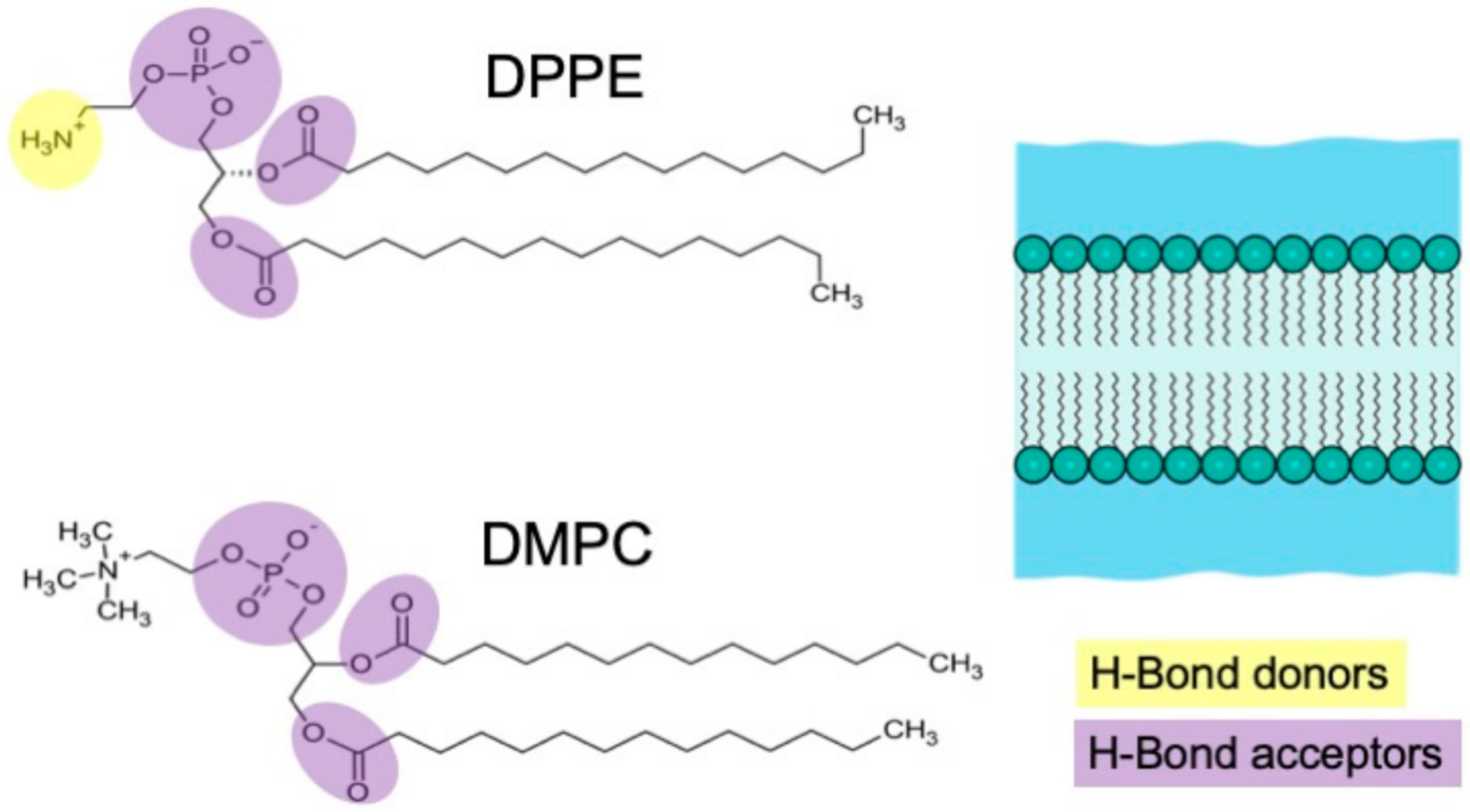
On the other hand, the α and β bands, observed exclusively in the spectra of the DPPE, require additional considerations for their assignment. The DPPE membrane exhibits an enhanced lipid–lipid hydrogen-bonding capability of the PE group with respect to the PC group, which can differently form hydrogen bonds exclusively with water. When PE phospholipids aggregate, the headgroups align so that the ammonium group interacts directly with the phosphate group of the adjacent lipid via an intermolecular hydrogen bond, though other combinations are possible—e.g., interactions involving the carbonyl oxygen of ester groups [
8,
9]. Additionally, though DPPE lipids mainly interact via direct intermolecular
or
contacts, water molecules may act as bridges in mediating these interactions. It is well known that in DPPE, the ensemble of the hydrogen bond linkages between adjacent phospholipids produces a very compact and rigid structure at the bilayer surface that is largely unaffected by hydration, contrary to the considerably flexible network of DMPC [
8]. We thus tentatively assigned the α and β bands to vibrations of the network of DPPE phospholipids linked by intermolecular hydrogen bonds. In particular, the frequency of the α band (162 cm
−1) was found to be very close to the frequency (166 cm
−1) measured in a protic ionic liquid for the hydrogen bond stretching (N−H···O) occurring between the N−H group from the N-methylpyrrolidinium cation and the oxygen on the acetate anion [
16]. Based on that, we tentatively ascribed the α mode to the hydrogen bond stretching N−H···O where the N−H group from the ammonium group acted as a hydrogen bond donor, while the oxygen on the carbonyl esterified group acted as a hydrogen bond acceptor. Furthermore, we tentatively assigned the higher frequency β band to the N−H···O−P hydrogen bond stretching between the ammonium group and the oxygen of the phosphate moiety. This assignment was based on the following considerations: the N−H···O−P hydrogen bond is usually stronger than the N−H···O−C bond [
17], so a higher frequency is expected for the corresponding stretching band; it is more favorable for DPPE to form intermolecular hydrogen bonds between amine groups and phosphate groups than between amine groups and carbonyl groups [
8,
9]; this finding corresponds with the higher intensity of the β band with respect to the α band (
Figure 4).
It is worth noting that such a rigid network of lipid–lipid intermolecular contacts dramatically reduces the capability of hydration in DPPE. This is evident when analyzing the number of water molecules per lipid that only achieves the value n
W = 1.03 at the highest relative humidity (RH = 75%). On the other hand, the DMPC lipids have a higher number of water molecules per lipid, n
W = 4.0, that may act as bridges between lipids, contributing to establish an extended and more flexible network in the bilayer [
10]. Thus, in DPPE, differently from DMPC, we observed a reduced capability of hydration together with a lower sensitivity to the water content of the spectral fingerprints at THz frequencies. This situation was reflected in the minor changes observed for the fwhm of all 10 identified far-infrared components (data not shown) when the hydration was changed. The impenetrability to water maintained the bilayer rigidity and kept the acyl chains well-ordered.
However, the influence of the hydration degree on the low-frequency vibrations can be investigated through the inspection of the area of the Gaussian bands as a function of the relative humidity, as shown in
Figure 5. This trend can be more easily examined by looking at
Figure 5b, which shows the relative variation in the areas in the wet state (RH = 75%) compared to the dry state. It is immediately noticeable how this trend differs considerably from that observed in the DMPC samples. Firstly, the range of the fractional variation for the different components was much smaller in the case of DPPE, and this confirmed the lower sensitivity of these samples to hydration compared to DMPC. Secondly, while for the DMPC samples all of the higher frequency bands (from D to H) were significantly affected by the water content, for DPPE, only two bands, i.e., F and H, that correspond to 189 and 263 cm
−1, showed an important variation with respect to the dry sample, albeit very limited when compared to DMPC. The frequencies of these two bands were very close to the two bands at higher frequencies that characterized the connectivity band of pure water (195, 251 cm
−1), as detailed above in
Section 3.1.
Interestingly, the intensities of the α and β bands showed a negative increase with increasing hydration. This can be interpreted as the result of competition between the water molecules and the or carbonyl groups in forming hydrogen bonding with moieties. However, this variation does not appear to be relevant, indicating that a strong intermolecular interaction between internal groups in DPPE was also maintained when lipids were hydrated. This may be a main limiting factor for the hydration of the phosphate groups with respect to what is observed in DMPC.