Comparison of the Chemical and Sensorial Evaluation of Dark Chocolate Bars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dark Chocolate Bars
2.2. Headspaces Sampling
2.3. Gas Chromatography-Mass Spectrometry (GC-MS) Analyses and Peaks Identification
2.4. Panel Test
2.5. Statistical Analyses
3. Results and Discussion
3.1. Headspace Compositions
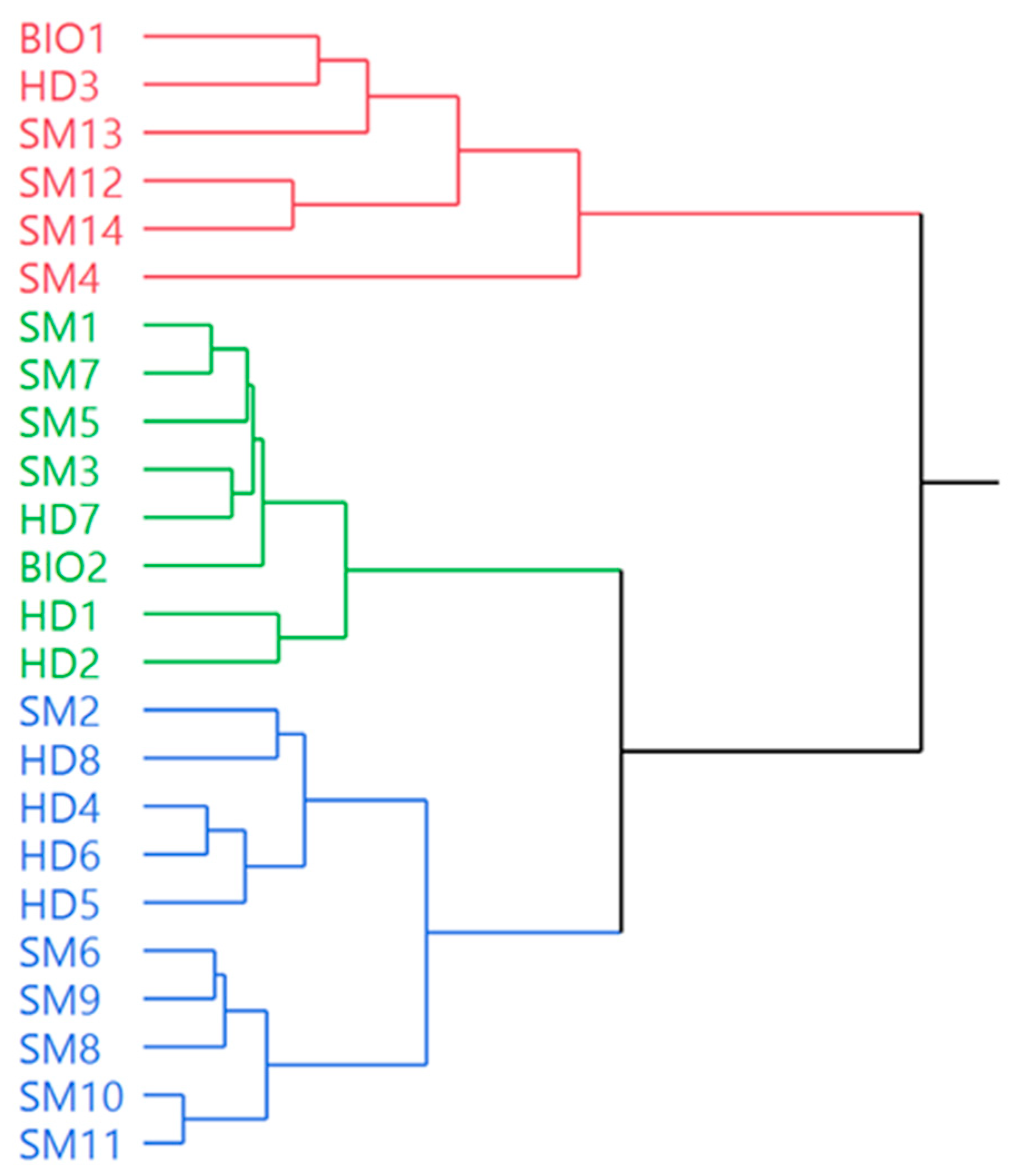
3.2. Statistical Analysis of Chemical Data
3.3. Panel Test
3.4. Statistical Analysis of Panel Test Data
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ascrizzi, R.; Pistelli, L.; Flamini, G. Aroma Evolution in Chocolate Production. In Food Aroma Evolution; Bordiga, M., Nollet, L., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 383–409. [Google Scholar]
- Afoakwa, E.O. Sensory character and flavour perception of chocolates. In Chocolate Science and Technology; Wiley-Blackwell: Chichester, UK, 2016; pp. 202–222. [Google Scholar]
- Qin, X.-W.; Lai, J.-X.; Tan, L.-H.; Hao, C.-Y.; Li, F.-P.; He, S.-Z.; Song, Y.-H. Characterization of volatile compounds in Criollo, Forastero and Trinitario cocoa seeds (Theobroma cacao L.) in China. Int. J. Food Prop. 2016, 2912, 2261–2275. [Google Scholar] [CrossRef] [Green Version]
- Ascrizzi, R.; Flamini, G.; Tessieri, C.; Pistelli, L. From the raw seed to chocolate: Volatile profile of Blanco de Criollo in different phases of the processing chain. Microchem. J. 2017, 133, 474–479. [Google Scholar] [CrossRef]
- Cidell, J.L.; Alberts, H.C. Constructing quality: The multinational histories of chocolate. Geoforum 2006, 37, 999–1007. [Google Scholar] [CrossRef]
- Januszewska, R.; Viaene, J. Acceptance of chocolate by preference cluster mapping across Belgium and Poland. J. Euromarketing 2002, 11, 61–86. [Google Scholar] [CrossRef]
- Lozano, J.; Santos, J.; Arroyo, T.; Aznar, M.; Cabellos, J.; Gil, M.; Horrillo, M. Correlating e-nose responses to wine sensorial descriptors and gas chromatography–mass spectrometry profiles using partial least squares regression analysis. Sens. Actuators B Chem. 2007, 127, 267–276. [Google Scholar] [CrossRef]
- Ziegleder, G. Linalool contents as characteristic of some flavor grade cocoas. Z. Für Leb. Und. Forsch. 1990, 191, 306–309. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Luca, S.V.; Miron, A. Flavor Chemistry of Cocoa and Cocoa Products-An Overview. Compr. Rev. Food Sci. Food Saf. 2016, 15, 73–91. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Ryan, A. Flavor Formation and Character in Cocoa and Chocolate: A Critical Review. Crit. Rev. Food Sci. Nutr. 2008, 48, 840–857. [Google Scholar] [CrossRef]
- Rodriguez-Campos, J.; Escalona-Buendía, H.B.B.; Contreras-Ramos, S.M.M.; Orozco-Avila, I.; Jaramillo-Flores, E.; Lugo-Cervantes, E. Effect of fermentation time and drying temperature on volatile compounds in cocoa. Food Chem. 2012, 132, 277–288. [Google Scholar] [CrossRef]
- Rodriguez-Campos, J.; Escalona-Buendia, H.B.; Orozco-Avila, I.; Lugo-Cervantes, E.; Jaramillo-Flores, M.E. Dynamics of volatile and non-volatile compounds in cocoa (Theobroma cacao L.) during fermentation and drying processes using principal components analysis. Food Res. Int. 2011, 44, 250–258. [Google Scholar] [CrossRef]
- Ramli, N.; Hassan, O.; Said, M.; Samsudin, W.; Idris, N. Influence of roasting conditions on volatile flavor of roasted Malaysian cocoa beans. J. Food Process. Preserv. 2006, 30, 280–298. [Google Scholar] [CrossRef]
- Ramos, C.L.; Dias, D.R.; Miguel, M.G.d.C.P.; Schwan, R.F. Impact of different cocoa hybrids (Theobroma cacao L.) and S. cerevisiae UFLA CA11 inoculation on microbial communities and volatile compounds of cocoa fermentation. Food Res. Int. 2014, 64, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Bonvehí, J.S. Investigation of aromatic compounds in roasted cocoa powder. Eur. Food Res. Technol. 2005, 221, 19–29. [Google Scholar] [CrossRef]
- Krings, U.; Berger, R.G.; Banavara, D.S. Thin layer high vacuum distillation to isolate the flavor of high-fat food. Eur. Food Res. Technol. 2003, 217, 70–73. [Google Scholar] [CrossRef]
- Di Natale, C.; Macagnano, A.; Paolesse, R.; Mantini, A.; Tarizzo, E.; D’Amico, A.; Sinesio, F.; Bucarelli, F.; Moneta, E.; Quaglia, G. Electronic nose and sensorial analysis: Comparison of performances in selected cases. Sens. Actuators B Chem. 1998, 50, 246–252. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 1932633219. [Google Scholar]
- National Institute of Standards and Technology. NIST/EPA/NIH Mass Spectral Library; The NIST Mass Spectrometry Data Center: Gaithersburg, MD, USA, 2014.
- Yuh, E. The Chocolate Tasting Guide; Chronicle Books LLC: San Francisco, CA, USA, 2014. [Google Scholar]
- Lenfant, F.; Hartmann, C.; Watzke, B.; Breton, O.; Loret, C.; Martin, N. Impact of the shape on sensory properties of individual dark chocolate pieces. LWT Food Sci. Technol. 2013, 51, 545–552. [Google Scholar] [CrossRef]
- Choi, Y.H.; Kim, H.K.; Hazekamp, A.; Erkelens, C.; Lefeber, A.W.M.; Verpoorte, R. Metabolomic Differentiation of Cannabis sativa Cultivars Using 1H NMR Spectroscopy and Principal Component Analysis. J. Nat. Prod. 2004, 67, 953–957. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Flamini, G.; Giusiani, M.; Stefanelli, F.; Deriu, V.; Chericoni, S. VOCs as fingerprints for the chemical profiling of hashish samples analyzed by HS-SPME/GC–MS and multivariate statistical tools. Forensic. Toxicol. 2018, 36, 243–260. [Google Scholar] [CrossRef]
- Beckett, S.T. The Science of Chocolate, 2nd ed.; Royal Society of Chemistry: Cambridge, UK, 2015; ISBN 9781788012355. [Google Scholar]
- Yu, A.N.; Zhang, A.D. The effect of pH on the formation of aroma compounds produced by heating a model system containing l-ascorbic acid with l-threonine/l-serine. Food Chem. 2010, 119, 214–219. [Google Scholar] [CrossRef]
- Frauendorfer, F.; Schieberle, P. Changes in key aroma compounds of Criollo cocoa beans during roasting. J. Agric. Food Chem. 2008, 56, 10244–10251. [Google Scholar] [CrossRef]
- Frauendorfer, F.; Schieberle, P. Identification of the key aroma compounds in cocoa powder based on molecular sensory correlations. J. Agric. Food Chem. 2006, 54, 5521–5529. [Google Scholar] [CrossRef] [PubMed]
- Slaghenaufi, D.; Ugliano, M. Norisoprenoids, Sesquiterpenes and Terpenoids Content of Valpolicella Wines During Aging: Investigating Aroma Potential in Relationship to Evolution of Tobacco and Balsamic Aroma in Aged Wine. Front. Chem. 2018, 6, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; CRC Press: Boca Raton, FL, USA, 2010; ISBN 978-1-4200-9077-2. [Google Scholar]
- Schwan, R.F.; Wheals, A.E. The microbiology of cocoa fermentation and its role in chocolate quality. Crit. Rev. Food Sci. Nutr. 2004, 44, 205–221. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Vieira, J. Effects of tempering and fat crystallisation behaviour on microstructure, mechanical properties and appearance in dark chocolate systems. J. Food Eng. 2008, 89, 128–136. [Google Scholar] [CrossRef]
- Afoakwa, E.O. Chocolate Manufacturing and Processing Technology. In Cocoa Production and Processing Technology; CRC Press: Boca Raton, FL, USA, 2014; pp. 183–194. [Google Scholar]
- Afoakwa, E.O. Chocolate Quality and Defects. In Cocoa Production and Processing Technology; CRC Press: Boca Raton, FL, USA, 2014; pp. 197–204. [Google Scholar]
- Hendrickx, H.; De Moor, H.; Huyghebaert, A.; Janssen, G. Manufacture of chocolate containing hydrogenated butterfat. Rev. Int. La Choc. 1971, 26, 186–197. [Google Scholar]
- Afoakwa, E.O. The chemistry of flavour development during cocoa processing and chocolate manufacture. In Chocolate Science and Technology; Wiley-Blackwell Publishers: Chichester, UK, 2016; pp. 154–170. [Google Scholar]
- Owusu, M.; Petersen, M.A.; Heimdal, H. Relationship of sensory and instrumental aroma measurements of dark chocolate as influenced by fermentation method, roasting and conching conditions. J. Food Sci. Technol. 2013, 50, 909–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charles, M.; Romano, A.; Yener, S.; Barnabà, M.; Navarini, L.; Märk, T.D.; Biasoli, F.; Gasperi, F. Understanding flavour perception of espresso coffee by the combination of a dynamic sensory method and in-vivo nosespace analysis. Food Res. Int. 2015, 69, 9–20. [Google Scholar] [CrossRef]
- Acierno, V.; Liu, N.; Alewijn, M.; Stieger, M.; van Ruth, S.M. Which cocoa bean traits persist when eating chocolate? Real-time nosespace analysis by PTR-QiToF-MS. Talanta 2019, 195, 676–682. [Google Scholar] [CrossRef] [PubMed]


| Sense | Descriptors | |
|---|---|---|
| Sight | Color | Light brown |
| Medium brown | ||
| Dark brown | ||
| Shininess | Glossy | |
| Matte | ||
| Presence of air bubbles | Yes/No | |
| Presence of white spots | Yes/No | |
| Presence of stripes | Yes/No | |
| Hearing | Snap at break | Hard |
| Crunchy | ||
| Crisp | ||
| Light | ||
| Mellow | ||
| Muted | ||
| High-pitched | ||
| Low | ||
| Smell | Aroma | Flowery (aromatic notes resembling flowers) |
| Fruity (aromatic notes resembling fruits) | ||
| Caramel (aromatic sweet notes resembling caramel, brown sugar) | ||
| Nutty (aromatic notes reminiscent of nut products, such as hazelnuts) | ||
| Herbaceous (“green” aromatic notes, reminiscent of cut grass) | ||
| Dairy (perception of “fat” notes, reminiscent of butter and cheese) | ||
| Fermentation/oxidation (unpleasant, pungent perception of fermented food) | ||
| Liqueur (reminiscent of alcoholic notes) | ||
| Plastic (unpleasant perception of “medicine-like” aroma notes) | ||
| Animal (unpleasant perception of animal odors) | ||
| Taste | Bitter | |
| Sweet | ||
| Salty | ||
| Acid | ||
| Umami | ||
| Touch | Lips | Smooth |
| Velvety | ||
| Grainy | ||
| Tongue | Smooth | |
| Velvety | ||
| Grainy | ||
| In-mouth sensation | Astringent | |
| Burning | ||
| Warm | ||
| Sparkling | ||
| Stinging | ||
| Metallic | ||
| Fatty | ||
| Oily | ||
| Melting | Immediate | |
| Delayed | ||
| Compounds | l.r.i. 1 | Relative Abundance (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HD1 | HD2 | HD3 | HD4 | HD5 | HD6 | HD7 | HD8 | ||
| ethyl acetate | 616 | - 2 | - | 10.7 | 9.9 | 5.9 | 12.6 | 3.3 | 5.3 |
| 2,3-butanediol | 787 | 3.3 | - | 4.8 | 2.0 | 4.8 | 1.6 | 5.9 | 1.2 |
| isovaleric acid | 834 | 2.6 | - | 2.5 | 1.3 | - | - | 3.0 | 0.4 |
| isopentyl acetate | 876 | - | - | 1.7 | 0.2 | - | 1.0 | 1.6 | 0.9 |
| 2,6-dimethylpyrazine | 914 | - | - | 4.0 | 0.3 | - | - | - | - |
| 2,5-dimethylpyrazine | 915 | 6.0 | 1.5 | - | - | - | - | 3.0 | 0.3 |
| benzaldehyde | 963 | 1.9 | - | - | 1.2 | - | - | 1.3 | - |
| myrcene | 990 | - | - | 0.2 | - | - | - | 0.6 | - |
| n-decane | 1000 | - | - | 0.6 | - | - | - | - | - |
| 2-ethyl-3-methylpyrazine | 1002 | - | - | - | - | - | - | 0.3 | - |
| 2,3,5-trimethylpyrazine | 1004 | 13.4 | 5.6 | 12.1 | 6.2 | 7.1 | 9.4 | 12.9 | 5.5 |
| limonene | 1031 | 1.6 | 7.2 | - | - | - | - | 0.2 | - |
| (Z)-β-ocimene | 1041 | - | - | - | - | - | - | 0.2 | - |
| 2-heptyl acetate | 1043 | - | 2.4 | 1.1 | 1.2 | 1.3 | 1.3 | 0.5 | 0.5 |
| 2-acetylpyrrole | 1060 | 3.2 | 2.3 | 0.8 | - | - | - | - | - |
| acetophenone | 1067 | - | - | - | - | 1.2 | - | 1.0 | - |
| 3-ethyl-2,5-dimethylpyrazine | 1080 | 2.3 | 1.5 | - | 0.7 | 1.0 | - | 1.6 | - |
| 2-ethyl-3,5-dimethylpyrazine | 1085 | - | - | 2.0 | - | - | - | - | - |
| tetramethylpyrazine | 1086 | 43.7 | 47.3 | 37.1 | 65.7 | 59.9 | 62.0 | 50.8 | 58.1 |
| linalool | 1100 | - | - | - | - | 0.2 | 0.7 | 0.2 | - |
| nonanal | 1103 | 6.3 | 6.6 | 1.5 | 0.3 | 3.0 | - | 1.1 | - |
| phenylethyl alcohol | 1110 | 3.1 | 7.4 | 1.8 | 1.5 | 4.7 | 1.9 | 1.7 | 0.7 |
| 3,5-diethyl-2-methyl pyrazine | 1160 | 1.2 | 0.5 | 1.2 | 1.3 | 1.0 | - | 1.2 | 1.8 |
| trans-linalool oxide (pyranoid) | 1177 | 0.1 | - | - | - | - | - | - | - |
| ethyl octanoate | 1197 | - | 2.7 | - | 0.7 | 2.6 | - | 0.5 | 1.0 |
| n-dodecane | 1200 | - | - | 2.4 | - | - | - | - | - |
| decanal | 1205 | 0.6 | 0.8 | 0.3 | 0.2 | 0.7 | 0.7 | - | 0.4 |
| butanoic acid, 2-methyl-3-oxo-, methyl ester | 1223 | 0.7 | 0.6 | 0.6 | 0.3 | 0.8 | 0.4 | 0.4 | 0.6 |
| ascaridole | 1237 | - | - | - | 0.1 | - | - | 0.2 | - |
| benzene acetic acid, ethyl ester | 1247 | 0.2 | - | 0.3 | 0.5 | - | - | 0.3 | 0.8 |
| (Z)-anethole | 1253 | - | - | 1.0 | - | - | - | - | - |
| 2-phenylethylacetate | 1258 | 3.4 | 2.9 | 2.1 | 4.6 | 3.6 | 4.2 | 2.6 | 12.2 |
| nonanoic acid | 1273 | 2.5 | 3.6 | - | - | - | 1.1 | 0.2 | 0.3 |
| (E)-anethole | 1287 | - | - | 6.2 | 1.1 | - | - | 1.2 | - |
| 2-undecanone | 1294 | - | - | - | 0.1 | - | 0.4 | - | - |
| n-tridecane | 1300 | - | - | - | - | 0.6 | - | 0.2 | - |
| undecanal | 1305 | - | - | - | 0.2 | - | - | - | - |
| δ-elemene | 1340 | - | - | - | - | - | - | 0.1 | - |
| α-terpinyl acetate | 1351 | - | - | - | - | - | 0.3 | - | - |
| α-copaene | 1376 | 0.6 | - | - | - | - | - | 0.2 | - |
| β-patchoulene | 1380 | - | - | - | - | - | - | - | 2.3 |
| ethyl decanoate | 1396 | - | - | - | - | 0.5 | - | - | - |
| n-tetradecane | 1400 | - | - | 1.3 | - | 0.6 | - | - | - |
| longifolene | 1403 | - | - | - | - | - | - | 0.2 | - |
| methyl eugenol | 1404 | - | - | - | - | - | 0.3 | - | - |
| (Z)-caryophyllene | 1405 | - | - | - | - | - | - | 0.2 | - |
| β-duprezianene | 1423 | - | - | - | - | - | - | - | 1.1 |
| (E)-geranyl acetone | 1454 | 1.0 | 1.4 | 0.5 | 0.3 | 0.4 | 0.8 | 0.3 | 2.6 |
| α-patchoulene | 1456 | - | - | - | - | - | - | 0.2 | - |
| alloaromadendrene | 1461 | - | - | - | - | - | 0.5 | - | - |
| drima-7,9(11)-diene | 1470 | - | - | - | - | - | - | - | 1.3 |
| 1-dodecanol | 1471 | - | 3.2 | - | - | - | - | - | - |
| trans-cadina-1(6),4-diene | 1475 | - | - | - | - | - | - | - | 1.9 |
| germacrene D | 1480 | - | - | - | - | - | - | 0.5 | - |
| cis-β-guaiene | 1489 | - | - | - | - | - | - | 0.2 | - |
| bicyclogermacrene | 1495 | - | - | - | - | - | 0.5 | - | - |
| trans-β-guaiene | 1501 | 0.4 | - | 0.6 | - | - | 0.5 | 0.5 | - |
| trans-γ-cadinene | 1513 | - | - | - | - | - | - | 0.4 | - |
| δ-cadinene | 1523 | - | - | 0.6 | - | - | - | 0.5 | - |
| ethyl dodecanoate | 1595 | - | - | 0.2 | 0.1 | - | - | 0.2 | 0.8 |
| n-hexadecane | 1600 | - | - | 0.2 | - | - | - | - | - |
| citronellyl pentanoate | 1625 | - | - | 0.8 | - | - | - | - | - |
| cadalene | 1675 | 1.9 | 2.5 | 1.0 | - | - | - | 0.4 | - |
| Monoterpene hydrocarbons | 1.6 | 7.2 | 0.2 | - | - | - | 1.0 | - | |
| Oxygenated monoterpenes | 0.1 | - | 0.8 | 0.1 | 0.2 | 1.0 | 0.4 | - | |
| Sesquiterpene hydrocarbons | 2.9 | 2.5 | 2.2 | - | - | 1.5 | 3.4 | 6.7 | |
| Pyrazines | 66.6 | 56.4 | 56.3 | 74.2 | 69.0 | 71.3 | 69.9 | 65.6 | |
| Pyrroles | 3.2 | 2.3 | 0.8 | - | - | - | - | - | |
| Apocarotenoids | 1.0 | 1.4 | 0.5 | 0.3 | 0.4 | 1.1 | 0.3 | 2.6 | |
| Phenylpropanoids | - | - | 7.2 | 1.1 | - | - | 1.2 | - | |
| Non-terpene acids | 5.2 | 3.6 | 2.5 | 1.3 | - | 1.1 | 3.2 | 0.8 | |
| Non-terpene alcohols | 6.4 | 10.6 | 6.6 | 3.6 | 9.6 | 3.4 | 7.5 | 1.9 | |
| Non-terpene aldehydes | 8.8 | 7.4 | 1.9 | 1.8 | 3.7 | 0.7 | 2.4 | 0.4 | |
| Non-terpene esters | 4.3 | 8.5 | 16.6 | 17.5 | 14.7 | 19.5 | 9.4 | 22.1 | |
| Non-terpene ketones | - | - | - | 0.1 | 1.2 | 0.4 | 1.0 | - | |
| Non-terpene hydrocarbons | - | - | 4.4 | - | 1.2 | - | 0.2 | - | |
| Total identified (%) | 100.0 | 99.9 | 100.0 | 99.9 | 100.0 | 100.0 | 99.9 | 100.0 | |
| Compounds | l.r.i. 1 | Relative Abundance (%) | |
|---|---|---|---|
| BIO1 | BIO2 | ||
| ethyl acetate | 616 | 22.3 | - 2 |
| 2,3-butanediol | 787 | 1.1 | 2.2 |
| isovaleric acid | 834 | 2.6 | 2.1 |
| isopentyl acetate | 876 | 1.0 | 1.0 |
| 2,6-dimethylpyrazine | 914 | - | 2.9 |
| 2,5-dimethylpyrazine | 915 | 0.9 | - |
| benzaldehyde | 963 | 0.6 | - |
| myrcene | 990 | - | 0.4 |
| 2,3,5-trimethylpyrazine | 1004 | 5.9 | 16.5 |
| limonene | 1031 | 0.1 | 2.6 |
| 2-heptyl acetate | 1043 | 2.0 | 1.1 |
| acetophenone | 1067 | 0.6 | 1.0 |
| trans-linalool oxide (furanoid) | 1076 | 1.6 | - |
| 3-ethyl-2,5-dimethylpyrazine | 1080 | 0.2 | 3.0 |
| tetramethylpyrazine | 1086 | 44.3 | 51.5 |
| trans-sabinene hydrate | 1099 | - | 2.6 |
| linalool | 1100 | - | 0.4 |
| nonanal | 1103 | 1.1 | - |
| isopentyl isovalerate | 1105 | 0.8 | - |
| phenylethyl alcohol | 1110 | 3.0 | 0.5 |
| 3,5-diethyl-2-methyl pyrazine | 1160 | 0.8 | 2.5 |
| cis-pinocarveol | 1184 | 0.1 | - |
| ethyl octanoate | 1197 | 0.7 | 0.7 |
| decanal | 1205 | 0.4 | 0.3 |
| butanoic acid, 2-methyl-3-oxo-, methyl ester | 1223 | 0.4 | 0.5 |
| ascaridole | 1237 | 0.2 | - |
| benzene acetic acid, ethyl ester | 1247 | 0.8 | - |
| 2-phenylethylacetate | 1258 | 4.7 | 5.5 |
| nonanoic acid | 1273 | 0.9 | - |
| (E)-anethole | 1287 | - | 2.5 |
| n-tridecane | 1300 | 0.6 | - |
| undecanal | 1305 | 0.4 | - |
| n-tetradecane | 1400 | 0.4 | - |
| dodecanal | 1408 | 0.2 | - |
| (E)-geranyl acetone | 1454 | 0.7 | 0.4 |
| n-pentadecane | 1500 | 0.2 | - |
| benzoic acid, 4-ethoxy-, ethyl ester | 1522 | 0.4 | - |
| Monoterpene hydrocarbons | 0.1 | 3.0 | |
| Oxygenated monoterpenes | 2.0 | 2.9 | |
| Pyrazines | 52.0 | 76.4 | |
| Apocarotenoids | 0.7 | 0.4 | |
| Phenylpropanoids | - | 2.5 | |
| Non-terpene acids | 3.5 | 2.1 | |
| Non-terpene alcohols | 4.2 | 2.7 | |
| Non-terpene aldehydes | 2.5 | 0.3 | |
| Non-terpene esters | 33.3 | 8.8 | |
| Non-terpene ketones | 0.6 | 1.0 | |
| Non-terpene hydrocarbons | 1.2 | - | |
| Total identified (%) | 100.0 | 100.0 | |
| Compounds | l.r.i. 1 | Relative Abundance (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SM1 | SM2 | SM3 | SM4 | SM5 | SM6 | SM7 | SM8 | SM9 | SM10 | SM11 | SM12 | SM13 | SM14 | ||
| ethyl acetate | 616 | - 2 | 0.1 | - | - | - | - | - | - | - | 0.2 | - | 6.7 | 20.1 | 3.8 |
| pentanal | 695 | - | - | - | - | - | - | - | - | - | - | - | 13.5 | - | 7.0 |
| 2,3-butanediol | 787 | 4.4 | 2.2 | 2.0 | - | - | - | 2.1 | - | - | 0.4 | 1.6 | 5.5 | - | 5.9 |
| isovaleric acid | 834 | 4.1 | 2.5 | 4.1 | 3.1 | - | - | 2.6 | 1.2 | 1.1 | 0.7 | 0.9 | 5.4 | - | 3.7 |
| isopentyl acetate | 876 | - | - | 1.9 | - | - | 0.5 | - | 4.5 | - | 0.2 | 0.5 | 9.4 | - | 6.7 |
| 2,6-dimethylpyrazine | 914 | 3.9 | 1.0 | - | - | - | - | 2.6 | - | 1.2 | - | - | - | - | - |
| 2,5-dimethylpyrazine | 915 | - | - | 3.1 | 1.6 | 3.6 | - | - | - | - | 0.7 | 0.6 | 3.2 | - | 3.6 |
| ethyl acetoacetate | 940 | - | 8.7 | - | - | - | - | - | - | - | - | - | - | - | - |
| benzaldehyde | 963 | 1.1 | - | 2.4 | - | - | 0.3 | 1.5 | - | 0.2 | 0.2 | - | 0.3 | - | 0.1 |
| myrcene | 990 | - | - | 1.4 | - | - | 0.2 | 1.9 | - | 0.9 | - | - | 0.6 | - | 0.3 |
| 2-ethyl-6-methylpyrazine | 994 | - | - | 0.6 | - | - | - | - | - | - | - | - | - | - | 0.3 |
| m-mentha-1(7),8-diene | 1001 | - | - | - | - | - | - | - | - | - | - | - | 0.4 | - | - |
| 2,3,5-trimethylpyrazine | 1004 | 9.2 | 7.2 | 9.6 | 4.8 | 13.1 | 9.1 | 9.9 | 5.1 | 7.9 | 8.6 | 6.5 | 8.1 | 3.7 | 14.7 |
| limonene | 1031 | 0.5 | - | 0.3 | - | - | - | - | - | - | - | - | - | 3.2 | - |
| sylvestrene | 1032 | 0.2 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1,8-cineole | 1035 | - | 0.4 | - | - | 1.7 | - | 1.0 | - | - | - | - | - | - | - |
| (Z)-β-ocimene | 1041 | - | - | - | - | - | - | 0.3 | - | - | - | - | 0.2 | - | - |
| 2-heptyl acetate | 1043 | 2.0 | - | - | 0.5 | - | 0.8 | - | - | 0.8 | - | - | 1.8 | - | 0.7 |
| 2-acetylpyrrole | 1060 | 1.8 | - | 4.7 | 1.1 | 2.3 | - | 2.9 | 1.6 | 1.7 | - | - | 0.9 | - | 0.8 |
| acetophenone | 1067 | - | 0.8 | - | - | - | - | - | - | - | - | 0.4 | - | - | - |
| trans-linalool oxide (furanoid) | 1076 | - | 1.3 | - | - | - | - | - | - | - | 0.7 | - | - | - | - |
| 3-ethyl-2,5-dimethylpyrazine | 1080 | 1.1 | - | - | 0.4 | 2.7 | 1.0 | 1.8 | 0.5 | 1.0 | 0.8 | 0.6 | - | - | 1.5 |
| 2,6-diethylpyrazine | 1081 | - | - | 0.9 | - | - | - | - | - | - | - | - | - | - | - |
| 2-ethyl-3,5-dimethylpyrazine | 1085 | - | - | - | - | - | - | - | - | - | - | - | 0.9 | - | - |
| tetramethylpyrazine | 1086 | 56.3 | 59.7 | 51.2 | 24.4 | 56.5 | 70.7 | 55.5 | 69.1 | 66.7 | 77.0 | 75.1 | 34.2 | 27.8 | 45.2 |
| linalool | 1100 | 2.4 | - | - | - | - | - | - | - | 3.4 | - | - | 1.9 | - | - |
| nonanal | 1103 | - | 2.3 | 2.9 | 1.3 | 3.4 | 0.6 | 3.5 | 3.0 | - | - | - | - | 8.0 | - |
| phenylethyl alcohol | 1110 | 4.0 | 3.8 | 2.7 | 2.8 | 3.4 | 3.6 | 2.3 | 2.3 | 1.4 | 1.3 | 2.0 | 0.6 | 7.6 | 0.8 |
| 3,5-diethyl-2-methyl pyrazine | 1160 | 0.7 | 1.3 | 0.5 | - | 1.1 | 0.6 | 1.0 | 1.3 | 1.3 | 1.8 | 1.2 | 0.7 | - | 1.6 |
| ethyl benzoate | 1173 | 0.2 | - | 0.2 | - | - | 0.3 | - | - | - | - | - | - | - | - |
| trans-linalool oxide (pyranoid) | 1177 | - | - | - | - | - | - | - | 0.1 | 0.3 | - | - | - | 0.4 | - |
| butanedioic acid, diethyl ester | 1185 | 0.2 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1-dodecene | 1192 | - | - | - | - | 0.6 | - | - | - | - | - | - | - | - | - |
| ethyl octanoate | 1197 | 1.2 | 0.8 | 0.4 | 0.3 | - | 0.7 | 0.8 | 0.6 | 0.8 | 1.0 | 0.6 | 0.6 | 1.6 | 0.6 |
| n-dodecane | 1200 | - | - | - | - | 1.8 | - | - | - | - | - | - | - | - | - |
| decanal | 1205 | 0.6 | 0.6 | 0.7 | 0.3 | 1.3 | - | 0.4 | 0.3 | 0.2 | 0.2 | 0.2 | - | 1.5 | - |
| butanoic acid, 2-methyl-3-oxo-, methyl ester | 1223 | 0.8 | 0.6 | 0.6 | 0.4 | 0.7 | - | 0.6 | 0.6 | 0.6 | 0.5 | - | 0.5 | 1.3 | 0.4 |
| ascaridole | 1237 | - | - | 0.2 | - | - | - | 0.3 | 0.1 | 0.3 | - | - | 0.2 | - | - |
| benzene acetic acid, ethyl ester | 1247 | - | - | 0.4 | - | - | - | - | - | 0.5 | - | 0.7 | 0.2 | 1.7 | 0.2 |
| (Z)-anethole | 1253 | - | - | - | - | - | - | - | - | - | - | - | 1.2 | - | - |
| 2-phenylethylacetate | 1258 | 3.9 | 6.1 | 4.5 | 1.6 | 2.0 | 7.2 | 5.2 | 5.5 | 5.6 | 4.0 | 5.8 | 2.3 | 11.1 | 1.6 |
| nonanoic acid | 1273 | - | - | 0.8 | - | 0.4 | 0.6 | 1.8 | - | 1.2 | - | - | - | - | - |
| (E)-anethole | 1287 | - | - | - | - | - | - | - | - | - | 0.9 | 2.6 | 0.3 | 6.9 | 0.3 |
| n-tridecane | 1300 | - | - | - | - | 0.6 | - | 0.3 | - | 0.1 | - | - | - | - | - |
| undecanal | 1305 | - | - | - | - | - | - | - | - | - | - | - | - | 0.3 | - |
| undec-9-en-1-al | 1315 | - | - | - | - | - | - | 0.2 | - | - | - | - | - | - | - |
| dihydrocitronellol acetate | 1321 | - | - | - | - | 0.5 | - | - | - | - | - | - | - | - | - |
| α-copaene | 1376 | - | - | - | - | - | - | 0.3 | 0.1 | 0.2 | - | - | - | 0.5 | - |
| β-patchoulene | 1380 | - | - | - | - | - | - | - | - | - | - | - | - | 0.2 | - |
| β-panasinsene | 1383 | - | - | 0.2 | - | - | - | - | - | - | - | - | - | - | - |
| vanillin | 1394 | - | - | - | - | - | - | 0.3 | 3.1 | 1.4 | - | - | - | - | - |
| sativene | 1395 | - | - | 0.3 | - | - | - | - | - | - | - | - | - | - | - |
| ethyl decanoate | 1396 | - | - | - | - | - | 0.6 | - | - | - | 0.4 | 0.3 | - | - | - |
| n-tetradecane | 1400 | 0.5 | - | 0.5 | - | 2.1 | - | - | - | - | - | - | 0.2 | - | - |
| longifolene | 1403 | - | - | 0.1 | - | - | - | - | - | - | - | - | - | - | - |
| dodecanal | 1408 | 0.3 | - | - | - | 0.6 | - | - | - | - | - | - | - | - | - |
| β-ylangene | 1414 | - | - | - | - | - | - | - | - | - | - | - | - | 0.4 | - |
| (E)-caryophyllene | 1418 | 0.2 | 0.2 | - | - | - | - | - | - | - | - | - | - | - | - |
| (trans,cis)-iridolactone | 1446 | - | - | - | 6.3 | - | - | - | - | - | - | - | - | - | - |
| (E)-geranyl acetone | 1454 | 0.4 | 0.2 | 1.8 | 4.7 | 1.0 | - | 0.2 | 0.4 | 0.3 | - | 0.2 | - | 2.1 | - |
| (E)-ethyl cinnamate | 1462 | - | - | - | - | - | - | - | 0.1 | 0.2 | - | - | - | - | - |
| (E)-2-dodecen-1-ol | 1465 | - | - | - | 0.7 | - | - | - | - | - | - | - | - | - | - |
| γ-muurolene | 1477 | - | - | - | - | - | - | 0.3 | - | - | - | - | - | - | - |
| n-pentadecane | 1500 | - | - | - | - | - | - | 0.2 | - | - | - | - | - | - | - |
| (Z)-α-bisabolene | 1503 | - | - | - | - | 0.3 | - | - | - | - | - | - | - | - | - |
| benzoic acid, 4-ethoxy-, ethyl ester | 1522 | - | - | 0.8 | - | - | - | - | - | - | - | - | - | - | - |
| δ-cadinene | 1523 | - | - | - | - | - | - | - | - | - | 0.1 | - | - | 0.6 | - |
| trans-cadinene ether | 1559 | - | - | - | - | - | - | - | - | 0.3 | - | - | - | - | - |
| (E)-dehydroapofarnesol | 1591 | - | - | - | 19.4 | - | - | - | - | - | - | - | - | - | - |
| ethyl dodecanoate | 1595 | - | - | - | - | - | - | 0.5 | 0.3 | 0.4 | 0.2 | 0.2 | 0.1 | 1.0 | 0.2 |
| epi-cedrol | 1598 | - | - | - | 9.4 | - | - | - | - | - | - | - | - | - | - |
| n-hexadecane | 1600 | - | - | - | - | - | 2.5 | - | - | - | - | - | - | - | - |
| tetradecanal | 1612 | - | - | - | 16.5 | - | - | - | - | - | - | - | - | - | - |
| cadalene | 1675 | - | - | - | - | - | - | - | - | - | - | 0.2 | - | - | 0.2 |
| tetradecanol | 1676 | - | 0.4 | - | - | - | - | - | 0.1 | - | - | - | - | - | - |
| Monoterpene hydrocarbons | 0.7 | - | 1.7 | - | - | 0.2 | 2.2 | - | 0.9 | - | - | 1.2 | 3.2 | 0.3 | |
| Oxygenated monoterpenes | 2.4 | 1.7 | 0.2 | - | 2.1 | - | 1.3 | 0.3 | 4.0 | 0.7 | - | 2.1 | 0.4 | - | |
| Sesquiterpene hydrocarbons | 0.2 | 0.2 | 0.6 | - | 0.3 | - | 0.6 | 0.1 | 0.2 | 0.1 | 0.2 | - | 1.8 | 0.2 | |
| Oxygenated sesquiterpenes | - | - | - | 28.8 | - | - | - | - | 0.3 | - | - | - | - | - | |
| Pyrazines | 71.2 | 69.1 | 65.9 | 31.2 | 77.0 | 81.4 | 70.7 | 76.0 | 78.1 | 88.9 | 83.9 | 47.1 | 31.5 | 67.0 | |
| Pyrroles | 1.8 | - | 4.7 | 1.1 | 2.3 | - | 2.9 | 1.6 | 1.7 | - | - | 0.9 | - | 0.8 | |
| Apocarotenoids | 0.4 | 0.2 | 1.8 | 4.7 | 1.0 | - | 0.2 | 0.4 | 0.3 | - | 0.2 | 0.0 | 2.1 | - | |
| Phenylpropanoids | - | - | - | - | - | - | - | 0.1 | 0.2 | 0.9 | 2.6 | 1.6 | 6.9 | 0.3 | |
| Non-terpene acids | 4.1 | 2.5 | 4.9 | 3.1 | 0.4 | 0.6 | 4.4 | 1.2 | 2.4 | 0.7 | 0.9 | 5.4 | - | 3.7 | |
| Non-terpene alcohols | 8.5 | 6.3 | 4.8 | 3.4 | 3.4 | 3.6 | 4.4 | 2.4 | 1.4 | 1.8 | 3.6 | 6.2 | 7.6 | 6.7 | |
| Non-terpene aldehydes | 2.0 | 2.9 | 6.0 | 18.1 | 5.3 | 0.8 | 5.9 | 6.4 | 1.8 | 0.4 | 0.2 | 13.8 | 9.7 | 7.2 | |
| Non-terpene esters | 8.3 | 16.3 | 8.8 | 2.8 | 2.6 | 10.0 | 7.1 | 11.6 | 8.7 | 6.6 | 8.0 | 21.6 | 36.8 | 13.9 | |
| Non-terpene ketones | 0.0 | 0.8 | - | 6.3 | - | - | - | - | - | - | 0.4 | - | - | - | |
| Non-terpene hydrocarbons | 0.5 | - | 0.5 | - | 5.0 | 2.5 | 0.5 | - | 0.1 | - | - | 0.2 | - | - | |
| Total identified (%) | 100.0 | 100.0 | 99.9 | 99.5 | 99.4 | 99.1 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |
| Descriptors | BIO | HD | SM |
|---|---|---|---|
| Visual features | |||
| Light brown | 0.0 | 0.4 | 0.4 |
| Medium brown | 4.0 | 1.8 | 2.2 |
| Dark brown | 2.0 | 3.8 | 3.4 |
| Shiny | 2.5 | 4.0 | 2.2 |
| Matte | 3.5 | 2.0 | 3.8 |
| Presence of air bubbles | 0.0 | 2.0 | 0.7 |
| Absence of air bubbles | 6.0 | 4.0 | 5.3 |
| Presence of white spots | 1.5 | 1.3 | 1.6 |
| Absence of white spots | 4.5 | 4.8 | 4.4 |
| Presence of stripes | 2.5 | 1.6 | 2.1 |
| Absence of stripes | 3.5 | 4.4 | 3.9 |
| Snap sound at breakage | |||
| Hard | 2.0 | 0.9 | 2.0 |
| Crunchy | 2.5 | 0.6 | 1.1 |
| Crisp | 0.5 | 0.6 | 0.5 |
| Light | 2.5 | 1.6 | 1.8 |
| Mellow | 1.0 | 1.8 | 0.6 |
| Muted | 0.0 | 0.6 | 0.1 |
| High-pitched | 0.0 | 0.8 | 1.0 |
| Low | 0.0 | 1.0 | 1.0 |
| Odor attributes | |||
| Flowery | 0.0 | 0.9 | 0.9 |
| Fruity | 2.5 | 2.0 | 1.8 |
| Caramel | 2.0 | 0.8 | 1.3 |
| Nutty | 1.5 | 0.6 | 1.1 |
| Herbaceous | 1.0 | 1.0 | 0.8 |
| Dairy | 0.0 | 0.3 | 0.1 |
| Fermentation | 0.0 | 0.0 | 0.3 |
| Malty | 0.0 | 0.0 | 0.1 |
| Animal aroma | 0.0 | 0.4 | 0.0 |
| Aroma attributes | |||
| Flowery | 0.0 | 0.3 | 0.6 |
| Fruity | 1.5 | 1.5 | 1.5 |
| Caramel | 0.5 | 1.4 | 1.2 |
| Nutty | 2.0 | 1.3 | 1.1 |
| Herbaceous | 1.5 | 0.8 | 0.9 |
| Dairy | 0.0 | 0.4 | 0.3 |
| Fermentation | 0.5 | 0.4 | 0.7 |
| Malty | 0.0 | 0.0 | 0.1 |
| Plastic | 0.0 | 0.0 | 0.1 |
| Animal | 0.0 | 0.6 | 0.1 |
| Texture and melting attributes | |||
| Smooth (lips) | 4.0 | 4.1 | 3.6 |
| Velvety (lips) | 1.5 | 1.6 | 1.9 |
| Grainy (lips) | 0.5 | 0.5 | 0.5 |
| Smooth (tongue) | 2.5 | 2.8 | 2.4 |
| Velvety (tongue) | 3.0 | 3.3 | 2.9 |
| Grainy (tongue) | 0.5 | 0.1 | 0.6 |
| Immediate melting | 1.0 | 2.6 | 1.1 |
| Delayed melting | 5.0 | 3.4 | 4.9 |
| Taste perception | |||
| Bitter taste | 5.0 | 3.4 | 3.5 |
| Sweet taste | 2.0 | 2.8 | 2.4 |
| Salty taste | 0.5 | 0.8 | 0.4 |
| Acidic taste | 0.0 | 1.1 | 1.6 |
| Umami taste | 0.0 | 0.4 | 0.2 |
| In-mouth sensation | |||
| Astringent sensation | 2.0 | 0.9 | 0.9 |
| Burnt sensation | 0.0 | 0.1 | 0.1 |
| Warmth sensation | 1.5 | 1.6 | 1.7 |
| Sparkling sensation | 0.5 | 0.4 | 0.8 |
| Acrid sensation | 0.0 | 0.3 | 0.4 |
| Metallic sensation | 0.0 | 0.1 | 0.2 |
| Fatty sensation | 0.0 | 0.6 | 0.4 |
| Oily sensation | 2.0 | 1.6 | 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieracci, Y.; Ascrizzi, R.; Pistelli, L.; Flamini, G. Comparison of the Chemical and Sensorial Evaluation of Dark Chocolate Bars. Appl. Sci. 2021, 11, 9964. https://doi.org/10.3390/app11219964
Pieracci Y, Ascrizzi R, Pistelli L, Flamini G. Comparison of the Chemical and Sensorial Evaluation of Dark Chocolate Bars. Applied Sciences. 2021; 11(21):9964. https://doi.org/10.3390/app11219964
Chicago/Turabian StylePieracci, Ylenia, Roberta Ascrizzi, Luisa Pistelli, and Guido Flamini. 2021. "Comparison of the Chemical and Sensorial Evaluation of Dark Chocolate Bars" Applied Sciences 11, no. 21: 9964. https://doi.org/10.3390/app11219964







