Influence of Oxygen Admixture on Plasma Nitrocarburizing Process and Monitoring of an Active Screen Plasma Treatment
Abstract
:1. Introduction
2. Materials and Methods
3. Results
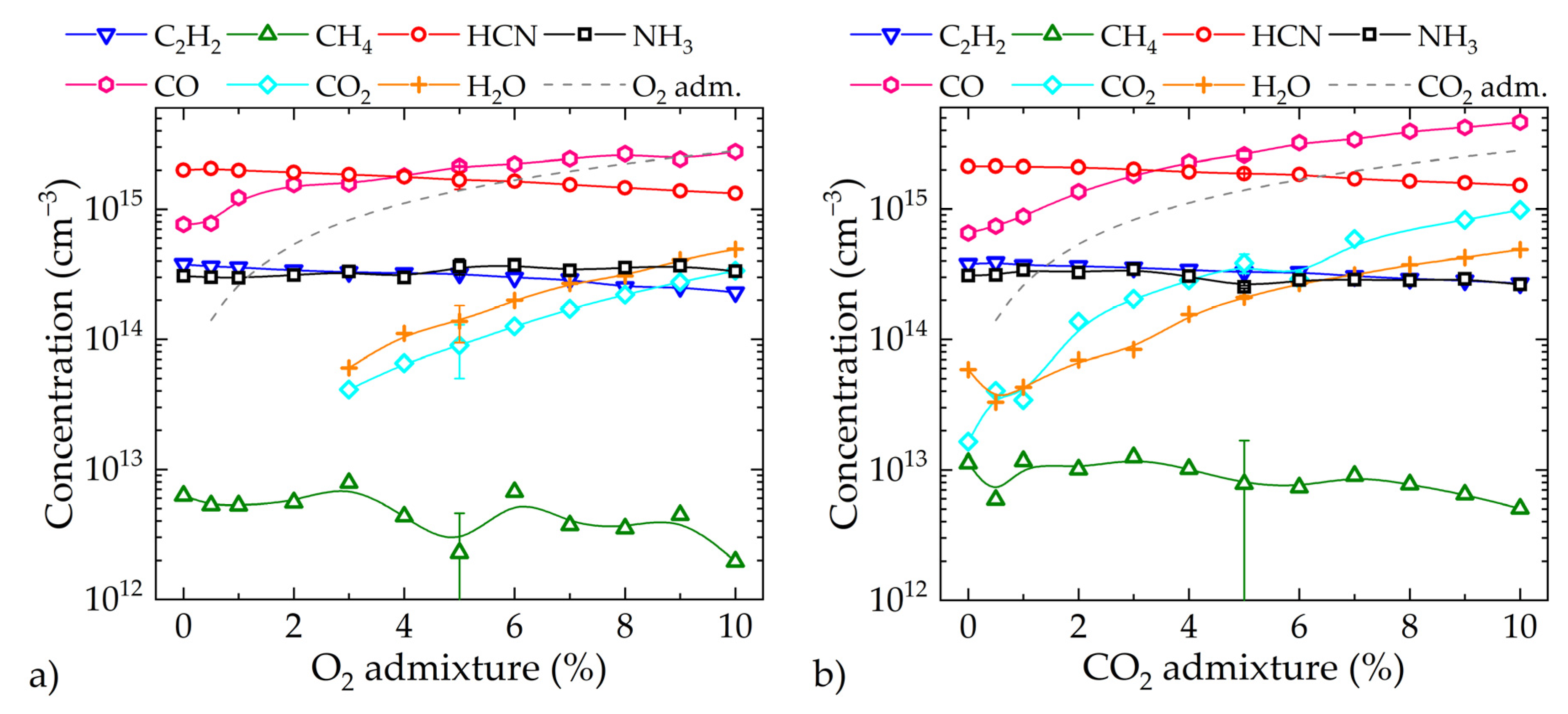
3.1. Spectroscopic Investigations
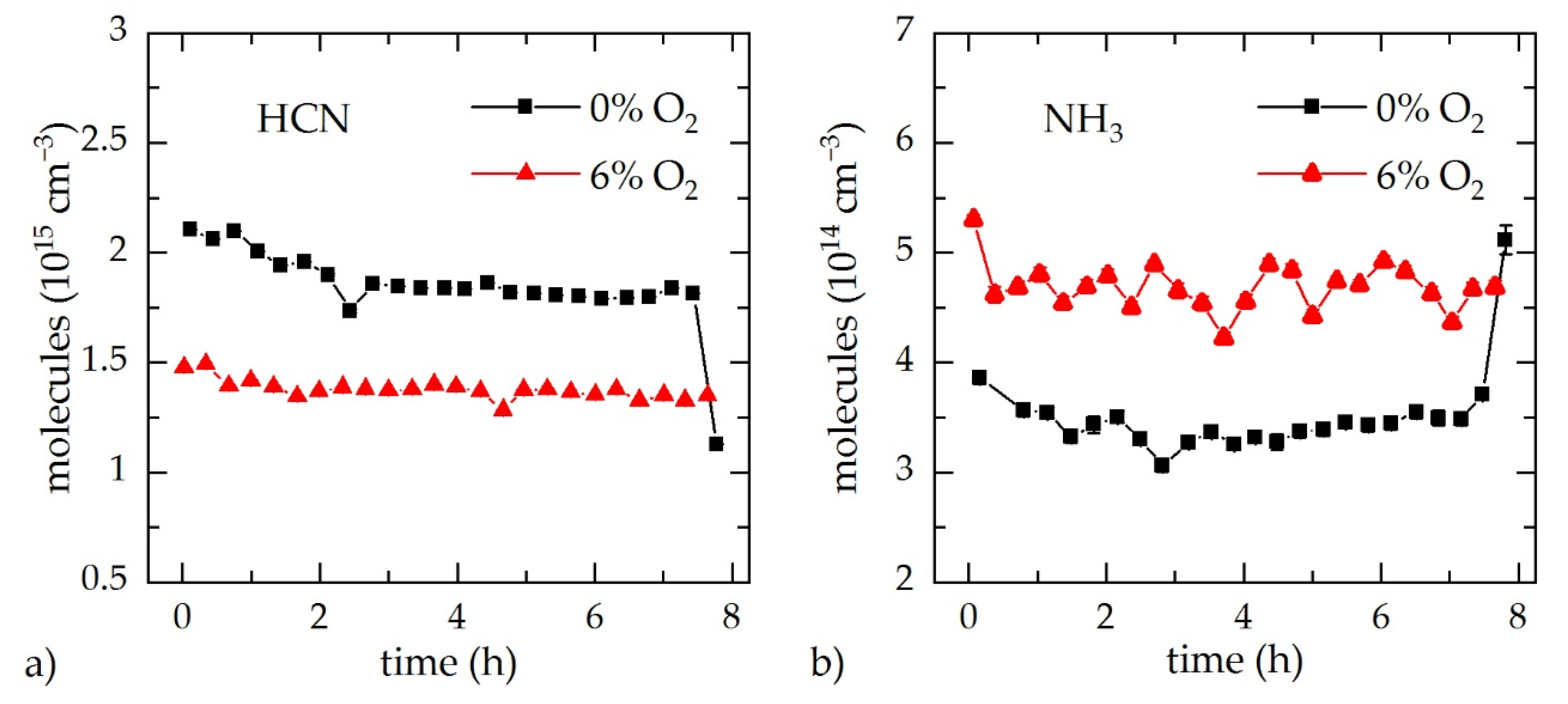
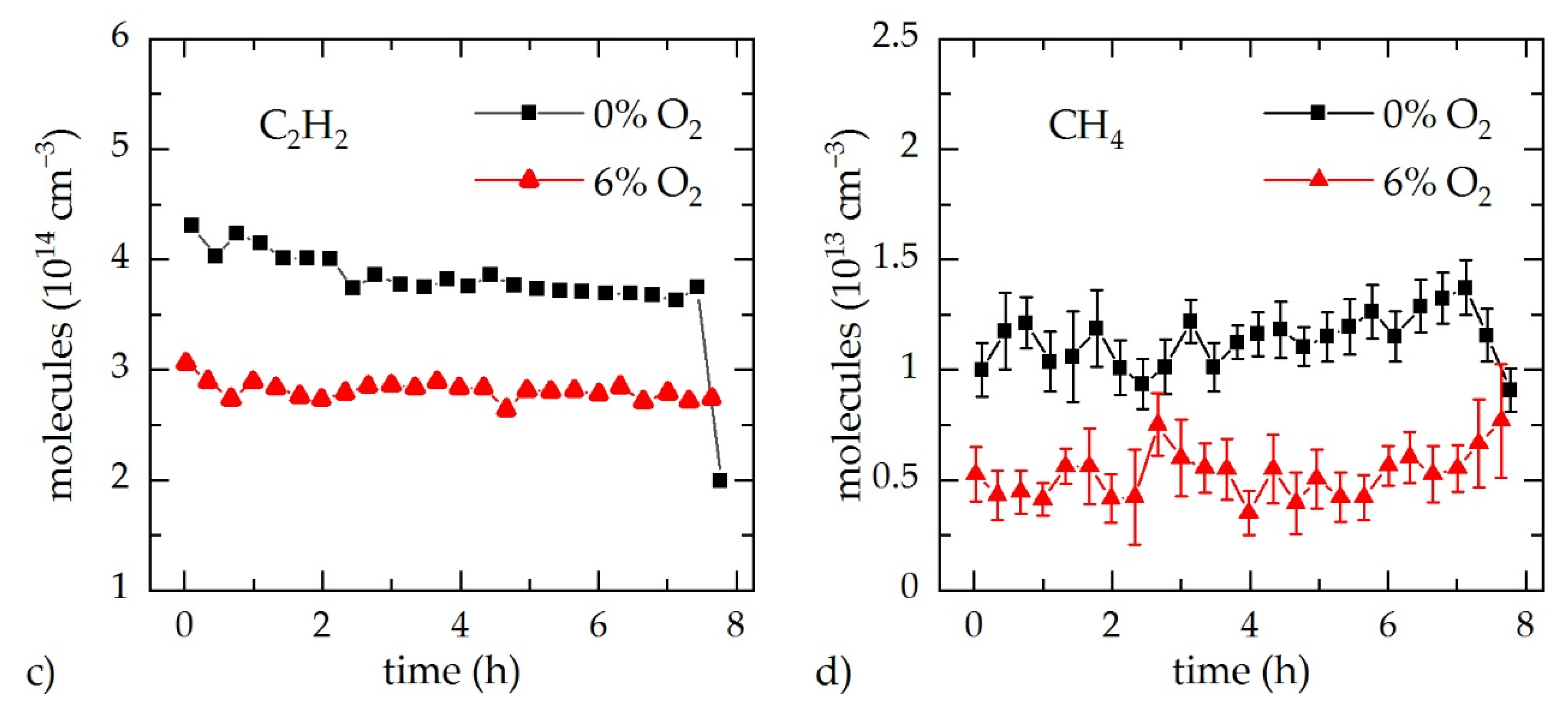
3.2. In Situ Gas Analysis during Process Monitoring
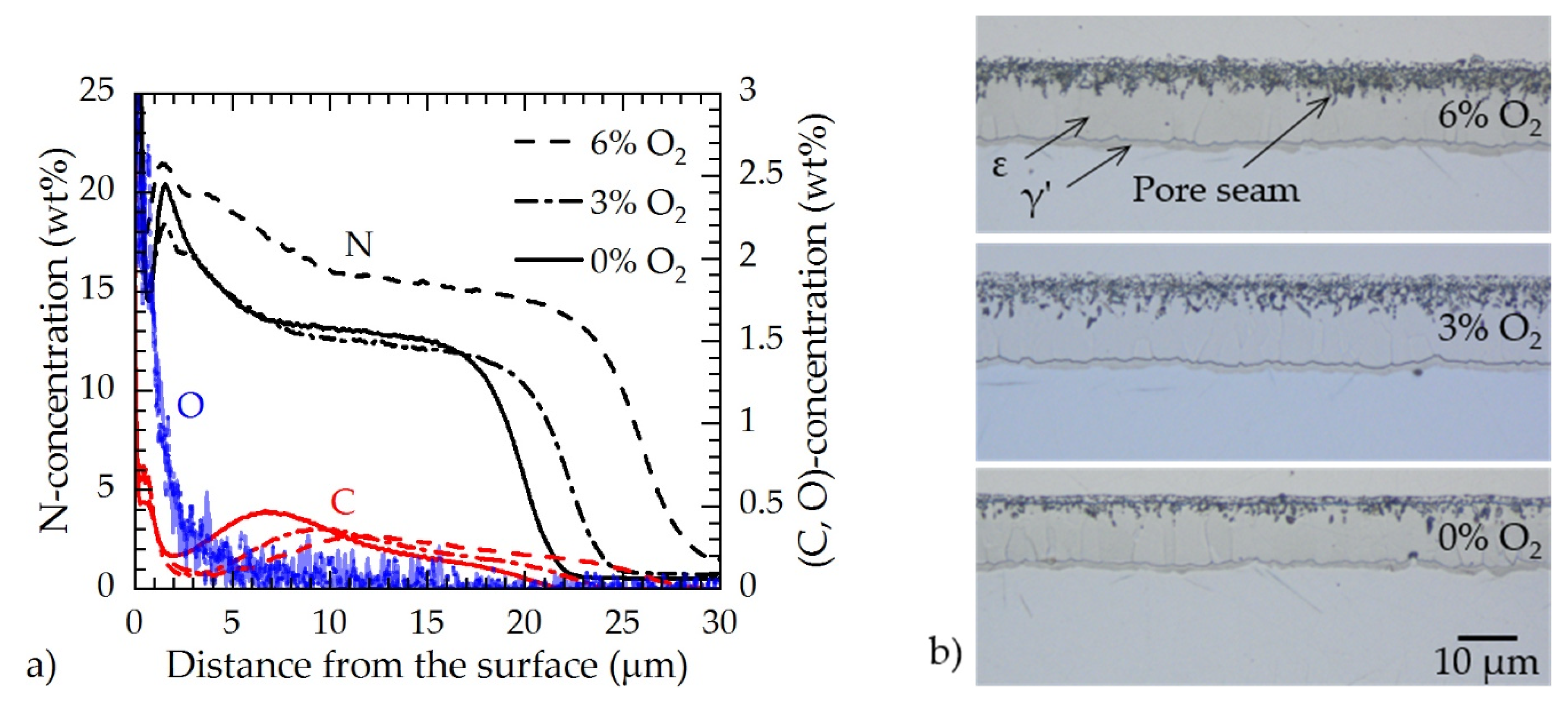
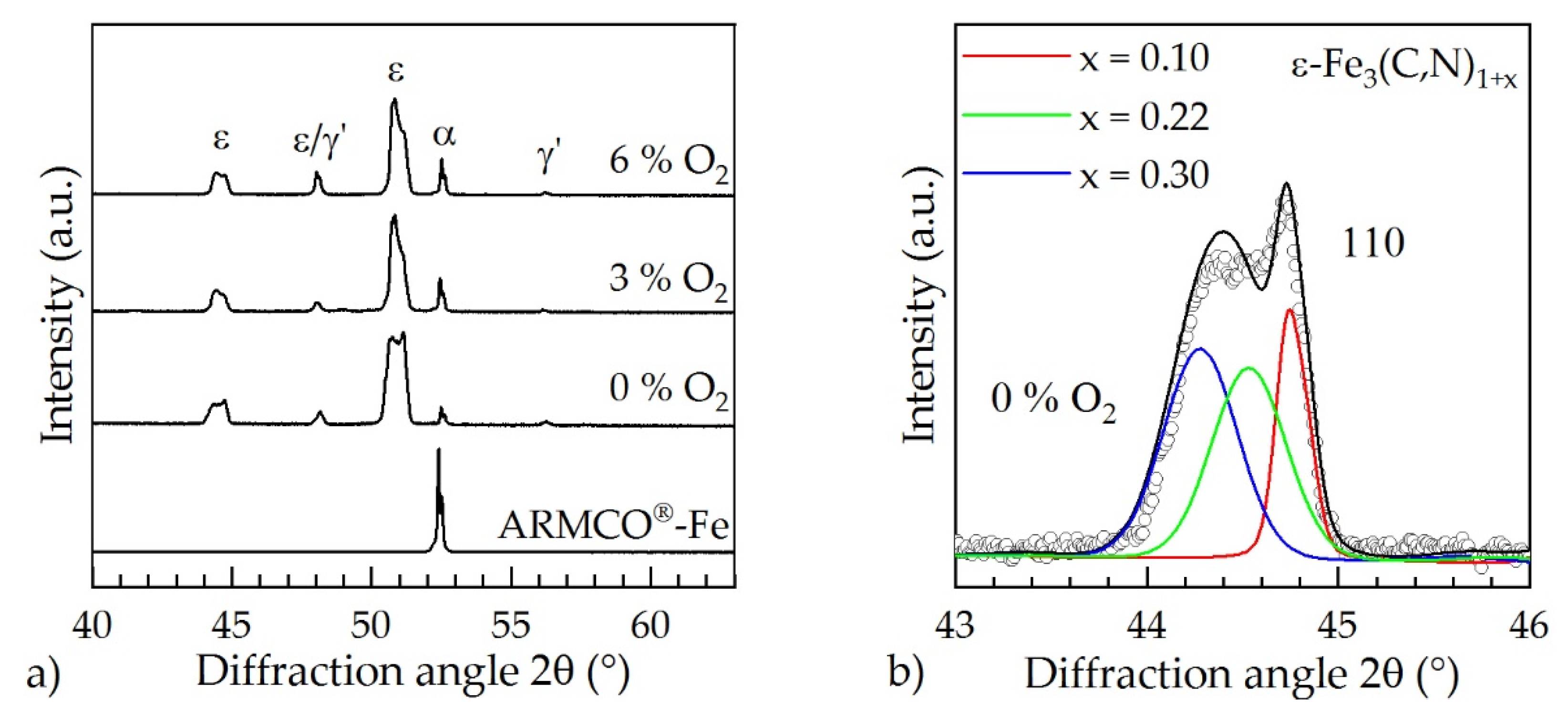
3.3. Surface Modification of ARMCO® Iron
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mittemeijer, E.; Somers, M.A.J. (Eds.) Thermochemical Surface Engineering of Steels; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780857095923. [Google Scholar]
- Bell, T.; Sun, Y.; Suhadi, A. Environmental and technical aspects of plasma nitrocarburising. Vacuum 2000, 59, 14–23. [Google Scholar] [CrossRef]
- Basso, R.L.O.; Figueroa, C.A.; Zagonel, L.F.; Pastore, H.O.; Wisnivesky, D.; Alvarez, F. Effect of Carbon on the Compound Layer Properties of AISI H13 Tool Steel in Pulsed Plasma Nitrocarburizing. Plasma Process. Polym. 2007, 4, S728–S731. [Google Scholar] [CrossRef]
- Dalke, A.; Burlacov, I.; Spies, H.-J.; Biermann, H. Use of a solid carbon precursor for DC plasma nitrocarburizing of AISI 4140 steel. Vacuum 2018, 149, 146–149. [Google Scholar] [CrossRef]
- Puth, A.; Hamann, S.; Kusýn, L.; Burlacov, I.; Dalke, A.; Spies, H.-J.; Biermann, H.; Röpcke, J. Spectroscopic investigations of plasma nitrocarburizing processes using an active screen made of carbon in a model reactor. Plasma Sources Sci. Technol. 2018, 27, 75017. [Google Scholar] [CrossRef]
- Bystrov, K.; Morgan, T.W.; Tanyeli, I.; de Temmerman, G.; van de Sanden, M.C.M. Chemical sputtering of graphite by low temperature nitrogen plasmas at various substrate temperatures and ion flux densities. J. Appl. Phys. 2013, 114, 133301. [Google Scholar] [CrossRef] [Green Version]
- Puth, A.; Kusýn, L.; Pipa, A.V.; Burlacov, I.; Dalke, A.; Hamann, S.; van Helden, J.H.; Biermann, H.; Röpcke, J. Spectroscopic study of plasma nitrocarburizing processes with an industrial-scale carbon active screen. Plasma Sources Sci. Technol. 2020, 29, 35001. [Google Scholar] [CrossRef]
- Hamann, S.; Burlacov, I.; Spies, H.-J.; Biermann, H.; Röpcke, J. Spectroscopic investigations of plasma nitriding processes: A comparative study using steel and carbon as active screen materials. J. Appl. Phys. 2017, 121, 153301. [Google Scholar] [CrossRef]
- Burlacov, I.; Hamann, S.; Spies, H.-J.; Dalke, A.; Röpcke, J.; Biermann, H. A Novel Approach of Plasma Nitrocarburizing Using a Solid Carbon Active Screen—A Proof of Concept. HTM J. Heat Treat. Mater. 2017, 72, 254–259. [Google Scholar] [CrossRef]
- Dalke, A.; Burlacov, I.; Hamann, S.; Puth, A.; Böcker, J.; Spies, H.-J.; Röpcke, J.; Biermann, H. Solid carbon active screen plasma nitrocarburizing of AISI 316L stainless steel: Influence of N2-H2 gas composition on structure and properties of expanded austenite. Surf. Coat. Technol. 2019, 357, 1060–1068. [Google Scholar] [CrossRef]
- Jafarpour, S.; Puth, A.; Dalke, A.; Böcker, J.; Pipa, A.; Röpcke, J.; van Helden, J.-P.H.; Biermann, H. Solid carbon active screen plasma nitrocarburizing of AISI 316L stainless steel in cold wall reactor: Influence of plasma conditions. J. Mater. Res. Technol. 2020, 9, 9195–9205. [Google Scholar] [CrossRef]
- Böcker, J.; Puth, A.; Dalke, A.; Röpcke, J.; van Helden, J.-P.H.; Biermann, H. Influence of the Active Screen Plasma Power during Afterglow Nitrocarburizing on the Surface Modification of AISI 316L. Coatings 2020, 10, 1112. [Google Scholar] [CrossRef]
- Haruman, E.; Bell, T.; Sun, Y. Compound layer characteristics resulting from plasma nitrocarburising in atmospheres containing carbon dioxide gas additions. Surf. Eng. 1992, 8, 275–282. [Google Scholar] [CrossRef]
- Hamann, S.; Börner, K.; Burlacov, I.; Hübner, M.; Spies, H.-J.; Röpcke, J. Spectroscopic studies of conventional and active screen N2–H2 plasma nitriding processes with admixtures of CH4 or CO2. Plasma Sources Sci. Technol. 2013, 22, 55022. [Google Scholar] [CrossRef]
- Burlacov, I.; Börner, K.; Spies, H.-J.; Biermann, H.; Lopatik, D.; Zimmermann, H.; Röpcke, J. In-Situ monitoring of plasma enhanced nitriding processes using infrared absorption and mass spectroscopy. Surf. Coat. Technol. 2012, 206, 3955–3960. [Google Scholar] [CrossRef]
- Hamann, S.; Börner, K.; Burlacov, I.; Spies, H.-J.; Röpcke, J. Spectroscopic diagnostics of active screen plasma nitriding processes: On the interplay of active screen and model probe plasmas. J. Phys. D Appl. Phys. 2015, 48, 345204. [Google Scholar] [CrossRef]
- Hamann, S.; Börner, K.; Burlacov, I.; Spies, H.-J.; Strämke, M.; Strämke, S.; Röpcke, J. Plasma nitriding monitoring reactor: A model reactor for studying plasma nitriding processes using an active screen. Rev. Sci. Instrum. 2015, 86, 123503. [Google Scholar] [CrossRef]
- Gordon, I.E.; Rothman, L.S.; Hill, C.; Kochanov, R.V.; Tan, Y.; Bernath, P.F.; Birk, M.; Boudon, V.; Campargue, A.; Chance, K.V.; et al. The HITRAN2016 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2017, 203, 3–69. [Google Scholar] [CrossRef]
- Ba, Y.A.; Wenger, C.; Surleau, R.; Boudon, V.; Rotger, M.; Daumont, L.; Bonhommeau, D.A.; Tyuterev, V.G.; Dubernet, M.-L. MeCaSDa and ECaSDa: Methane and ethene calculated spectroscopic databases for the virtual atomic and molecular data centre. J. Quant. Spectrosc. Radiat. Transf. 2013, 130, 62–68. [Google Scholar] [CrossRef]
- Cottaz, C.; Kleiner, I.; Tarrago, G.; Brown, L.R.; Margolis, J.S.; Poynter, R.L.; Pickett, H.M.; Fouchet, T.; Drossart, P.; Lellouch, E. Line Positions and Intensities in the 2nu(2)/nu(4) Vibrational System of (14)NH(3) near 5–7 µm. J. Mol. Spectrosc. 2000, 203, 285–309. [Google Scholar] [CrossRef] [PubMed]
- Toth, R.A. H2O Linelist. Available online: https://mark4sun.jpl.nasa.gov/h2o.html (accessed on 3 August 2021).
- Gomez, L.; Jacquemart, D.; Lacome, N.; Mandin, J.-Y. New line intensity measurements for 12C2H2 around 7.7μm and HITRAN format line list for applications. J. Quant. Spectrosc. Radiat. Transf. 2010, 111, 2256–2264. [Google Scholar] [CrossRef] [Green Version]
- Maki, A.G.; Mellau, G.C.; Klee, S.; Winnewisser, M.; Quapp, W. High-Temperature Infrared Measurements in the Region of the Bending Fundamental of H12C14N, H12C15N, and H13C14N. J. Mol. Spectrosc. 2000, 202, 67–82. [Google Scholar] [CrossRef] [Green Version]
- Maki, A.G.; Quapp, W.; Klee, S. Intensities of Hot-Band Transitions: HCN Hot Bands. J. Mol. Spectrosc. 2000, 171, 420–434. [Google Scholar] [CrossRef]
- Coxon, J.A.; Hajigeorgiou, P.G. Direct potential fit analysis of the X1sigma+ ground state of CO. J. Chem. Phys. 2004, 121, 2992–3008. [Google Scholar] [CrossRef]
- Kröcher, O.; Elsener, M. Hydrolysis and oxidation of gaseous HCN over heterogeneous catalysts. Appl. Catal. B Environ. 2009, 92, 75–89. [Google Scholar] [CrossRef]
- Leineweber, A.; Jacobs, H.; Hüning, F.; Lueken, H.; Kockelmann, W. Nitrogen ordering and ferromagnetic properties of ϵ-Fe3N1+x (0.10 ≤ x ≤ 0.39) and ϵ-Fe3(N0.80C0.20)1.38. J. Alloys Compd. 2001, 316, 21–38. [Google Scholar] [CrossRef]
- Jack, K.H. Binary and Ternary Interstitial Alloys. II. The Iron-Carbon-Nitrogen System. Proc. R. Soc. Lond. 1948, 195, 41–55. [Google Scholar]
- Jacobs, H.; Rechenbach, D.; Zachwieja, U. Structure determination of γ′-Fe4N and ϵ-Fe3N. J. Alloys Compd. 1995, 227, 10–17. [Google Scholar] [CrossRef]

| Species | Spectral Position (cm−1) | Absorption Line Strength (cm−1/molecules cm−2) | Limit of Detection (molecules cm−3) | Ref. |
|---|---|---|---|---|
| CH4(Q) | 1356.4868 | 1.784 × 10−20 | 2 × 1013 | [19] |
| CH4(Q) | 1356.5974 | 1.190 × 10−20 | 2 × 1013 | [19] |
| NH3(Q) | 1388.0552 | 2.726 × 10−22 | 2 × 1014 | [20] |
| NH3(Q) | 1767.5181 | 6.090 × 10−21 | 2 × 1013 | [20] |
| H2O(Q) | 1387.9337 | 8.769 × 10−23 | 5 × 1014 | [18] |
| H2O(Q) | 1388.3483 | 9.843 × 10−24 | 2 × 1015 | [21] |
| H2O(Q) | 1781.9619 | 1.167 × 10−21 | 1 × 1014 | [21] |
| C2H2(Q) | 1356.8305 | 5.899 × 10−22 | 5 × 1014 | [22] |
| C2H2(Q) | 1356.8881 | 8.920 × 10−21 | 2 × 1013 | [22] |
| HCN(Q) | 1356.9389 | 4.636 × 10−23 | 4 × 1014 | [23] |
| HCN(Q) | 1388.3225 | 3.592 × 10−22 | 1 × 1014 | [24] |
| CO(T) | 2150.3409 | 1.840 × 10−21 | 2 × 1011 | [25] |
| CO(T) | 2150.8560 | 1.826 × 10−19 | 2 × 1013 | [25] |
| CO2(T) | 606.2771 | 2.713 × 10−21 | 5 × 1013 | [5] |
| Fe3(N,C)1+x (x = 0.10) | ||||
| Oxygen (vol%) | a (nm) | c (nm) | rel. (vol%) | abs. (vol%) |
| 0 | 0.4716 (3) | 0.4407 (1) | 13 | 12 ± 2 |
| 3 | 0.4713 (2) | 0.4406 (1) | 27 | 25 ± 2 |
| 6 | 0.4713 (2) | 0.4409 (1) | 26 | 24 ± 2 |
| Fe3(N,C)1+x (x = 0.22) | ||||
| Oxygen (vol%) | a (nm) | c (nm) | rel. (vol%) | abs. (vol%) |
| 0 | 0.4738 (1) | 0.4407 (1) | 41 | 39 ± 3 |
| 3 | 0.4736 (2) | 0.4406 (1) | 35 | 32 ± 3 |
| 6 | 0.4734 (2) | 0.4409 (1) | 36 | 33 ± 3 |
| Fe3(N,C)1+x (x = 0.30) | ||||
| Oxygen (vol%) | a (nm) | c (nm) | rel. (vol%) | abs. (vol%) |
| 0 | 0.4764 (1) | 0.4407 (1) | 46 | 43 ± 4 |
| 3 | 0.4753 (2) | 0.4406 (1) | 37 | 34 ± 3 |
| 6 | 0.4754 (2) | 0.4409 (1) | 38 | 35 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Böcker, J.; Dalke, A.; Puth, A.; Schimpf, C.; Röpcke, J.; van Helden, J.-P.H.; Biermann, H. Influence of Oxygen Admixture on Plasma Nitrocarburizing Process and Monitoring of an Active Screen Plasma Treatment. Appl. Sci. 2021, 11, 9918. https://doi.org/10.3390/app11219918
Böcker J, Dalke A, Puth A, Schimpf C, Röpcke J, van Helden J-PH, Biermann H. Influence of Oxygen Admixture on Plasma Nitrocarburizing Process and Monitoring of an Active Screen Plasma Treatment. Applied Sciences. 2021; 11(21):9918. https://doi.org/10.3390/app11219918
Chicago/Turabian StyleBöcker, Jan, Anke Dalke, Alexander Puth, Christian Schimpf, Jürgen Röpcke, Jean-Pierre H. van Helden, and Horst Biermann. 2021. "Influence of Oxygen Admixture on Plasma Nitrocarburizing Process and Monitoring of an Active Screen Plasma Treatment" Applied Sciences 11, no. 21: 9918. https://doi.org/10.3390/app11219918
APA StyleBöcker, J., Dalke, A., Puth, A., Schimpf, C., Röpcke, J., van Helden, J.-P. H., & Biermann, H. (2021). Influence of Oxygen Admixture on Plasma Nitrocarburizing Process and Monitoring of an Active Screen Plasma Treatment. Applied Sciences, 11(21), 9918. https://doi.org/10.3390/app11219918






