Effects of Adding Active Elements to Aluminum-Based Filler Alloys on the Bonding of 6061 Aluminum Alloy and Alumina
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.T.; Cheng, Y.H.; Lin, C.C.; Lin, K.L. Direct bonding of aluminum to alumina using a nickel interlayer for power, electronics applications. Results Mater. 2000, 6, 100093. [Google Scholar] [CrossRef]
- Ning, X.S.; Lin, Y.; Xu, W.; Peng, R.; Zhou, H.; Chen, K. Development of a directly bonded aluminum/alumina power electronic substrate. Mater. Sci. Eng. B 2003, 99, 479–482. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Tuan, W.-H. Direct Bonding of Aluminum to Alumina for Thermal Dissipation Purposes. Int. J. Appl. Ceram. Technol. 2015, 13, 170–176. [Google Scholar] [CrossRef]
- Tuan, W.-H.; Lee, S.-K. Eutectic bonding of copper to ceramics for thermal dissipation applications—A review. J. Eur. Ceram. Soc. 2014, 34, 4117–4130. [Google Scholar] [CrossRef]
- Yoo, S.-Y.; Kim, S.-K.; Heo, S.-J.; Koak, J.-Y.; Kim, J.-G. Effects of Bonding Agents on Metal-Ceramic Bond Strength of Co-Cr Alloys Fabricated by Selective Laser Melting. Materials 2020, 13, 4322. [Google Scholar] [CrossRef] [PubMed]
- Aliya, D.; Walker, L.; Montz, E.; Pastor, S.; Abad, A.; Hashim, F.; Abdul-Latif, A.; Al-Roubaiy, A.; Oh, Y.; Garmestani, H.; et al. Characterization of the Effects of Active Filler-Metal Alloys in Joining Ceramic-to-Ceramic and Ceramic-to-Metal Materials. Defect Diffus. Forum 2014, 354, 167–173. [Google Scholar] [CrossRef]
- Ahn, B. Recent Advances in Brazing Fillers for Joining of Dissimilar Materials. Metals 2021, 11, 1037. [Google Scholar] [CrossRef]
- Phongpreecha, T.; Nicholas, J.D.; Bieler, T.R.; Qi, Y. Computational design of metal oxides to enhance the wetting and adhesion of silver-based brazes on yttria-stabilized-zirconia. Acta Mater. 2018, 152, 229–238. [Google Scholar] [CrossRef]
- Ali, M.; Knowles, K.M.; Mallinson, P.M.; Fernie, J.A. Microstructural evolution and characterisation of interfacial phases in Al 2 O 3 /Ag–Cu–Ti/Al 2 O 3 braze joints. Acta Mater. 2015, 96, 143–158. [Google Scholar] [CrossRef]
- Laik, A.; Mishra, P.; Bhanumurthy, K.; Kale, G.; Kashyap, B. Microstructural evolution during reactive brazing of alumina to Inconel 600 using Ag-based alloy. Acta Mater. 2013, 61, 126–138. [Google Scholar] [CrossRef]
- Koleňák, R.; Kostolný, I.; Drápala, J.; Sahul, M.; Urminský, J. Characterizing the Soldering Alloy Type In–Ag–Ti and the Study of Direct Soldering of SiC Ceramics and Copper. Metals 2018, 8, 274. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.Y.; Hung, Y.T.; Chuang, T.H. Joining Alumina to Inconel 600 and UMCo-50 Superalloys Using an Sn10Ag4Ti Active Filler Metal. J. Mater. Eng. Perform. 2003, 12, 123–127. [Google Scholar] [CrossRef]
- Bian, H.; Fu, W.; Lei, Y.; Song, X.; Liu, D.; Cao, J.; Feng, J. Wetting and low temperature bonding of zirconia metallized with Sn0.3Ag0.7Cu-Ti alloys. Ceram. Int. 2018, 44, 11456–11465. [Google Scholar] [CrossRef]
- Fu, W.; Song, X.G.; Zhao, Y.X.; Cao, J.; Feng, J.C.; Jin, C.; Wang, G.D. Effect of Ti content on the wetting behavior of Sn0.3Ag0.7Cu/AlN system. Effect of Ti content on the wetting behavior of Sn0.3Ag0.7Cu/AlN system. Mater. Des. 2017, 115, 1–7. [Google Scholar] [CrossRef]
- Chang, S.Y. Active Soldering of ZnS–SiO 2 Sputtering Targets to Copper Backing Plates Using an Sn56Bi4Ti(Ce, Ga) Filler. Mater. Manuf. Process. 2006, 21, 761–765. [Google Scholar] [CrossRef]
- Koleňák, R.; Kostolny, I.; Drapala, J.; Zackova, P.; Kuruc, M. Direct Ultrasonic Soldering of AlN Ceramics with Copper Substrate Using Zn–Al–Mg Solder. Metals 2020, 10, 160. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Jung, J.P. Possibility of Al-Si brazing alloys for industrial microjoining applications. J. Microelectron. Packag. Soc. 2017, 24, 35–40. [Google Scholar] [CrossRef]
- Dong, Z.L.; Luo, X.; Li, X.Q.; Li, J.M.; Nie, M.; Xiao, Q. Development of Al-Si-Cu-Zn-Mn filler metal for brazing 3003 aluminum alloy. In Proceedings of the 2nd Annual International Workshop on Materials Science and Engineering (IWMSE 2016), Guangzhou, China, 12–14 August 2016; pp. 299–304. [Google Scholar] [CrossRef]
- Chang, S.Y.; Tsao, L.C.; Li, T.Y.; Chuang, T.H. Joining 6061 aluminum alloy with Al-Si-Cu filler metals. J. Alloys Compd. 2009, 488, 174–180. [Google Scholar] [CrossRef]
- Niu, Z.W.; Huang, J.H.; Liu, K.K.; Xu, F.Z.; Chen, S.H.; Zhao, X.K. Brazing of 6061 aluminum alloy with the novel Al-Si-Ge-Zn filler metal. Mater. Lett. 2016, 179, 47–51. [Google Scholar] [CrossRef]
- Peng, C.; Zhu, D.; Li, K.; Du, X.; Zhao, F.; Wan, M.; Tan, Y. Research on a Low Melting Point Al-Si-Cu (Ni) Filler Metal for 6063 Aluminum Alloy Brazing. Appl. Sci. 2021, 11, 4296. [Google Scholar] [CrossRef]
- Sharma, A.; Xu, D.E.; Jung, J.P. Effect of different nanoparticles on microstructure, wetting and joint strength of Al–12Si–20Cu braze filler. Mater. Res. Express 2019, 6, 056526. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Zhao, M.Q.; Sun, L.H.; Liu, H.B.; Hu, J.L. Effect of single rare earth element Ce and mixed rare earth element RE on properties of Sn-Zn-Cu system Lead-free solder alloys. Mater. Rev. 2007, 21, 144–146. [Google Scholar]
- Desai, P.D. Thermodynamic Properties of Selected Binary Aluminum Alloy Systems. J. Phys. Chem. Ref. Data 1987, 16, 109–124. [Google Scholar] [CrossRef]
- Hallstedt, B.; Gröbner, J.; Hampl, M.; Schmid-Fetzer, R. Calorimetric measurements and assessment of the binary Cu-Si and ternary Al-Cu-Si phase diagrams. CALPHAD: Comput. Coupling Phase Diagr. Thermochem. 2016, 53, 25–38. [Google Scholar] [CrossRef]
- Qin, Q.D.; Zhao, Y.G.; Liu, C.; Zhou, W.; Jiang, Q.C. Development of aluminium composites with in situ formed AlTiSi reinforcements through infiltration. Mater. Sci. Eng. A 2007, 460, 604–610. [Google Scholar] [CrossRef]
- Samuel, A.M.; Elgallad, E.M.; Mahmoud, M.G.; Doty, H.W.; Valtierra, S.; Samuel, F.H. Rare Earth Metal-Based Intermetallics Formation in Al–Cu–Mg and Al–Si–Cu–Mg Alloys: A Metallographic Study. Adv. Mater. Sci. Eng. 2018, 2018, 7607465. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Zheng, L.; Chen, Z.P.; Zhang, J.Y. Effect of electromagnetic stirring way and rare earth on solidification structure of semi-solid A356 alloy. Nanoferrous Met. Sci. Eng. 2017, 8, 76–82. [Google Scholar] [CrossRef]
- Saleema, N.; Sarkar, D.K.; Paynter, R.W.; Gallant, D.; Eskandarian, M. A simple surface treatment and characterization of AA 6061 aluminum alloy surface for adhesive bonding applications. Appl. Surf. Sci. 2012, 261, 742–748. [Google Scholar] [CrossRef] [Green Version]
- Abid, J.; Raza, H.; Akhtar, A.; Gohar, G.A.; Ullah, S.; Akram, M.; Raza, Y.; Bukhari, M.D. Effect of Surface Roughness on Shear Strength of Bonded Joints of Aluminum AL 6061 T6 substrate. VW Appl. Sci. 2020, 2, 87–91. [Google Scholar] [CrossRef]
- Ahmad, R.; Bakar, M.A. Effect of a post-weld heat treatment on the mechanical and microstructure properties of AA6061 joints welded by the gas metal arc welding cold metal transfer method. Mater. Des. 2011, 32, 5120–5126. [Google Scholar] [CrossRef]

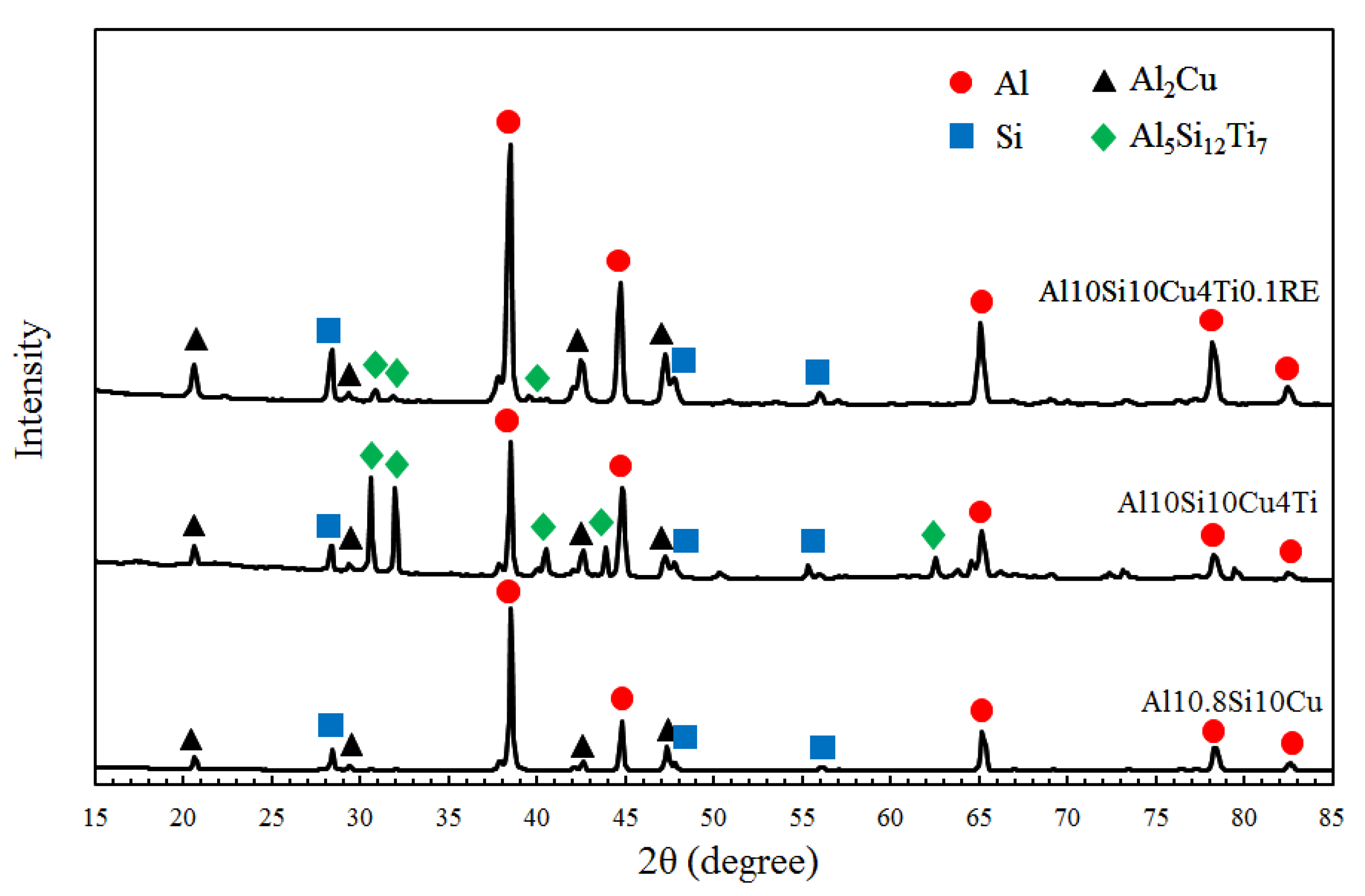
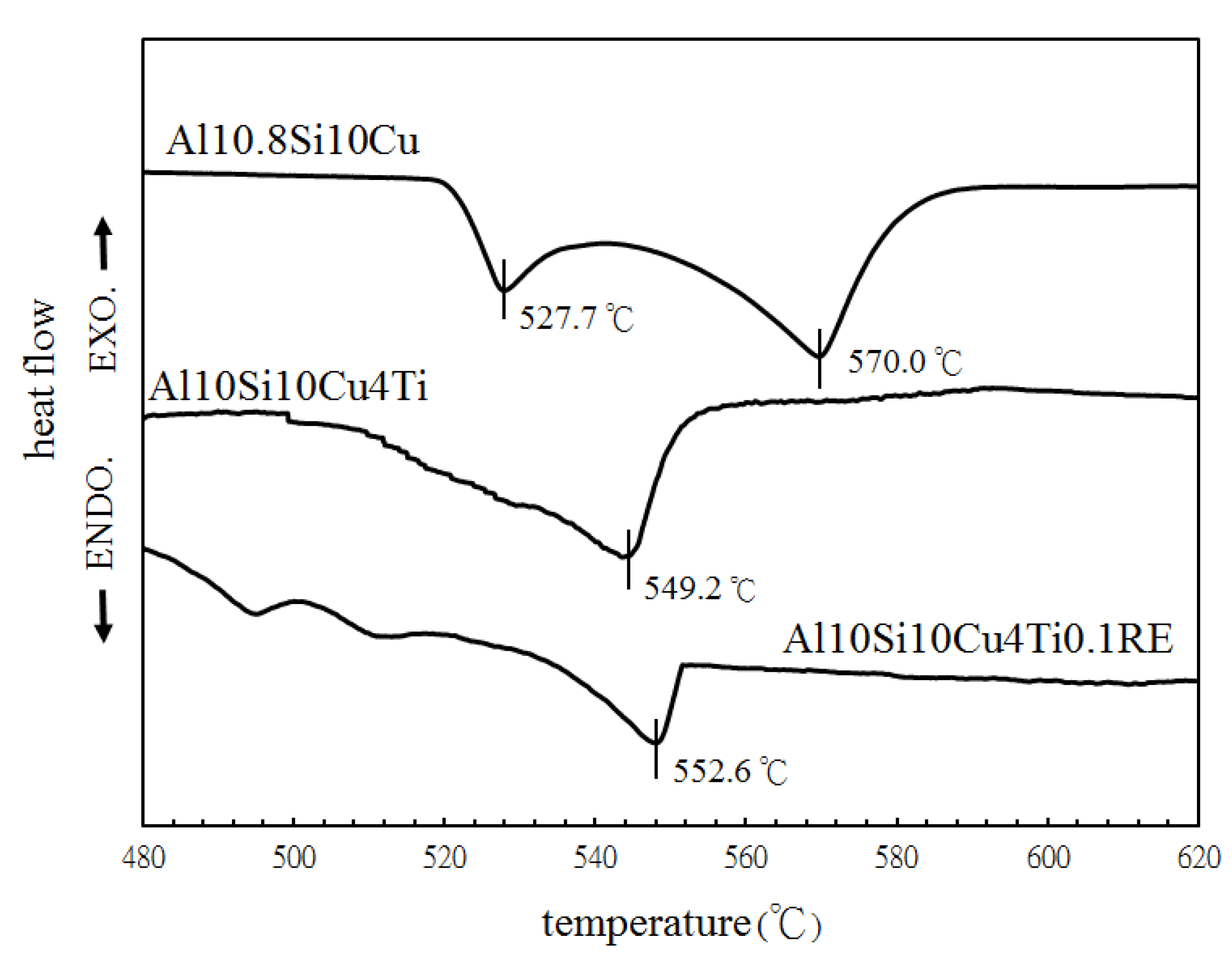
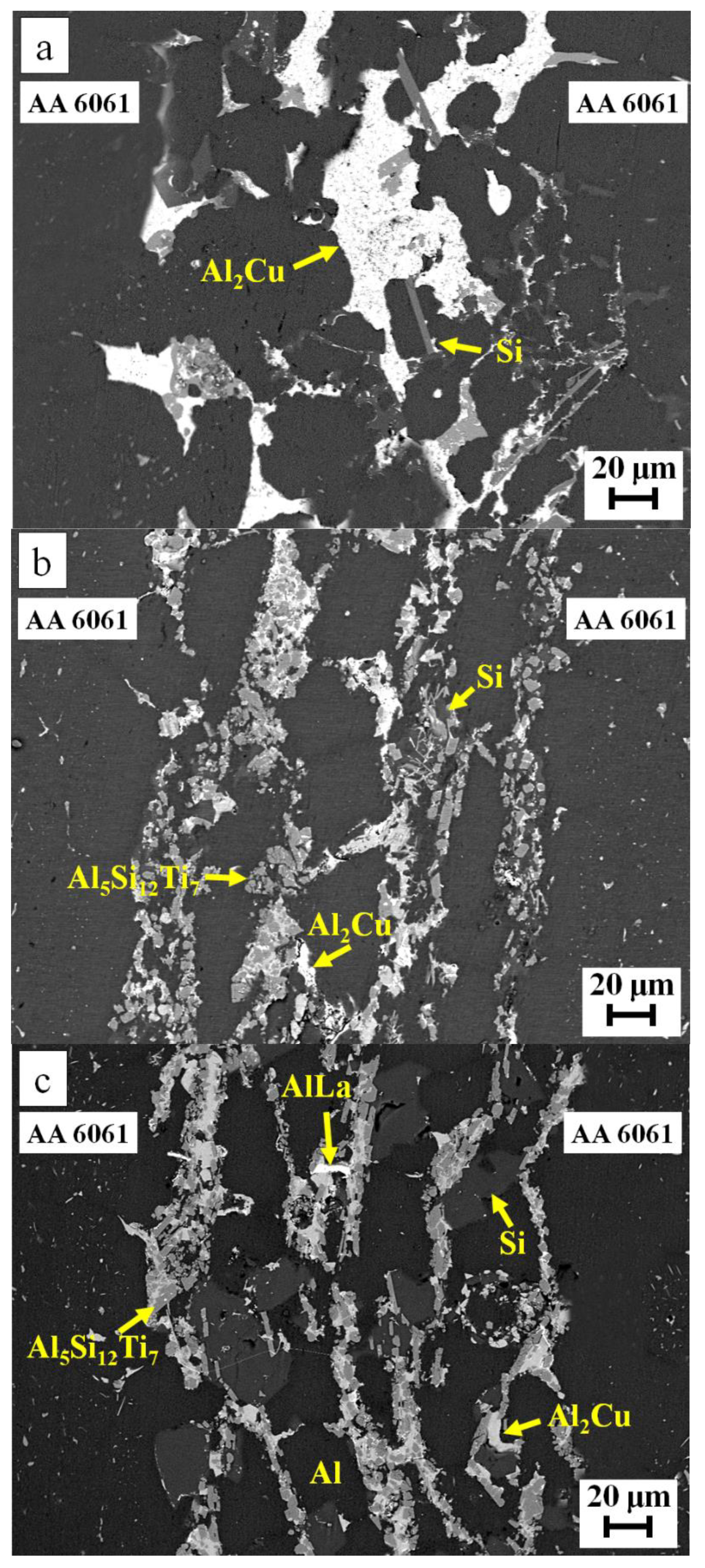
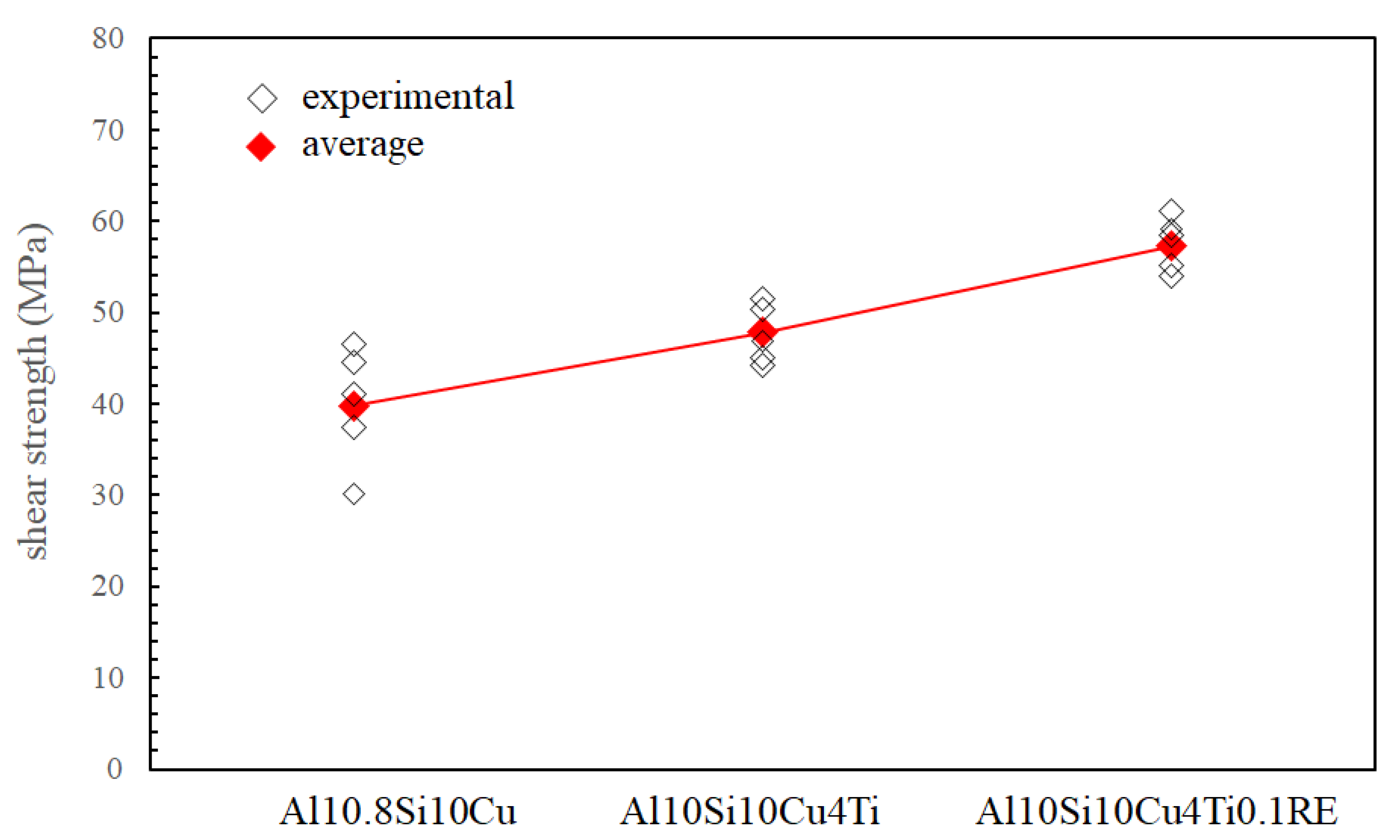


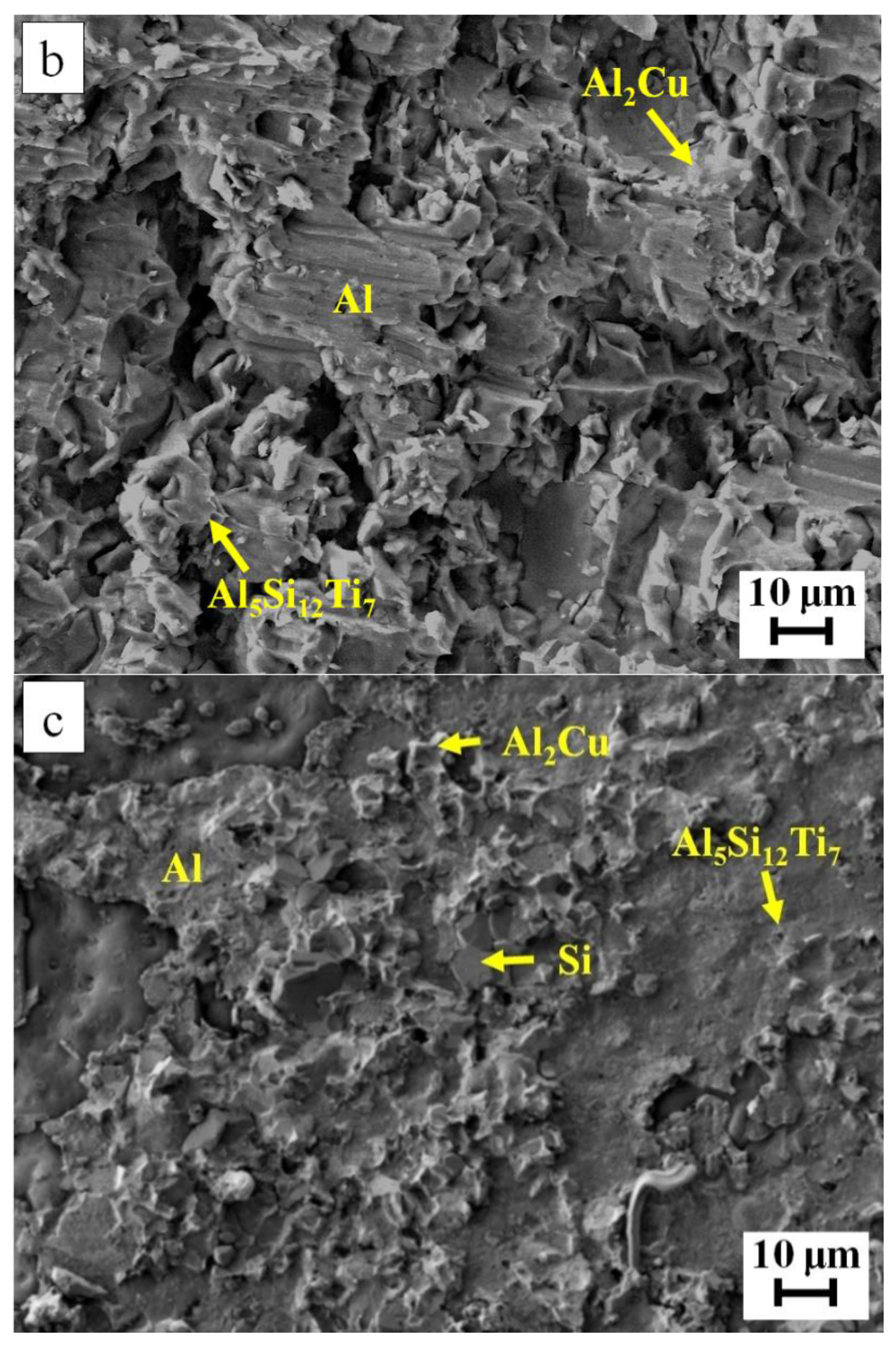
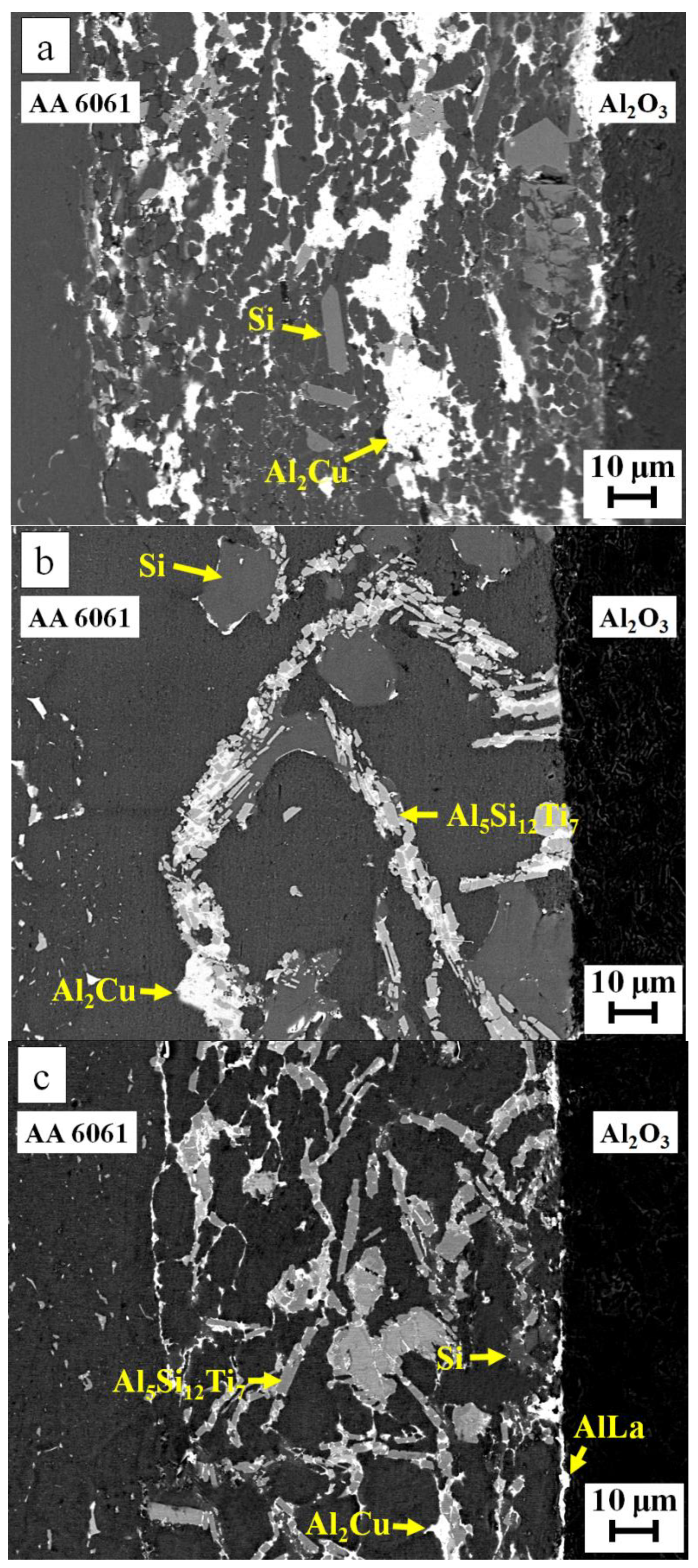
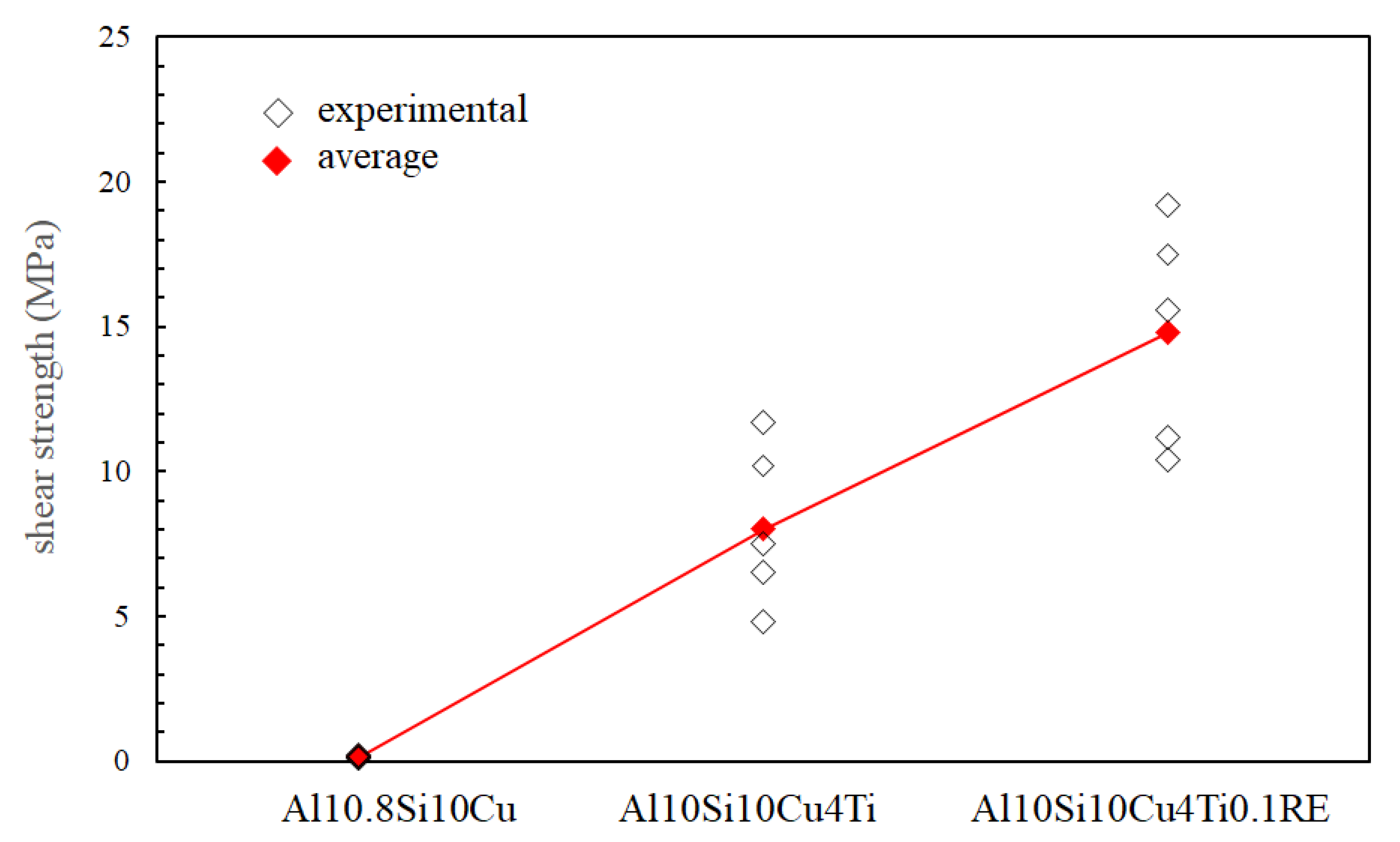
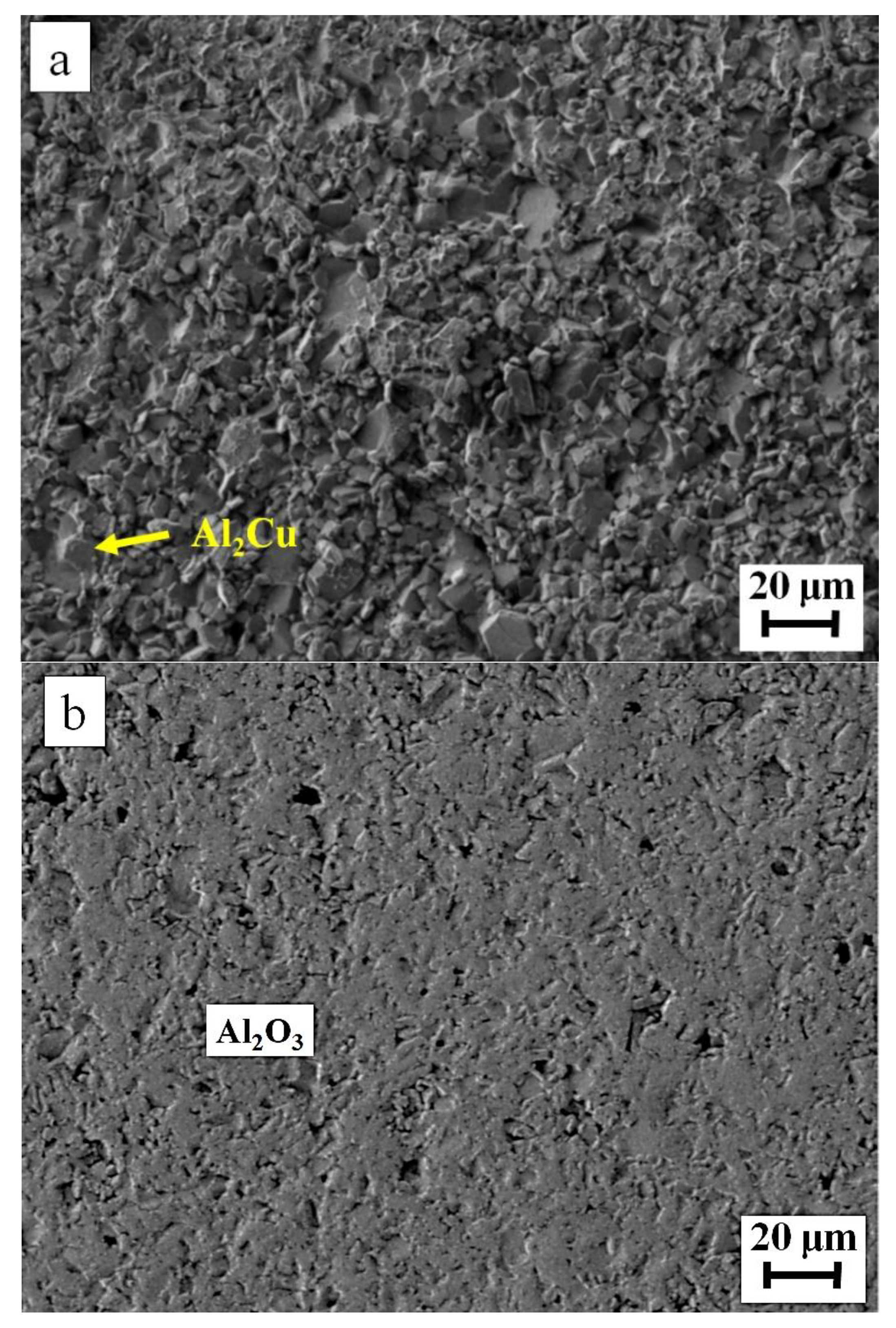
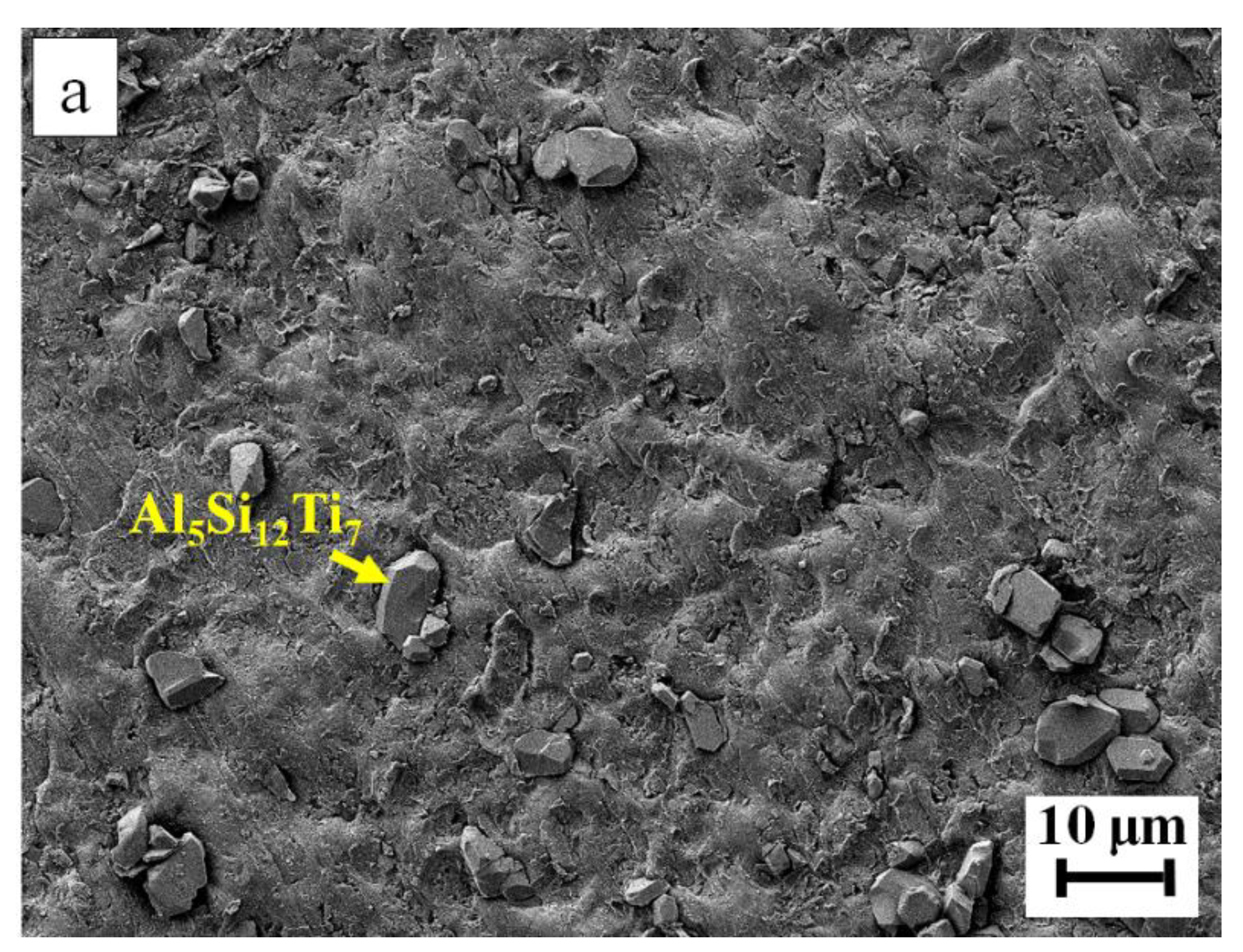
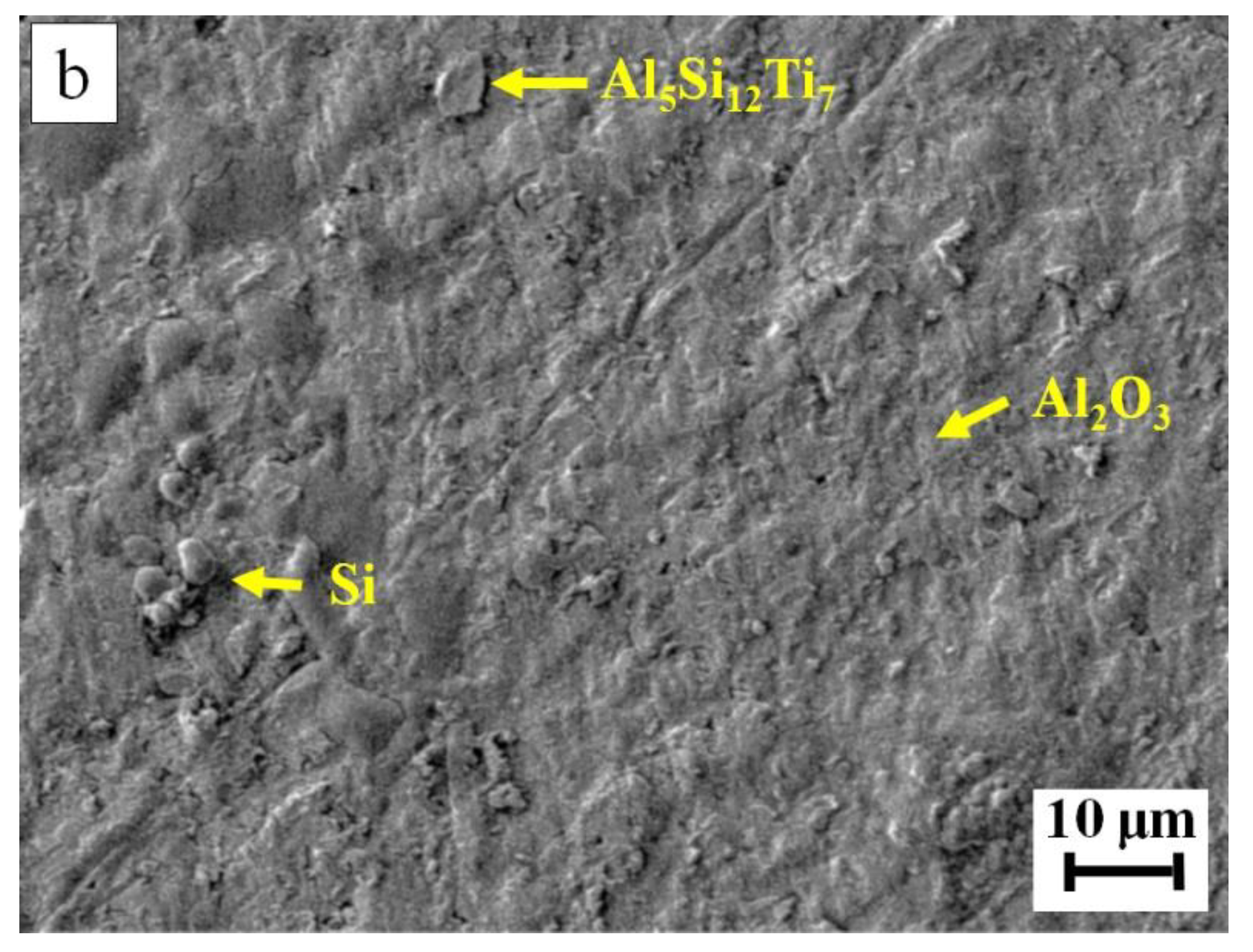

| Filler Alloy | Al | Si | Cu | Ti | La | Pr | Ce | Ne |
|---|---|---|---|---|---|---|---|---|
| Al10.8Si10Cu | Bal. | 10.8 | 10.0 | --- | --- | --- | --- | --- |
| Al10Si10Cu4Ti | Bal. | 10.0 | 10.0 | 4.0 | --- | --- | --- | --- |
| Al10Si10Cu4Ti0.1RE | Bal. | 10.0 | 10.0 | 4.0 | 0.078 | 0.017 | 0.003 | 0.002 |
| Al | Si | Cr | Cu | Fe | Mg | Mn | Ti | Zn |
|---|---|---|---|---|---|---|---|---|
| Bal. | 0.65 | 0.17 | 0.24 | 0.39 | 0.91 | 0.31 | 0.01 | 0.04 |
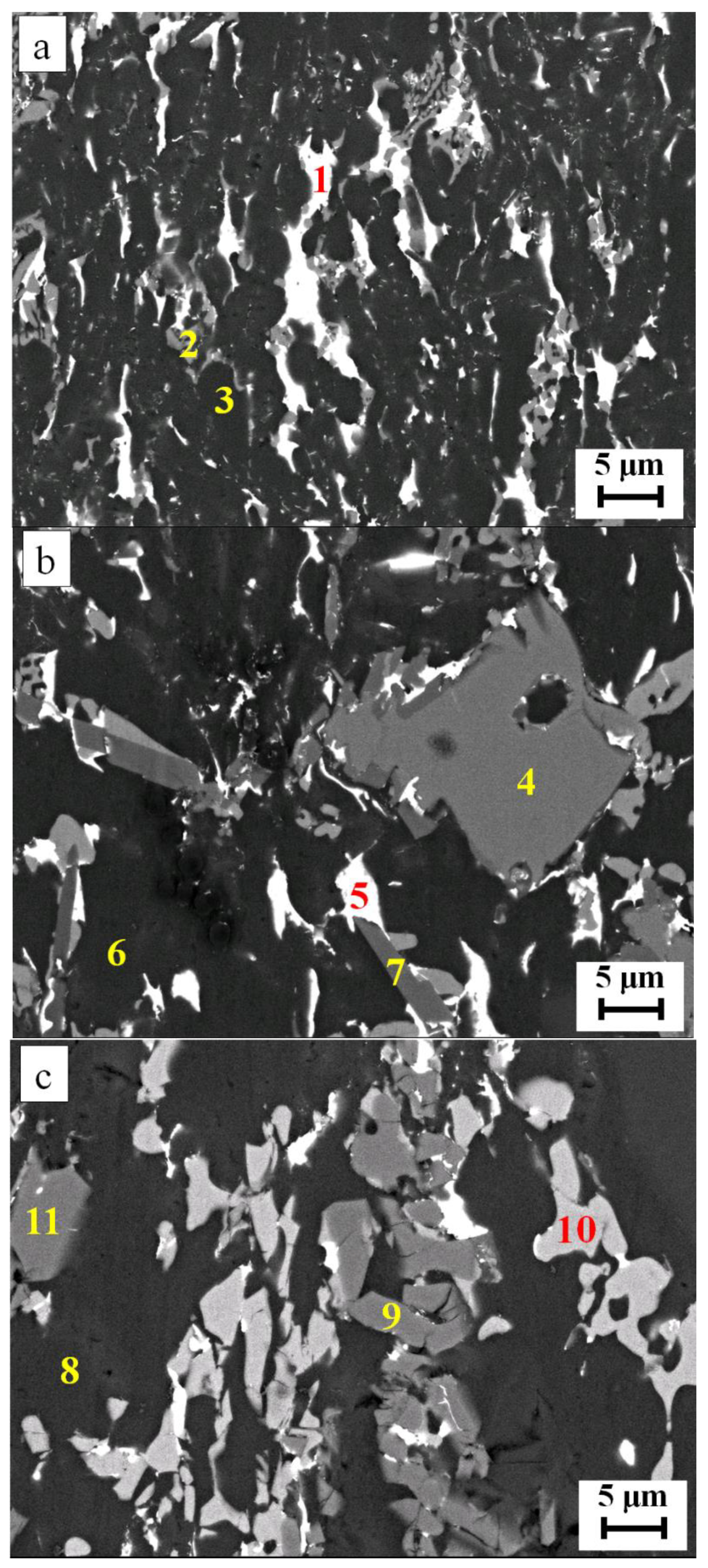
| Spot | Al | Si | Cu | Ti | Possible Phase |
|---|---|---|---|---|---|
| 1 | 66.3 | --- | 33.7 | --- | Al2Cu |
| 2 | 1.8 | 98.2 | --- | --- | Si-rich |
| 3 | 97.5 | 1.7 | 0.8 | --- | Al-rich |
| 4 | 0.7 | 99.3 | --- | --- | Si-rich |
| 5 | 64.0 | --- | 36.0 | --- | Al2Cu |
| 6 | 98.3 | 1.0 | 0.7 | --- | Al-rich |
| 7 | 14.8 | 55.0 | --- | 30.2 | Al5Si12Ti7 |
| 8 | 98.2 | 1.3 | 0.5 | --- | Al-rich |
| 9 | 14.6 | 55.7 | --- | 29.7 | Al5Si12Ti7 |
| 10 | 65.1 | --- | 34.9 | --- | Al2Cu |
| 11 | 1.1 | 98.9 | --- | --- | Si-rich |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.-K.; Chang, S.-Y.; Tsao, L.-C.; Chuang, T.-H.; Zhang, G.-Z.; Yeh, C.-Y. Effects of Adding Active Elements to Aluminum-Based Filler Alloys on the Bonding of 6061 Aluminum Alloy and Alumina. Appl. Sci. 2021, 11, 10440. https://doi.org/10.3390/app112110440
Sun Y-K, Chang S-Y, Tsao L-C, Chuang T-H, Zhang G-Z, Yeh C-Y. Effects of Adding Active Elements to Aluminum-Based Filler Alloys on the Bonding of 6061 Aluminum Alloy and Alumina. Applied Sciences. 2021; 11(21):10440. https://doi.org/10.3390/app112110440
Chicago/Turabian StyleSun, Yu-Kai, Shih-Ying Chang, Lung-Chuan Tsao, Tung-Han Chuang, Guo-Zhan Zhang, and Chih-Yi Yeh. 2021. "Effects of Adding Active Elements to Aluminum-Based Filler Alloys on the Bonding of 6061 Aluminum Alloy and Alumina" Applied Sciences 11, no. 21: 10440. https://doi.org/10.3390/app112110440





