An Investigation of Surface-Enhanced Raman Scattering of Different Analytes Adsorbed on Gold Nanoislands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Gold Nanoislands as SERS Substrates
2.2. Characterization of SERS Substrates
2.3. Sample Preparation for SERS Measurements
2.4. SERS Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rostron, P.; Gaber, S.; Gaber, D. Raman Spectroscopy, Review. Int. J. Eng. Tech. Res. (IJETR) 2016, 6, 50–64. [Google Scholar]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muehlethaler, C.; Leona, M.; Lombardi, J.R. Towards a validation of surface-enhanced Raman scattering (SERS) for use in forensic science: Repeatability and reproducibility experiments. Forensic Sci. Int. 2016, 268, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ouhibi, A.; Saadaoui, M.; Lorrain, N.; Guendouz, M.; Raouafi, N.; Moadhen, A. Application of Doehlert Matrix for an Optimized Preparation of a Surface-Enhanced Raman Spectroscopy (SERS) Substrate Based on Silicon Nanowires for Ultrasensitive Detection of Rhodamine 6G. Appl. Spectrosc. 2020, 74, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Stockman, M.I. Electromagnetic Theory of SERS. Surf.-Enhanc. Raman Scatt. Phys. Appl. 2006, 103, 47–66. [Google Scholar]
- Otto, A. The “chemical” (electronic) contribution to surface-enhanced Raman scattering. J. Raman Spectrosc. 2005, 36, 497–509. [Google Scholar] [CrossRef]
- Otto, A. Charge transfer in first layer enhanced Raman scattering and surface resistance. Q. Phys. Rev. 2017, 3, 1–14. [Google Scholar]
- Mosier-Boss, P.A. Review of SERS substrates for Chemical Sensing. Nanomaterials 2017, 7, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, M.; Andrade, G.F.S.; Brolo, A.G. A review on the fabrication of substrates for surface enhanced Raman spectroscopy and their applications in analytical chemistry. Anal. Chem. Acta 2011, 693, 7–25. [Google Scholar] [CrossRef]
- Bora, T. Recent Developments on Metal Nanoparticles for SERS. In Applications Noble and Precious Metals—Properties, Nanoscale Effects and Applications; IntechOpen: London, UK, 2018; pp. 117–133. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.-Y.; Yi, J.; Li, J.-F.; Ren, B.; Wu, D.-Y.; Panneerselvam, R.; Tian, Z.-Q. Nanostructure-based plasmonenhanced Raman spectroscopy for surface analysis of materials. Nat. Rev. Mater. 2016, 1, 1–16. [Google Scholar] [CrossRef]
- Pal, P.; Bonyár, A.; Veres, M.; Himics, L.; Balázs, L.; Juhász, L.; Csarnovics, I. A generalized exponential relationship between the surface-enhanced Raman scattering (SERS) efficiency of gold/silver nanoisland arrangements and their non-dimensional interparticle distance/particle diameter ratio. Sens. Actuators A Phys. 2020, 314, 112225. [Google Scholar] [CrossRef]
- Israelsen, N.D.; Hanson, C.; Vargis, E. Nanoparticle Properties and Synthesis Effects on Surface-Enhanced Raman Scattering Enhancement Factor: An Introduction. Sci. World J. 2015, 2015, 124582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, V.; Matschulat, A.; Polte, J.; Rolf, S.; Emmerling, F.; Kneipp, J. SERS enhancement of gold nanospheres of defined size. J. Raman Spectrosc. 2011, 42, 1736–1742. [Google Scholar] [CrossRef]
- Kundu, S. A new route for the formation of Au nanowires and application of shape-selective Au nanoparticles in SERS studies. J. Mater. Chem. C 2013, 1, 831–842. [Google Scholar] [CrossRef]
- Sharma, B.; Cardinal, M.F.; Kleinman, S.L.; Greeneltch, N.G.; Frontiera, R.R.; Blaber, M.G.; Schatz, G.C.; Van Duyne, R.P. High-performance SERS substrates: Advances and challenges. Mater. Res. Soc. 2013, 38, 615–624. [Google Scholar] [CrossRef] [Green Version]
- Gunnarsson, L.; Bjerneld, E.J.; Xu, H.; Petronis, S.; Kasemo, B.; Käll, M. Interparticle coupling effects in nanofabricated substrates for surface-enhanced Raman scattering. Appl. Phys. Lett. 2001, 78, 802–804. [Google Scholar] [CrossRef] [Green Version]
- Belhout, S.A.; Baptista, F.R.; Devereux, S.J.; Parker, A.W.; Ward, A.D.; Quinn, S.J. Preparation of polymer gold nanoparticle composites with tunable plasmon coupling and their application as SERS substrates. Nanoscale 2019, 11, 19884–19894. [Google Scholar] [CrossRef]
- Wang, J.F.; Wu, X.Z.; Xiao, R.; Dong, P.T.; Wang, C.G. Performance-Enhancing Methods for Au Film over Nanosphere Surface-Enhanced Raman Scattering Substrate and Melamine Detection Application. PLoS ONE 2014, 9, e97967. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Liu, L.; Dai, Z.; Liu, J.; Yang, S.; Zhou, L.; Xiao, X.; Jiang, C.; Roy, V.A.L. Low-Cost, Disposable, Flexible and Highly Reproducible Screen Printed SERS Substrates for the Detection of Various Chemicals. Sci. Rep. 2015, 5, 10208. [Google Scholar] [CrossRef]
- Holthoff, E.L.; Stratis-Cullum, D.N.; Hankus, M.E. A Nanosensor for TNT Detection Based on Molecularly Imprinted Polymers and Surface Enhanced Raman Scattering. Sensors 2011, 11, 2700–2714. [Google Scholar] [CrossRef] [Green Version]
- Wackerbarth, H.; Salb, C.; Gundrum, L.; Niederkrüger, M.; Christou, K.; Beushausen, V.; Viöl, W. Detection of Explosives Based on Surface-Enhanced Raman Spectroscopy. Appl. Opt. 2010, 49, 4362–4366. [Google Scholar] [CrossRef]
- Sulk, R.A.; Corcoran, R.C.; Carron, K.T. Surface-Enhanced Raman Scattering Detection of Amphetamine and Methamphetamine by Modification with 2-Mercaptonicotinic Acid. Appl. Spectrosc. 1999, 53, 954–959. [Google Scholar] [CrossRef]
- Kline, N.D.; Tripathi, A.; Mirsafavi, R.; Pardoe, I.; Moskovits, M.; Meinhart, C.; Guicheteau, J.A.; Christesen, S.D.; Fountain, A.W., III. Optimization of Surface-Enhanced Raman Spectroscopy Conditions for Implementation into a Microfluidic Device for Drug Detection. Anal. Chem. 2016, 88, 10513–10522. [Google Scholar] [CrossRef] [PubMed]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, E.A.; Barveen, N.R.; Wang, T.-J.; Kokulnathan, T.; Chang, Y.-H. Development of SERS platform based on ZnO multipods decorated with Ag nanospheres for detection of 4-nitrophenol and rhodamine 6G in real samples. Microchem. J. 2021, 170, 106660. [Google Scholar] [CrossRef]
- Barbillon, G. Fabrication and SERS Performances of Metal/Si and Metal/ZnO Nanosensors: A Review. Coatings 2019, 9, 86. [Google Scholar] [CrossRef] [Green Version]
- Ogundare, S.A.; van Zyl, W.E. Nanocrystalline cellulose as reducing- and stabilizing agent in the synthesis of silver nanoparticles: Application as a surface-enhanced Raman scattering (SERS) substrate. Surf. Interfaces 2018, 13, 1–10. [Google Scholar] [CrossRef]
- Švecová, M.; Ulbrich, P.; Dendisová, M.; Matějka, P. SERS study of riboflavin on green-synthesized silver nanoparticles prepared by reduction using different flavonoids: What is the role of flavonoid used? Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 195, 236–245. [Google Scholar] [CrossRef]
- Liu, F.; Gu, H.; Lin, Y.; Qi, Y.; Dong, X.; Gao, J.; Cai, T. Surface-enhanced Raman scattering study of riboflavin on borohydride-reduced silver colloids: Dependence of concentration, halide anions and pH values. Spectrochim. Acta Part A 2012, 85, 111–119. [Google Scholar] [CrossRef]
- Kokaislová, A.; Matějka, P. Surface-enhanced vibrational spectroscopy of B vitamins: What is the effect of SERS-active metals used? Anal. Bioanal. Chem. 2012, 403, 985–993. [Google Scholar] [CrossRef]
- Strelchuk, V.V.; Kolomys, O.F.; Kaganovich, E.B.; Krishchenko, I.M.; Golichenko, B.O.; Boyko, M.I.; Kravchenko, S.O.; Kruglenko, I.V.; Lytvyn, O.S.; Manoilov, E.G.; et al. Optical Characterization of SERS Substrates Based on Porous Au Films Prepared by Pulsed Laser Deposition. J. Nanomater. 2015, 2015, 203515. [Google Scholar] [CrossRef] [Green Version]
- Dendisova-Vyškovska, M.; Kokaislová, A.; Ončak, M.; Matějka, P. SERS and in situ SERS spectroscopy of riboflavin adsorbed on silver, gold and copper substrates. Elucidation of variability of surface orientation based on both experimental and theoretical approach. J. Mol. Struct. 2012, 1038, 19–28. [Google Scholar] [CrossRef]
- Dustov, M.; Golovina, D.I.; Polyakov, A.Y.; Goldt, A.E.; Eliseev, A.A.; Kolesnikov, E.A.; Sukhorukova, I.V.; Shtansky, D.V.; Grünert, W.; Grigorieva, A.V. Silver Eco-Solvent Ink for Reactive Printing of Polychromatic SERS and SPR Substrates. Sensors 2018, 18, 521. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.K.; Lee, Y.H.; Koh, C.S.L.; Phan-Quang, G.C.; Han, X.; Lay, C.L.; Ling, X.Y.; Sim, H.Y.F.; Kao, Y.-C.; An, Q.; et al. Designing surface-enhanced Raman scattering (SERS) platforms beyond hot-spot engineering: Emerging opportunities in analyte manipulations and hybrid materials. Chem. Soc. Rev. 2019, 48, 731–756. [Google Scholar] [CrossRef]
- Bonyár, A.; Csarnovics, I.; Veres, M.; Himics, L.; Csik, A.; Kaman, J.; Balázs, L.; Kökényesi, S. Investigation of the performance of thermally generated gold nanoislands for LSPR and SERS applications. Sens. Actuators B Chem. 2018, 255, 433–439. [Google Scholar] [CrossRef]
- Clark, D.E. What has polar surface area ever done for drug discovery? Future Med. Chem. 2011, 3, 469–484. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 493570, Riboflavin. 2021. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Riboflavin (accessed on 26 March 2021).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 13806, Rhodamine 6G. 2021. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Rhodamine-6G (accessed on 26 March 2021).
- Van Dyck, C.; Fu, B.; Van Duyne, R.P.; Schatz, G.C.; Ratner, M.A. Deducing the Adsorption Geometry of Rhodamine 6G from the Surface-Induced Mode Renormalization in Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2017, 122, 465–473. [Google Scholar] [CrossRef]
- Monserud, J.H.; Schwartz, D.K. Effects of molecular size and surface hydrophobicity on oligonucleotide interfacial dynamics. Biomacromolecules 2012, 13, 4002–4011. [Google Scholar] [CrossRef] [Green Version]
- Tian, F.; Bonnier, F.; Casey, A.; Shanahan, A.E.; Byrne, H.J. Surface enhanced Raman scattering with gold nanoparticles: Effect of particle shape. Anal. Methods 2014, 6, 9116–9123. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Qiu, C.; Mu, X.; Pang, H.; Chen, X.; Liu, D. Ultrasensitive SERS detection of rhodamine 6G and p-nitrophenol based on electrochemically roughened nano-Au film. Talanta 2019, 210, 120631. [Google Scholar] [CrossRef]
- Xu, D.; Jiang, H.; Yang, W.; Zhang, S.; Chen, J. SERS effect of Rhodamine 6G molecular probe on AgAu alloy nanowire arrays by a solid-state ionics method. Phys. E Low-Dimens. Syst. Nanostruct. 2018, 102, 132–136. [Google Scholar] [CrossRef]
- Volochanskyi, O.; Švecová, M.; Bartůněk, V.; Prokopec, V. Electroless deposition via galvanic displacement as a simple way for the preparation of silver, gold, and copper SERS-active substrates. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126310. [Google Scholar] [CrossRef]

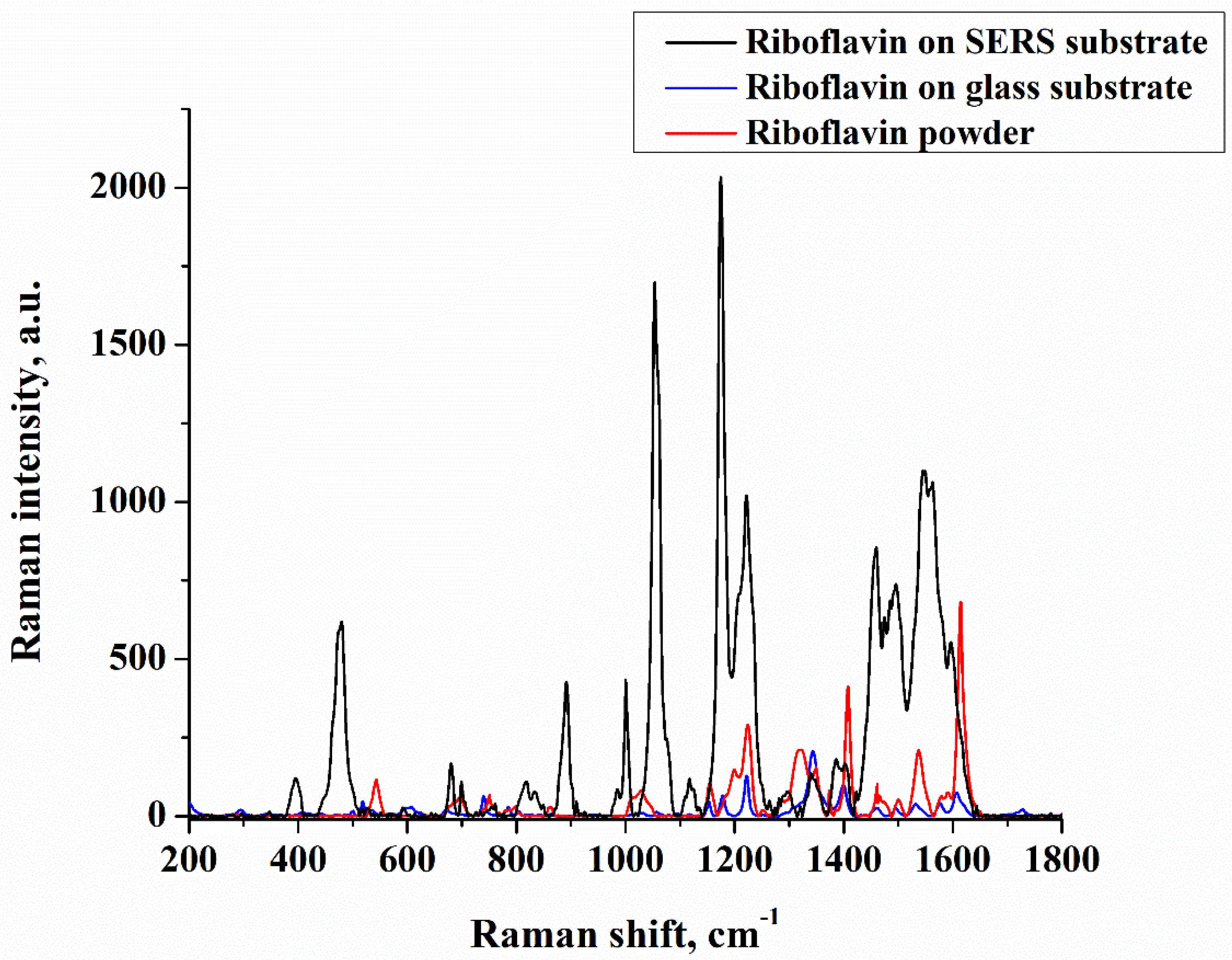
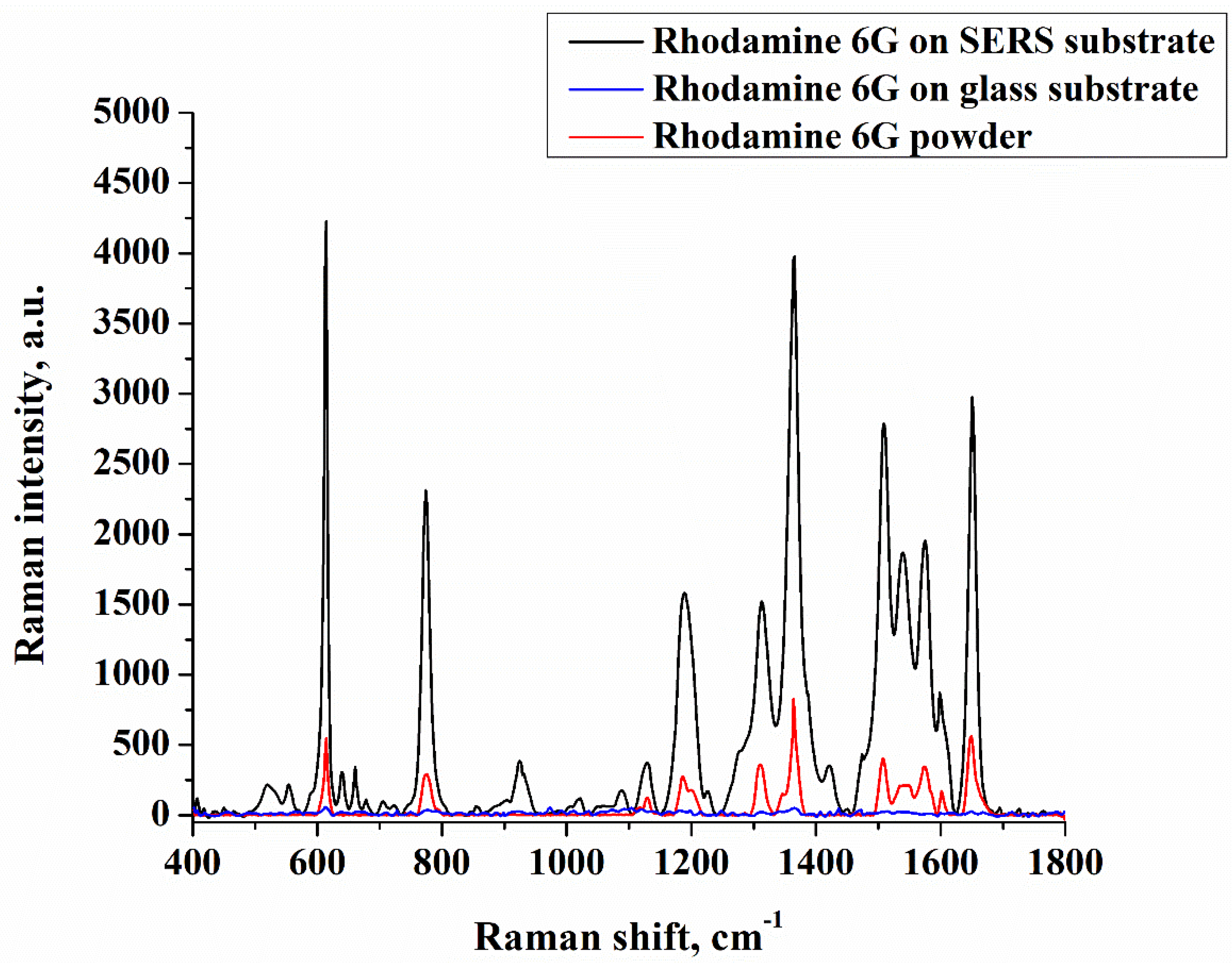
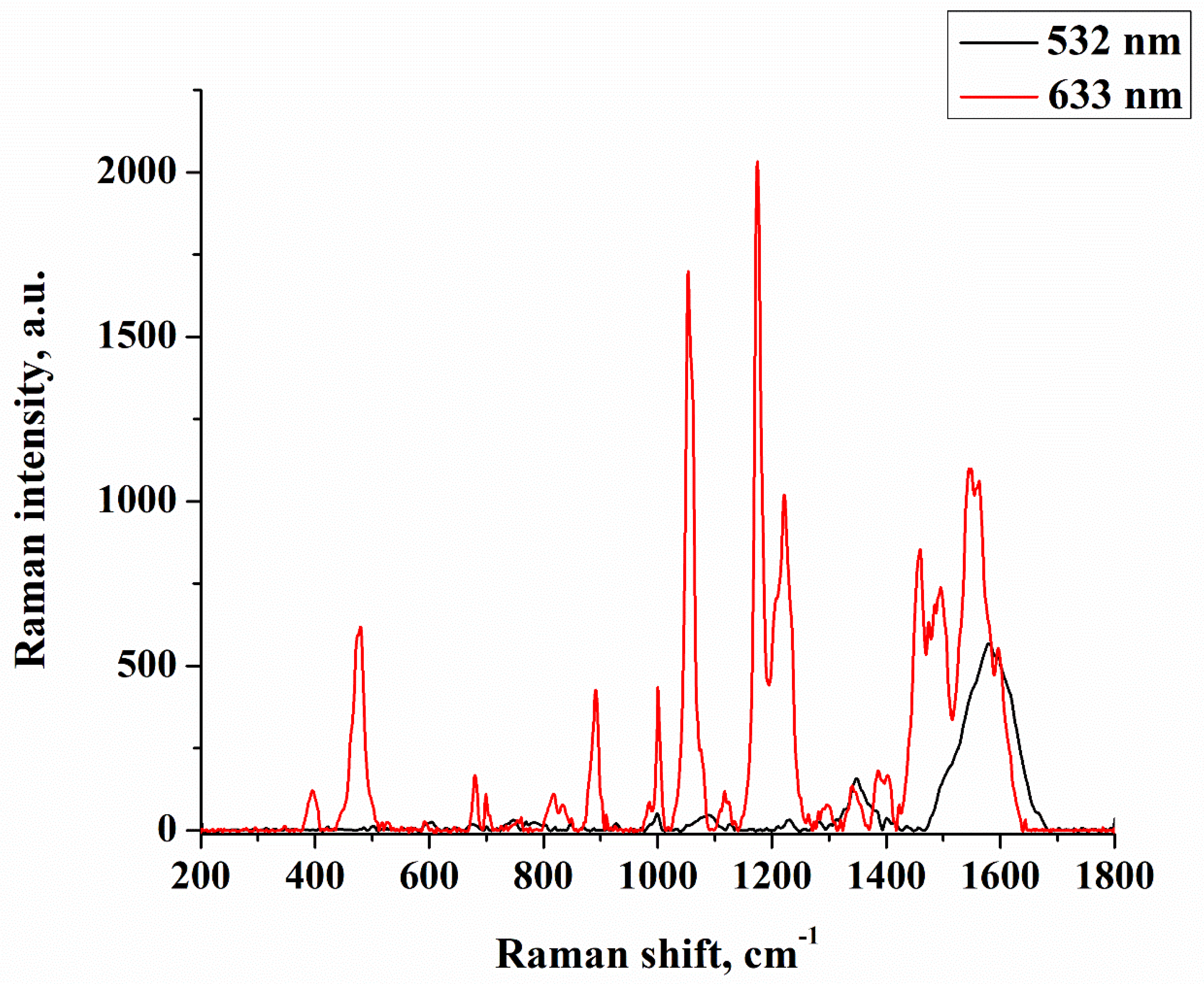


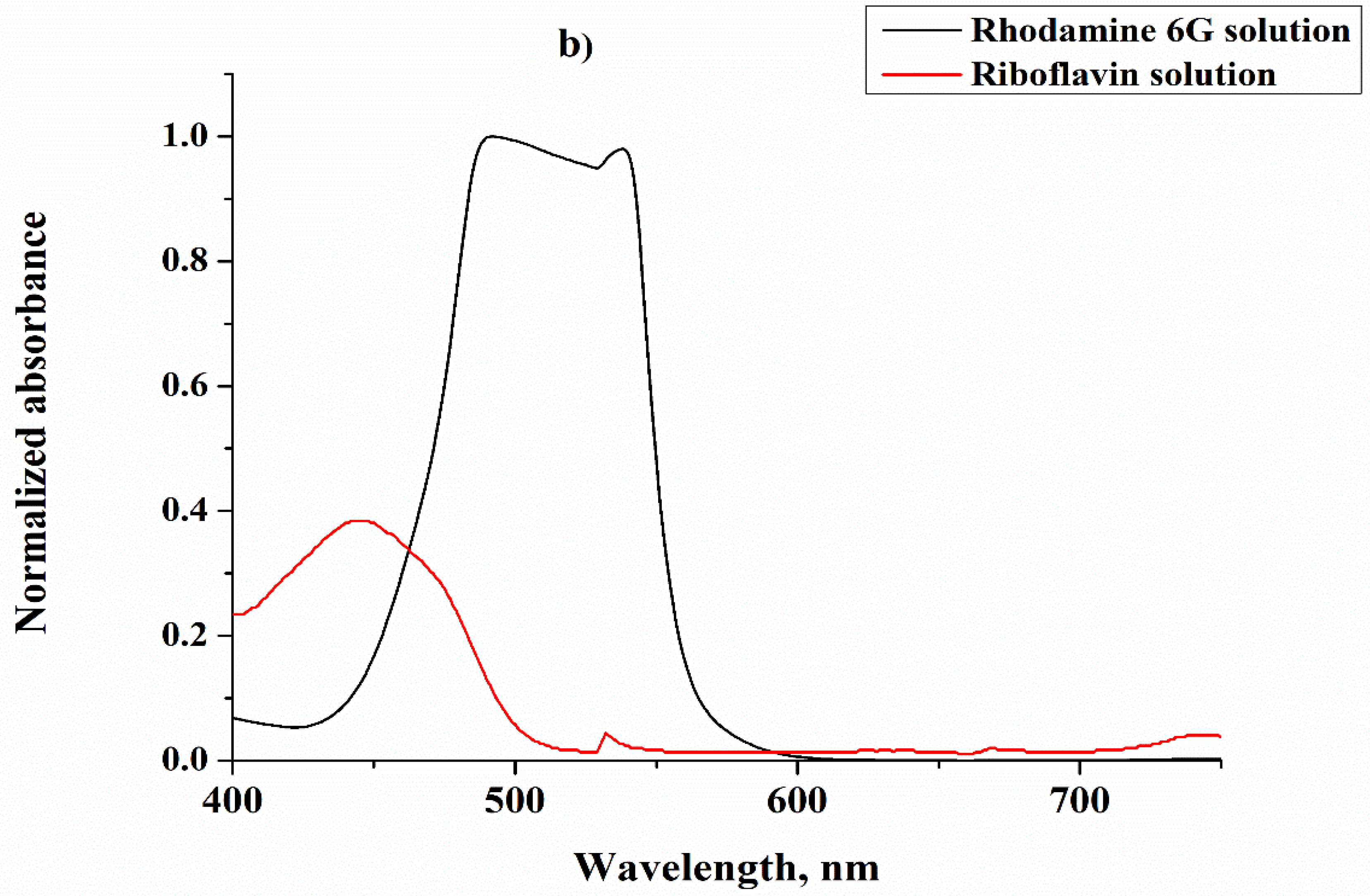
| Sample No. | Initial Layer Thickness (nm) | Annealing Temperature (°C) | Annealing Time (min) |
|---|---|---|---|
| 1 | 9 | 500 | 15 |
| 2 | 9 | 500 | 60 |
| 3 | 9 | 550 | 15 |
| 4 | 12 | 450 | 15 |
| 5 | 9 | 550 | 60 |
| 6 | 12 | 450 | 120 |
| 7 | 9 | 450 | 60 |
| 8 | 12 | 550 | 60 |
| 9 | 12 | 500 | 60 |
| 10 | 12 | 550 | 15 |
| 11 | 12 | 500 | 30 |
| 12 | 9 | 450 | 30 |
| 13 | 9 | 450 | 15 |
| 14 | 9 | 450 | 120 |
| 15 | 12 | 500 | 15 |
| 16 | 9 | 500 | 30 |
| 17 | 9 | 550 | 60 |
| 18 | 12 | 550 | 120 |
| Riboflavin | Rhodamin 6G | |||
|---|---|---|---|---|
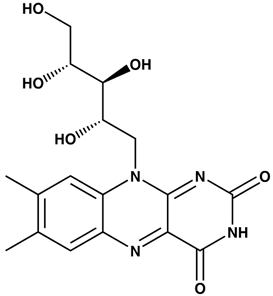 | 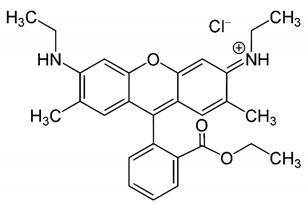 | |||
| Excitation wavelength | 532 nm | 633 nm | 532 nm | 633 nm |
| Laser intensity | 3.2%, 0.9 mW/cm2 | 1%, 0.06 mW/cm2 | 0.1%, 0.03 mW/cm2 | 1%, 0.06 mW/cm2 |
| Measurement time | 30 s | 40 s | 30 s | 30 s |
| Accumulation | 5 | 7 | 10 | 10 |
| Sample No. | Plasmon Wavelength of Gold Nanoisland Substrate with R6G, nm | SERS Enhancement Factor for Different Excitations | Plasmon Wavelength of Gold Nanoisland Substrate with Riboflavin, nm | SERS Enhancement Factor for Different Excitations | ||
|---|---|---|---|---|---|---|
| 532 nm | 633 nm | 532 nm | 633 nm | |||
| 1 | 569 | 59.98 +/− 1.81 | 1.69 +/− 0.04 | 593 | 10.46 +/− 0.15 | 16.28 +/− 0.29 |
| 2 | 545 | 85.31 +/− 2.99 | 31.99 +/− 0.80 | 586 | 14.71 +/− 0.22 | 33.76 +/− 0.89 |
| 3 | 539 | 85.06 +/− 2.98 | 2.67 +/− 0.09 | 570 | 2.04 +/− 0.09 | 1.89 +/− 0.03 |
| 4 | 593 | 145.06 +/− 2.45 | 54.12 +/− 1.89 | 607 | 5.93 +/− 0.23 | 69.82 +/− 1.51 |
| 5 | 532 | 44.85 +/− 1.57 | 1.5 +/− 0.03 | 558 | 1.57 +/− 0.03 | 4.51 +/− 0.08 |
| 6 | 636 | 172.58 +/− 2.59 | 12.30 +/− 0.18 | 643 | 9.81 +/− 0.30 | 207.12 +/− 4.11 |
| 7 | 589 | 87.97 +/− 3.08 | 4.08 +/− 0.06 | 622 | 2.71 +/− 0.11 | 100.48 +/− 1.92 |
| 8 | 548 | 154.11 +/− 5.39 | 28.15 +/− 0.62 | 569 | 2.21 +/− 0.09 | 3.53 +/− 0.07 |
| 9 | 582 | 183.94 +/− 3.68 | 10.96 +/− 0.24 | 600 | 9.65 +/− 0.28 | 22.37 +/− 0.42 |
| 10 | 547 | 259.17 +/− 10.37 | 32.30 +/− 0.71 | 571 | 5.50 +/− 0.20 | 35.64 +/− 0.75 |
| 11 | 557 | 86.20 +/− 3.02 | 1.76 +/− 0.04 | 592 | 28.69 +/− 0.67 | 21.87 +/− 0.52 |
| 12 | 559 | 93.40 +/− 2.34 | 3.79 +/− 0.05 | 595 | 15.64 +/− 0.39 | 27.55 +/− 0.65 |
| 13 | 557 | 158.83 +/− 5.56 | 54.43 +/− 1.72 | 587 | 9.80 +/− 0.35 | 15.29 +/− 0.35 |
| 14 | 556 | 104.82 +/− 2.62 | 6.58 +/− 0.25 | 584 | 16.90 +/− 0.42 | 40.06 +/− 0.82 |
| 15 | 582 | 109.25 +/− 2.73 | 18.09 +/− 0.41 | 613 | 2.82 +/− 0.03 | 7.71 +/− 0.42 |
| 16 | 613 | 61.91 +/− 3.10 | 5.48 +/− 0.21 | 655 | 2.75 +/− 0.04 | 127.75 +/− 2.11 |
| 17 | 540 | 67.35 +/− 3.37 | 22.45 +/− 0.49 | 586 | 12.33 +/− 0.29 | 18.88 +/− 0.42 |
| 18 | 573 | 56.23 +/− 2.53 | 2.54 +/− 0.06 | 627 | 4.63 +/− 0.19 | 76.47 +/− 2.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pál, P.; Bonyár, A.; Veres, M.; Juhász, L.; Szalóki, M.; Csarnovics, I. An Investigation of Surface-Enhanced Raman Scattering of Different Analytes Adsorbed on Gold Nanoislands. Appl. Sci. 2021, 11, 9838. https://doi.org/10.3390/app11219838
Pál P, Bonyár A, Veres M, Juhász L, Szalóki M, Csarnovics I. An Investigation of Surface-Enhanced Raman Scattering of Different Analytes Adsorbed on Gold Nanoislands. Applied Sciences. 2021; 11(21):9838. https://doi.org/10.3390/app11219838
Chicago/Turabian StylePál, Petra, Attila Bonyár, Miklós Veres, Laura Juhász, Melinda Szalóki, and István Csarnovics. 2021. "An Investigation of Surface-Enhanced Raman Scattering of Different Analytes Adsorbed on Gold Nanoislands" Applied Sciences 11, no. 21: 9838. https://doi.org/10.3390/app11219838







