Global Dynamics of a Stochastic Viral Infection Model with Latently Infected Cells
Abstract
:1. Introduction
2. Model Formulation
- (i).
- and for some , for every
- (ii).
- such that
- the diffusion matrix is strictly positive definite for all .
- there exists a non-negative function such that is negative for any .
3. Existence of Ergodic Stationary Distribution
4. Extinction
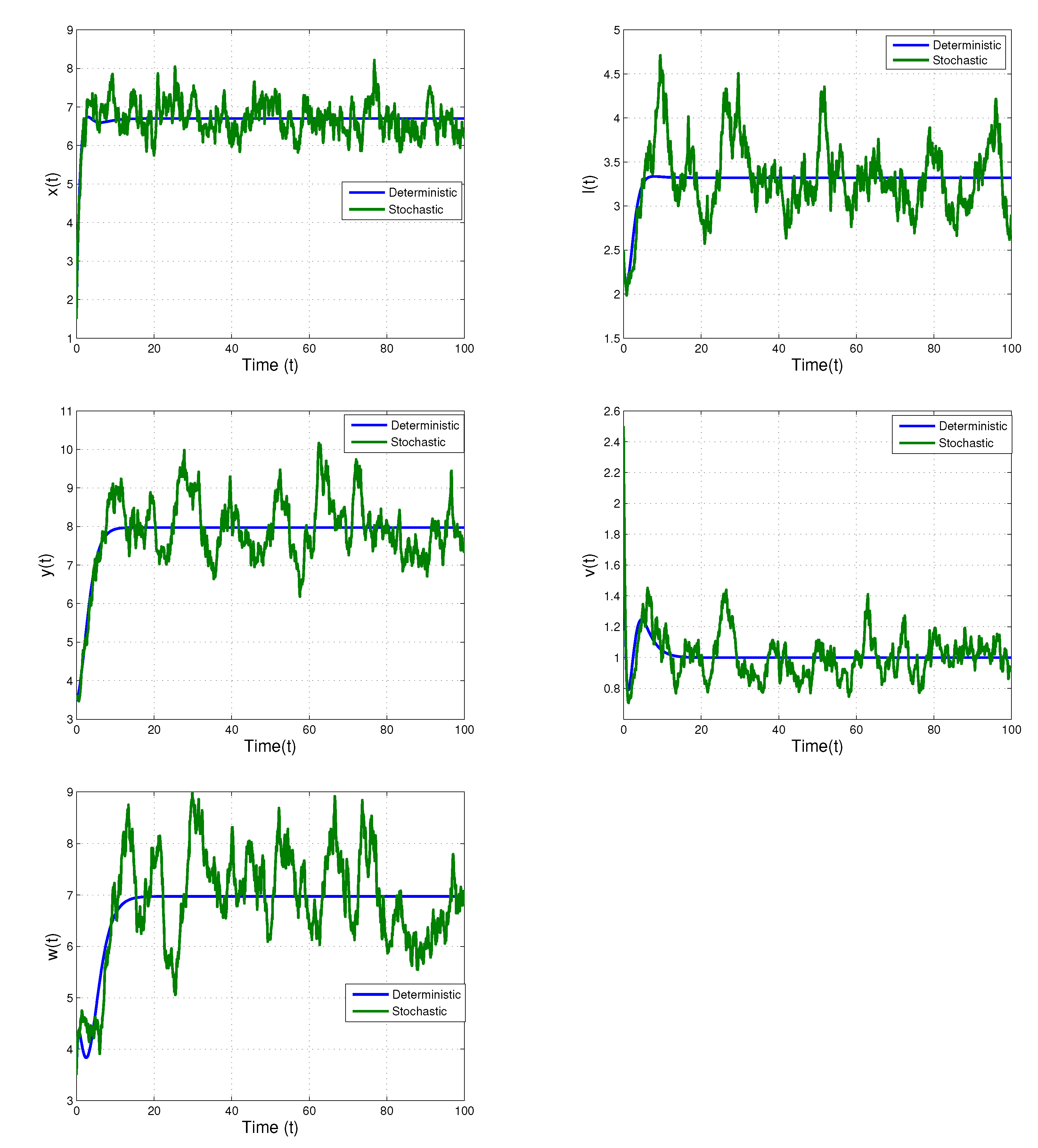
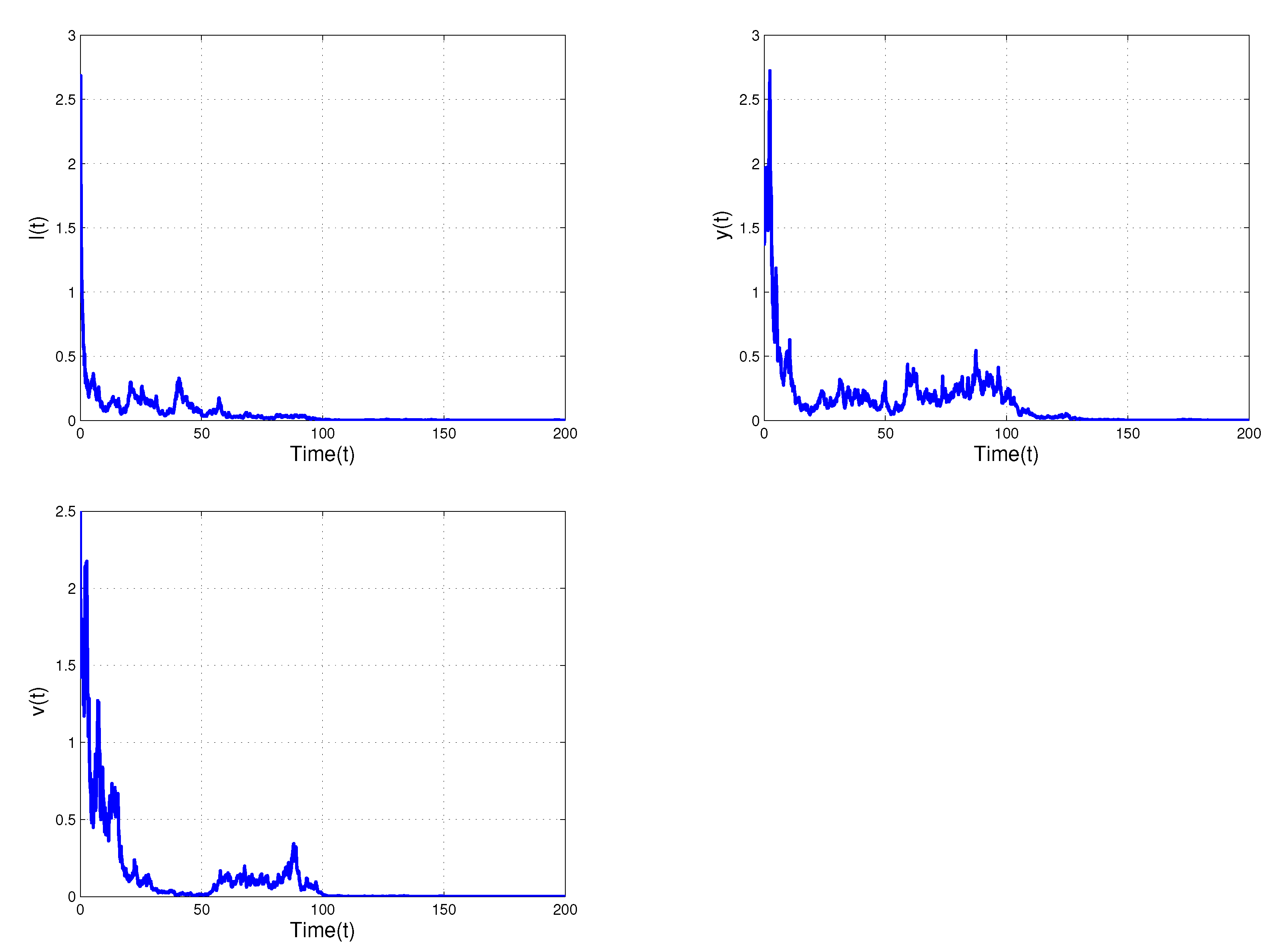
5. Numerical Simulations
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, C.; Miao, H.; Chen, X. Global stability analysis for a delayed HIV infection model with general incidence rate and cell immunity. Engin. Lett. 2016, 24, 392–398. [Google Scholar]
- Rihan, F.A.; Velmurugan, G. Dynamics and Sensitivity of Fractional-Order Delay Differential Model for Coronavirus (COVID-19) Infection. Prog. Fract. Differ. Appl. 2021, 7, 43–61. [Google Scholar]
- Rihan, F.; Alsakaji, H. Dynamics of a stochastic delay differential model for COVID-19 infection with asymptomatic infected and interacting peoples: Case study in the UAE. Results Phys. 2021, 28, 104658. [Google Scholar] [CrossRef]
- Dahari, H.; Loa, A.; Ribeiroa, R.; Perelson, A. Modeling hepatitis C virus dynamics: Liver regeneration and critical drug efficacy. J. Theor. Biol. 2007, 247, 371–381. [Google Scholar] [CrossRef] [Green Version]
- Rihan, F.A.; Arafa, A.A.; Rakkiyappan, R.; Rajivganthi, C.; Xu, Y. Fractional-order delay differential equations for thedynamics of hepatitis C virus infection with IFN-atreatment. Alex. Eng. J. 2021, 60, 4761–4774. [Google Scholar] [CrossRef]
- Yousfi, N.; Hattaf, K.; Tridane, A. Modeling the adaptative immune response in HBV infection. J. Math. Biol. 2011, 63, 933–957. [Google Scholar] [CrossRef]
- Stilianakis, N.I.; Seydel, J. Modeling the T-cell dynamics and pathogenesis of HTLV-I infection. Bull. Math. Biol. 1999, 61, 935–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Tang, S.; Song, X.; Rong, L. Mathematical analysis of an HIV latent infection model including both virus-to-cell infection and cell-to-cell transmission. J. Bio. Dyn. 2017, 11, 455–483. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Lou, J. The global dynamics of a model about HIV-1 infection in vivo. Ric. Mat. 2009, 58, 77–90. [Google Scholar] [CrossRef]
- Pan, S.; Chakrabarty, S.P. Threshold dynamics of HCV model with cell-to-cell transmission and a non-cytolytic cure in the presence of humoral immunity. Commun. Nonlinear Sci. Numer. Simulat. 2018, 61, 180–197. [Google Scholar] [CrossRef]
- Elaiw, A.M.; AlShamrani, N.H. Global properties of nonlinear humoral immunity viral infection models. Int. J. Biomath. 2015, 8, 1550058. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, L.; Zheng, T.; Teng, Z. Analysis of a diffusive virus infection model with humoral immunity, cell-to-cell transmission and nonlinear incidence. Phys. A 2019, 535, 122415. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, M.; Jiang, D. Viral dynamics of a latent HIV infection model with Beddington-DeAngelis incidence function, B-cell immune response and multiple delays. Math. Biosci. Eng. 2021, 18, 274–299. [Google Scholar] [CrossRef]
- Hattaf, K. Global stability and Hopf bifurcation of a generalized viral infection model with multi-delays and humoral immunity. Phys. A 2020, 545, 123689. [Google Scholar] [CrossRef]
- Rihan, F.A.; Rajivganthi, C. Dynamics of Tumor-Immune System with Random Noise. Mathematics 2021, 9, 2707. [Google Scholar] [CrossRef]
- Hui, H.; Nie, L.F. Analysis of a stochastic HBV infection model with nonlinear incidence rate. J. Bio. Syst. 2019, 27, 399–421. [Google Scholar] [CrossRef]
- Ji, C. The stationary distribution of hepatitis B virus with stochastic perturbation. Appl. Math. Lett. 2020, 100, 106017. [Google Scholar] [CrossRef]
- Rihan, F.A.; Alsakaji, H.J.; Rajivganthi, C. Stochastic SIRC epidemic model with time-delay for COVID-19. Adv. Differ. Equ. 2020, 2020, 502. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, K.; Jiang, D. An HIV latent infection model with cell-to-cell transmission and stochastic perturbation. Chaos Solitons Fractals 2021, 151, 111215. [Google Scholar] [CrossRef]
- Din, A.; Li, Y.; Yusuf, A. Delayed hepatitis B epidemic model with stochastic analysis. Chaos Solitons Fractals 2021, 146, 110839. [Google Scholar] [CrossRef]
- Sun, J.; Gao, M.; Jiang, D. Threshold Dynamics of a Non-Linear Stochastic Viral Model with Time Delay and CTL Responsiveness. Life 2021, 11, 766. [Google Scholar] [CrossRef]
- Rihan, F.A.; Alsakaji, H.J. Analysis of a stochastic HBV infection model with delayed immune response. Math. Biosci. Eng. 2021, 18, 5194–5220. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, W.; Liu, X. Global stability in a viral infection model with lytic and nonlytic immune responses. Comput. Math. Appl. 2006, 51, 1593–1610. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Wang, W.; Liu, X. Viral infection model with periodic lytic immune response. Chaos Solitons Fractals 2006, 28, 90–99. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W. An HIV infection model based on a vectored immunoprophylaxis experiment. J. Theoret. Biol. 2012, 313, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Wodarz, D. Hepatitis C virus dynamics and pathology: The role of CTL and antibody responses. J. Gen. Virol. 2003, 84, 1743–1750. [Google Scholar] [CrossRef]
- Murase, A.; Sasaki, T.; Kajiwara, T. Stability analysis of pathogen-immune interaction dynamics. J. Math. Biol. 2005, 51, 247–267. [Google Scholar] [CrossRef]
- Rihan, F.A. Delay Differential Equations and Applications to Biology; Springer: Singapore, 2021. [Google Scholar]
- Song, X.; Wang, S.; Dong, J. Stability properties and Hopf bifurcation of a delayed viral infection model with lytic immune response. J. Math. Anal. Appl. 2011, 373, 345–355. [Google Scholar] [CrossRef] [Green Version]
- Holling, C.S. The functional response of predators to prey density and its role in mimicry and population regulations. Mem. Entomol. Soc. Can. 1965, 45, 3–60. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Jiang, D.; Hayat, T.; Alsaedi, A. Dynamics of a stochastic tuberculosis model with antibiotic resistance. Chaos Solitons Fractals 2018, 109, 223–230. [Google Scholar] [CrossRef]
- Mao, X. Stochastic Differential Equations and their Applications; Horwood: Chichester, UK, 1997. [Google Scholar]
- Oksendal, B. Stochastic Differential Equations: An Introduction with Applications, 6th ed.; Springer: New York, NY, USA, 2003. [Google Scholar]
- Hasminskii, R.Z. Stochastic Stability of Differential Equations; Sijthoff and Noordhoff: Alphen aan den Rijn, The Netherlands, 1980. [Google Scholar]
- Higham, D.J. An algorithmic introduction to numerical simulation of stochastic differential equations. SIAM Rev. 2001, 43, 525–546. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajivganthi, C.; Rihan, F.A. Global Dynamics of a Stochastic Viral Infection Model with Latently Infected Cells. Appl. Sci. 2021, 11, 10484. https://doi.org/10.3390/app112110484
Rajivganthi C, Rihan FA. Global Dynamics of a Stochastic Viral Infection Model with Latently Infected Cells. Applied Sciences. 2021; 11(21):10484. https://doi.org/10.3390/app112110484
Chicago/Turabian StyleRajivganthi, Chinnathambi, and Fathalla A. Rihan. 2021. "Global Dynamics of a Stochastic Viral Infection Model with Latently Infected Cells" Applied Sciences 11, no. 21: 10484. https://doi.org/10.3390/app112110484
APA StyleRajivganthi, C., & Rihan, F. A. (2021). Global Dynamics of a Stochastic Viral Infection Model with Latently Infected Cells. Applied Sciences, 11(21), 10484. https://doi.org/10.3390/app112110484






