Determination of Thermodynamic Parameters of Polylactic Acid by Thermogravimetry under Pyrolysis Conditions
Abstract
:1. Introduction
2. Methodology
2.1. Determination of Properties and Equipment
2.2. TGA Data Processing
2.2.1. Isoconversional Kinetic Models
Friedman Method (FR)
Kissinger-Akahira-Sunose Method (KAS)
Flynn-Wall-Ozawa (FWO) Method
2.2.2. Reaction Model
2.2.3. Statistical Adjustment and Validation of Kinetic Models
2.3. Thermodynamic Properties
2.3.1. Enthalpy
2.3.2. Gibbs Free Energy
2.3.3. Entropy
3. Results and Discussion
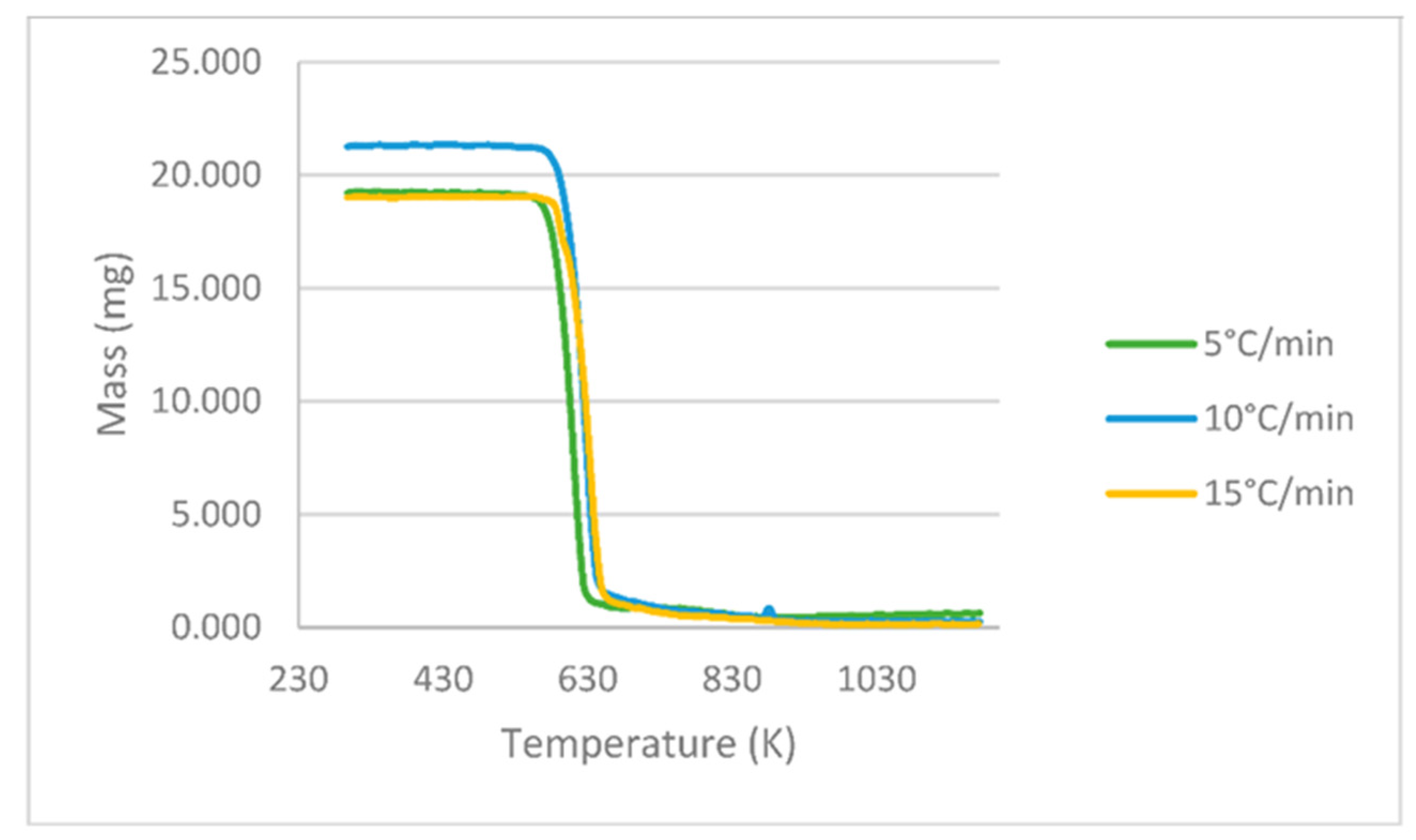
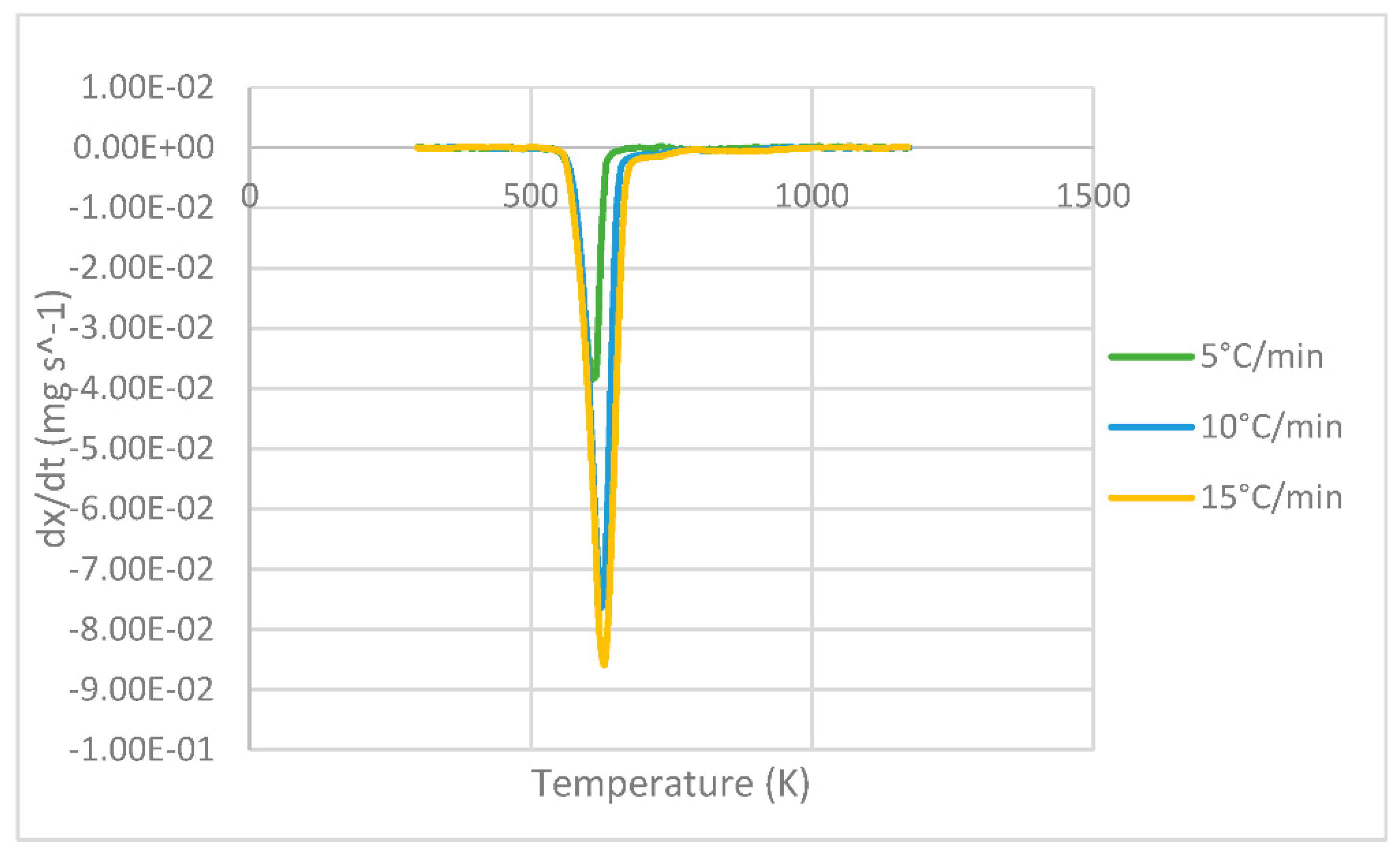
3.1. Characterization of the PLA Sample
3.1.1. Density and Specific Heat
3.1.2. Mechanical Properties
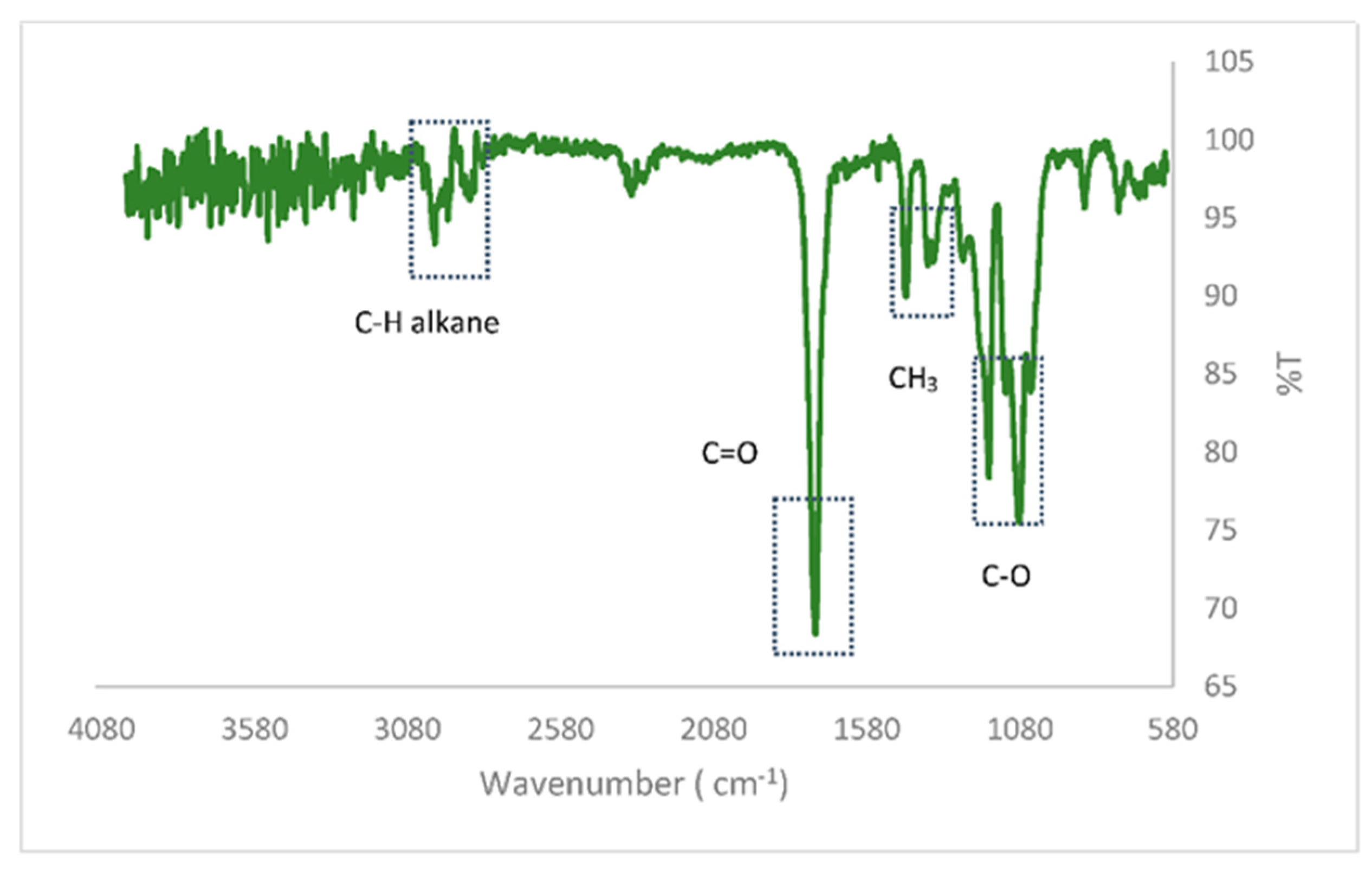
3.1.3. FT-IR Analysis
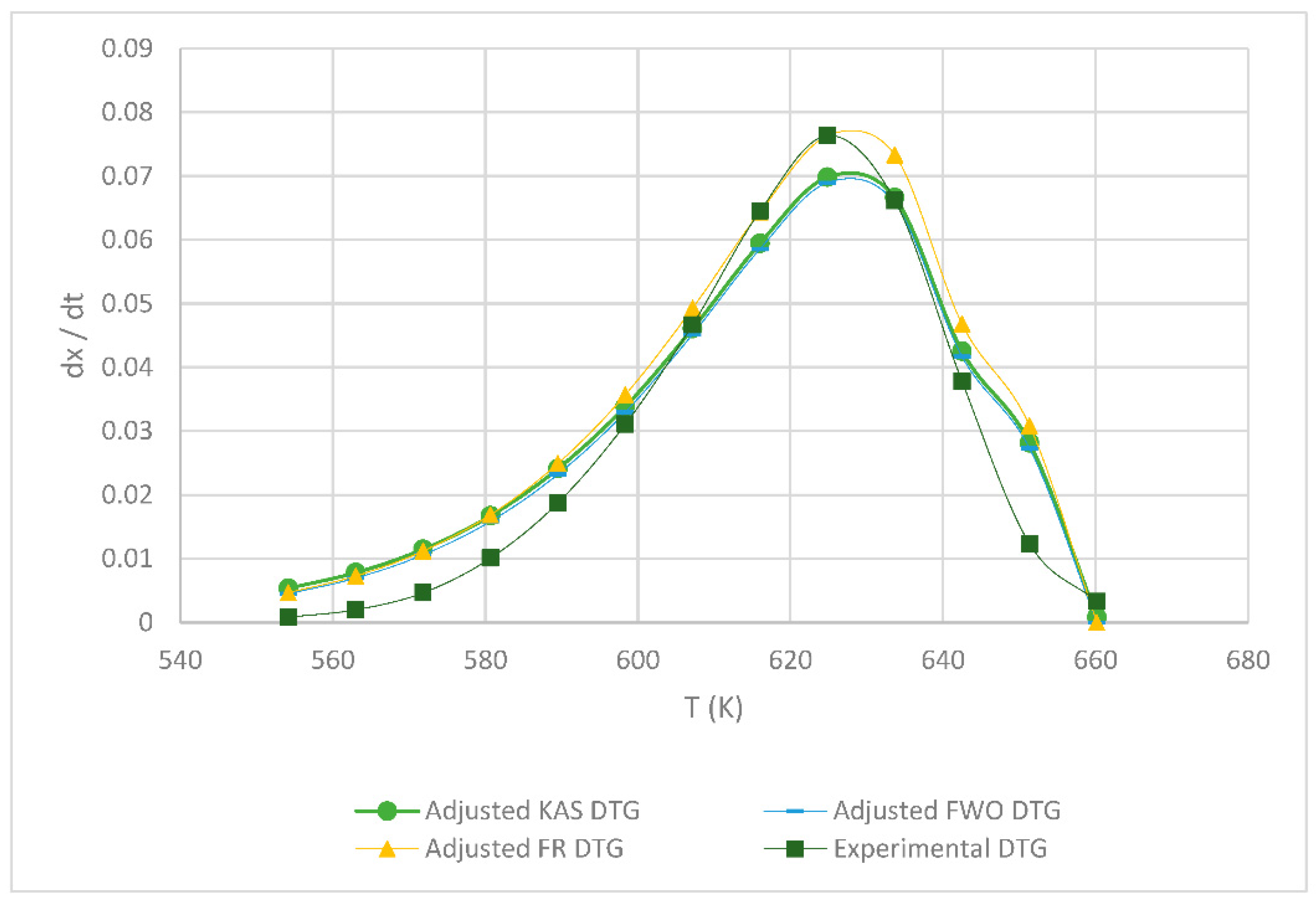
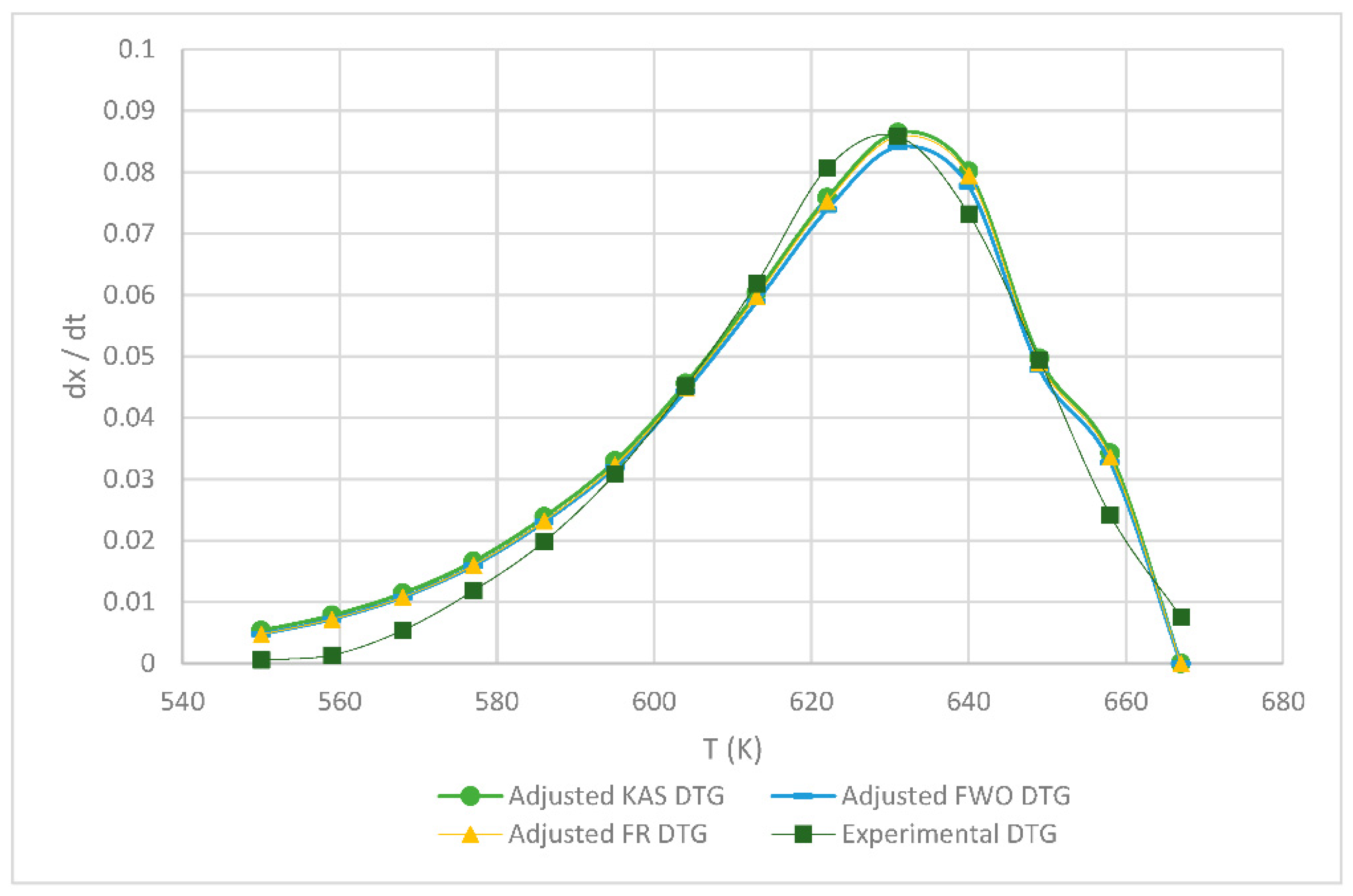
3.2. Kinetic Parameters of the FR, KAS and FWO Models
3.3. Thermodynamic Properties of PLA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plastics Europe. Plastics—The Facts 2018. An Analysis of European Plastics Production, Demand and Waste Data. Available online: https://www.plasticseurope.org; Plásticos–Situación en 2019. Plastic Europe. 2019. Available online: https://www.plasticseurope.org/es/resources/publications/2511-plasticos-situacion-en-201 (accessed on 1 September 2021).
- Plavec, R.; Hlaváčiková, S.; Omaníková, L.; Feranc, J.; Vanovčanová, Z.; Tomanová, K.; Bočkaj, J.; Kruželák, J.; Medlenová, E.; Gálisová, I.; et al. Recycling possibilities of bioplastics based on PLA/PHB blends. Polym. Test. 2020, 92, 106880. [Google Scholar] [CrossRef]
- Xiang, S.; Feng, L.D.; Bian, X.C.; Li, G.; Chen, X.S. Evaluation of PLA content in PLA/PBAT blends using TGA. Polym. Test. 2020, 81, 106211. [Google Scholar] [CrossRef]
- Fan, Y.; Nishida, H.; Hoshihara, S.; Shirai, Y.; Tokiwa, Y.; Endo, T. Pyrolysis kinetics of poly(l-lactide) with carboxyl and calcium salt end structures. Polym. Degrad. Stab. 2003, 79, 547–562. [Google Scholar] [CrossRef]
- Jin, Z.; Yin, L.; Chen, D.; Jia, Y.; Yuan, J.; Hu, Y. Co-pyrolysis characteristics of typical components of waste plastics in a falling film pyrolysis reactor. Chin. J. Chem. Eng. 2018, 26, 2176–2184. [Google Scholar] [CrossRef]
- Matias, Á.A.; Lima, M.S.; Pereira, J.; Pereira, P.; Barros, R.; Coelho, J.F.; Serra, A.C. Use of recycled polypropylene/poly(ethylene terephthalate) blends to manufacture water pipes: An industrial scale study. Waste Manag. 2020, 101, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Tyagi, V.; Singh, P.; Pandey, A. Estimation of thermodynamic characteristics for comprehensive dairy food processing plant: An energetic and exergetic approach. Energy 2020, 194, 116799. [Google Scholar] [CrossRef]
- Badia, J.; Blasco, L.S.; Moriana, R.; Ribes-Greus, A. Thermal analysis applied to the characterization of degradation in soil of polylactide: II. On the thermal stability and thermal decomposition kinetics. Polym. Degrad. Stab. 2010, 95, 2192–2199. [Google Scholar] [CrossRef]
- Brems, A.; Baeyens, J.; Beerlandt, J.; Dewil, R. Thermogravimetric pyrolysis of waste polyethylene-terephthalate and polystyrene: A critical assessment of kinetics modelling. Resour. Conserv. Recycl. 2011, 55, 772–781. [Google Scholar] [CrossRef]
- Das, P.; Tiwari, P. Thermal degradation kinetics of plastics and model selection. Thermochim. Acta 2017, 654, 191–202. [Google Scholar] [CrossRef]
- Xu, F.; Wang, B.; Yang, D.; Hao, J.; Qiao, Y.; Tian, Y. Thermal degradation of typical plastics under high heating rate conditions by TG-FTIR: Pyrolysis behaviors and kinetic analysis. Energy Convers. Manag. 2018, 171, 1106–1115. [Google Scholar] [CrossRef]
- Carrasco, F.; Pérez-Maqueda, L.; Sánchez-Jiménez, P.; Perejón, A.; Santana, O.; Maspoch, M. Enhanced general analytical equation for the kinetics of the thermal degradation of poly(lactic acid) driven by random scission. Polym. Test. 2013, 32, 937–945. [Google Scholar] [CrossRef]
- Duque, J.V.F.; Martins, M.F.; Debenest, G.; Orlando, M.T.D. The influence of the recycling stress history on LDPE waste pyrolysis. Polym. Test. 2020, 86, 106460. [Google Scholar] [CrossRef]
- María, L.; Ríos, A.; Victoria, P.; Muñoz, C.; Manuel, J.; Giraldo, C.; Gaviria, G.H.; Felipe, A.; González, R. Modelos cinéticos de degradación térmica de polímeros: Una revisión. Rev. Ing. Univ. De Medellín 2013, 12, 113–130. [Google Scholar] [CrossRef] [Green Version]
- Khedri, S.; Elyasi, S. Kinetic analysis for thermal cracking of HDPE: A new isoconversional approach. Polym. Degrad. Stab. 2016, 129, 306–318. [Google Scholar] [CrossRef]
- Valapa, R.; Pugazhenthi, G.; Katiyar, V. Thermal degradation kinetics of sucrose palmitate reinforced poly(lactic acid) biocomposites. Int. J. Biol. Macromol. 2014, 65, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Coats, A.W.; Redfern, J.P. Kinetic Parameters from Thermogravimetric Data. Nat. Cell Biol. 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. A quick, direct method for the determination of activation energy from thermogravimetric data. J. Polym. Sci. Part B Polym. Lett. 1966, 4, 323–328. [Google Scholar] [CrossRef]
- Ozawa, T. A New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef] [Green Version]
- Doyle, C.D. Kinetic analysis of thermogravimetric data. J. Appl. Polym. Sci. 1961, 5, 285–292. [Google Scholar] [CrossRef]
- Palmay, P.; Jaramillo-Uvidia, R.N.; Medina, C. Estudio cinético de la descomposición térmica del polietileno de alta densidad (HDPE) posconsumo a diferentes tasas de calentamiento. FIGEMPA Investig. y Desarro. 2021, 11, 61–68. [Google Scholar] [CrossRef]
- Kassargy, C.; Awad, S.; Burnens, G.; Kahine, K.; Tazerout, M. Experimental study of catalytic pyrolysis of polyethylene and polypropylene over USY zeolite and separation to gasoline and diesel-like fuels. J. Anal. Appl. Pyrolysis 2017, 127, 31–37. [Google Scholar] [CrossRef]
- Arjanggi, R.D.; Kansedo, J. Recent advancement and prospective of waste plastics as biodiesel additives: A review. J. Energy Inst. 2020, 93, 934–952. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [Green Version]
- NatureWorks|Home. Available online: https://www.natureworksllc.com/ (accessed on 12 October 2021).
- Choksi, N.; Desai, H. Synthesis of Biodegradable Polylactic Acid Polymer by Using Lactic Acid Monomer. Int. J. Appl. Chem. 2017, 13, 377–384. Available online: http://www.ripublication.com (accessed on 6 September 2021).
- Jumat, M.A.; Chevallier, P.; Mantovani, D.; Copes, F.; Razak, S.I.A.; Saidin, S. Three-dimensional printed biodegradable poly(l-lactic acid)/(poly(d-lactic acid) scaffold as an intervention of biomedical substitute. Polym. Technol. Mater. 2021, 60, 1005–1015. [Google Scholar] [CrossRef]
- Mehmood, M.A.; Ye, G.; Luo, H.; Liu, C.; Malik, S.; Afzal, I.; Xu, J.; Ahmad, M.S. Pyrolysis and kinetic analyses of Camel grass (Cymbopogon schoenanthus) for bioenergy. Bioresour. Technol. 2017, 228, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, Y.S.; Kim, S.H. Investigation of Thermodynamic Parameters in the Thermal Decomposition of Plastic Waste−Waste Lube Oil Compounds. Environ. Sci. Technol. 2010, 44, 5313–5317. [Google Scholar] [CrossRef]

| Kinetic Model | Experimental Condition | Results | |||
|---|---|---|---|---|---|
| Heating Rate β (°C min−1) | Tm (K) | E (kJ.mol−1) | A (s−1) | R2 | |
| FR | 5 | 607.2 | 146.70 | 1.192 × 1011 | 0.9840 |
| 10 | 624.8 | 130.94 | 5.147 × 109 | 0.9818 | |
| 15 | 631.0 | 119.44 | 5.224 × 108 | 0.9453 | |
| KAS | 5 | 607.2 | 145.59 | 8.958 × 1010 | 0.9909 |
| 10 | 624.8 | 128.27 | 2.781 × 109 | 0.9797 | |
| 15 | 631.0 | 119.44 | 5.224 × 108 | 0.9663 | |
| FWO | 5 | 607.2 | 145.59 | 8.958 × 1010 | 0.9909 |
| 10 | 624.8 | 128.28 | 2.789 × 109 | 0.9797 | |
| 15 | 631.0 | 118.59 | 4.353 × 108 | 0.9663 | |
| β (°C min−1) | E (kJ mol−1) | A (s−1) | ∆H (kJ mol−1) | ∆G (kJ mol−1) | ∆S (J mol−1) |
|---|---|---|---|---|---|
| 5 | 145.59 | 8.958 × 1010 | 140.54 | 170.58 | −49.47 |
| 10 | 128.27 | 2.781 × 109 | 123.08 | 172.17 | −78.57 |
| 15 | 119.44 | 5.224 × 108 | 114.19 | 172.60 | −92.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmay, P.; Mora, M.; Barzallo, D.; Bruno, J.C. Determination of Thermodynamic Parameters of Polylactic Acid by Thermogravimetry under Pyrolysis Conditions. Appl. Sci. 2021, 11, 10192. https://doi.org/10.3390/app112110192
Palmay P, Mora M, Barzallo D, Bruno JC. Determination of Thermodynamic Parameters of Polylactic Acid by Thermogravimetry under Pyrolysis Conditions. Applied Sciences. 2021; 11(21):10192. https://doi.org/10.3390/app112110192
Chicago/Turabian StylePalmay, Paul, Melissa Mora, Diego Barzallo, and Joan Carles Bruno. 2021. "Determination of Thermodynamic Parameters of Polylactic Acid by Thermogravimetry under Pyrolysis Conditions" Applied Sciences 11, no. 21: 10192. https://doi.org/10.3390/app112110192
APA StylePalmay, P., Mora, M., Barzallo, D., & Bruno, J. C. (2021). Determination of Thermodynamic Parameters of Polylactic Acid by Thermogravimetry under Pyrolysis Conditions. Applied Sciences, 11(21), 10192. https://doi.org/10.3390/app112110192







