A Nondestructive Method of Measuring Zebrafish Adipose Tissue Based on Micro-Computed Tomography (Micro-CT)
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Sample Preparation
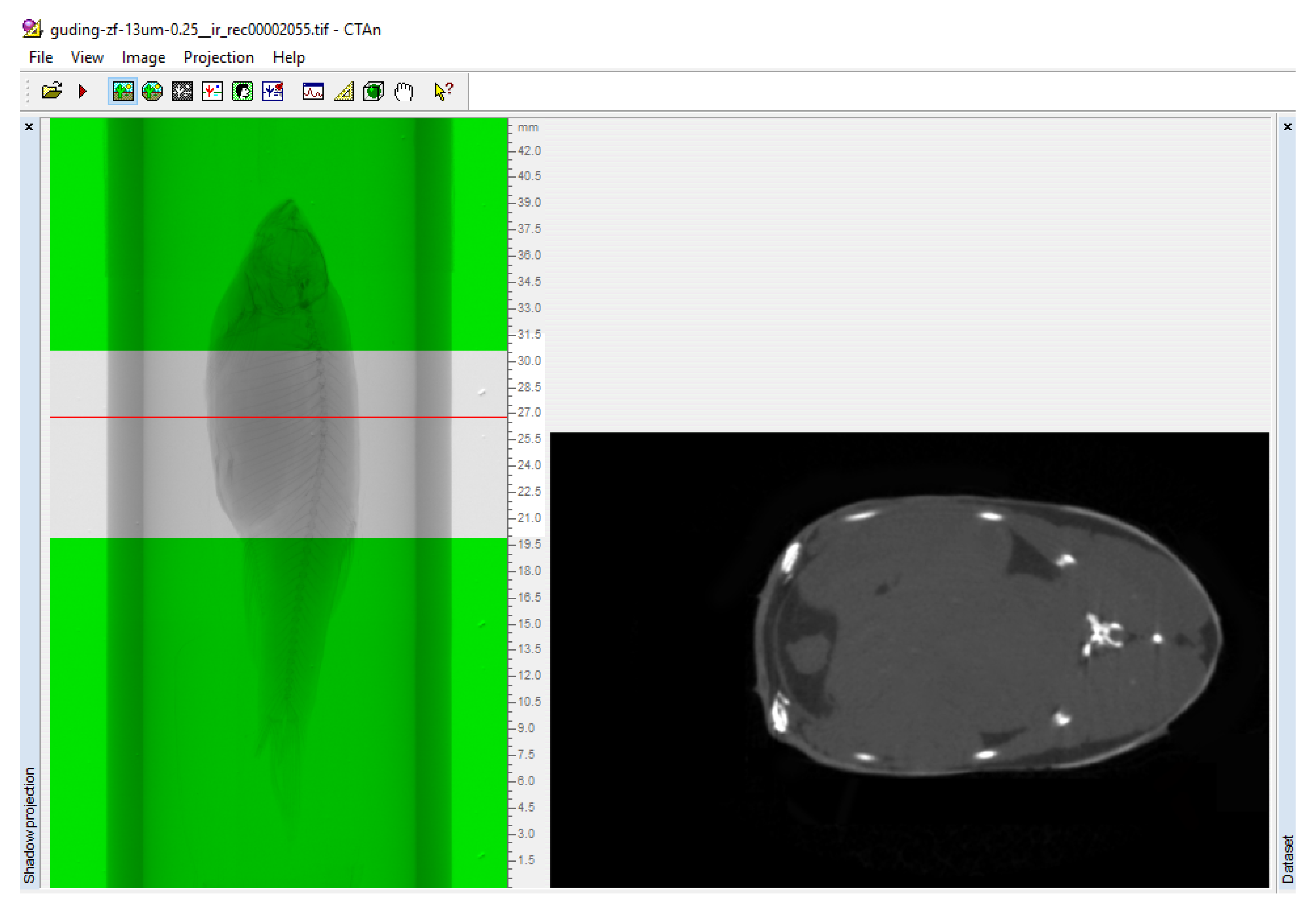
2.3. Optimization of CT-Scanning Imaging Parameters
2.4. Data Reconstruction
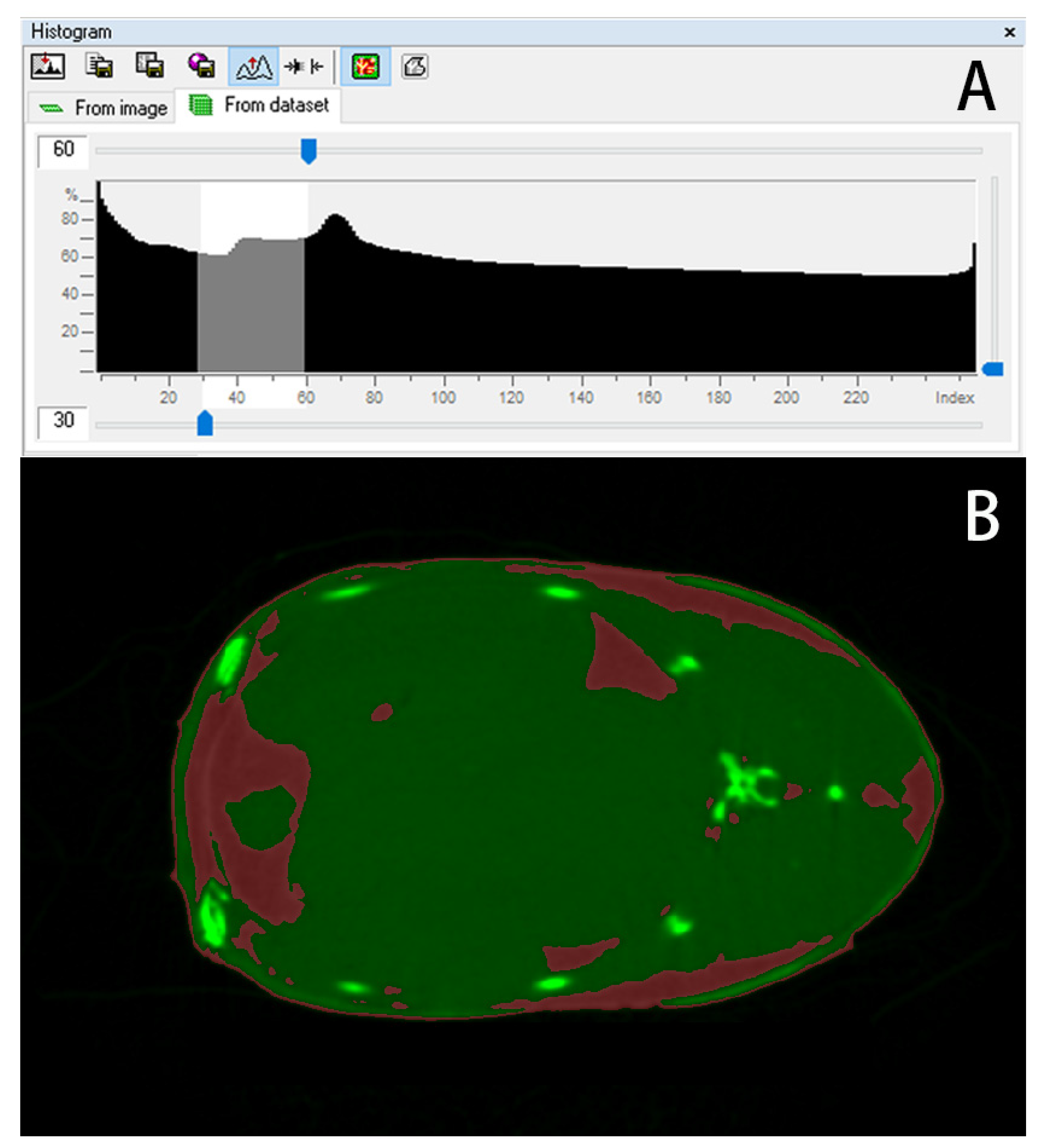
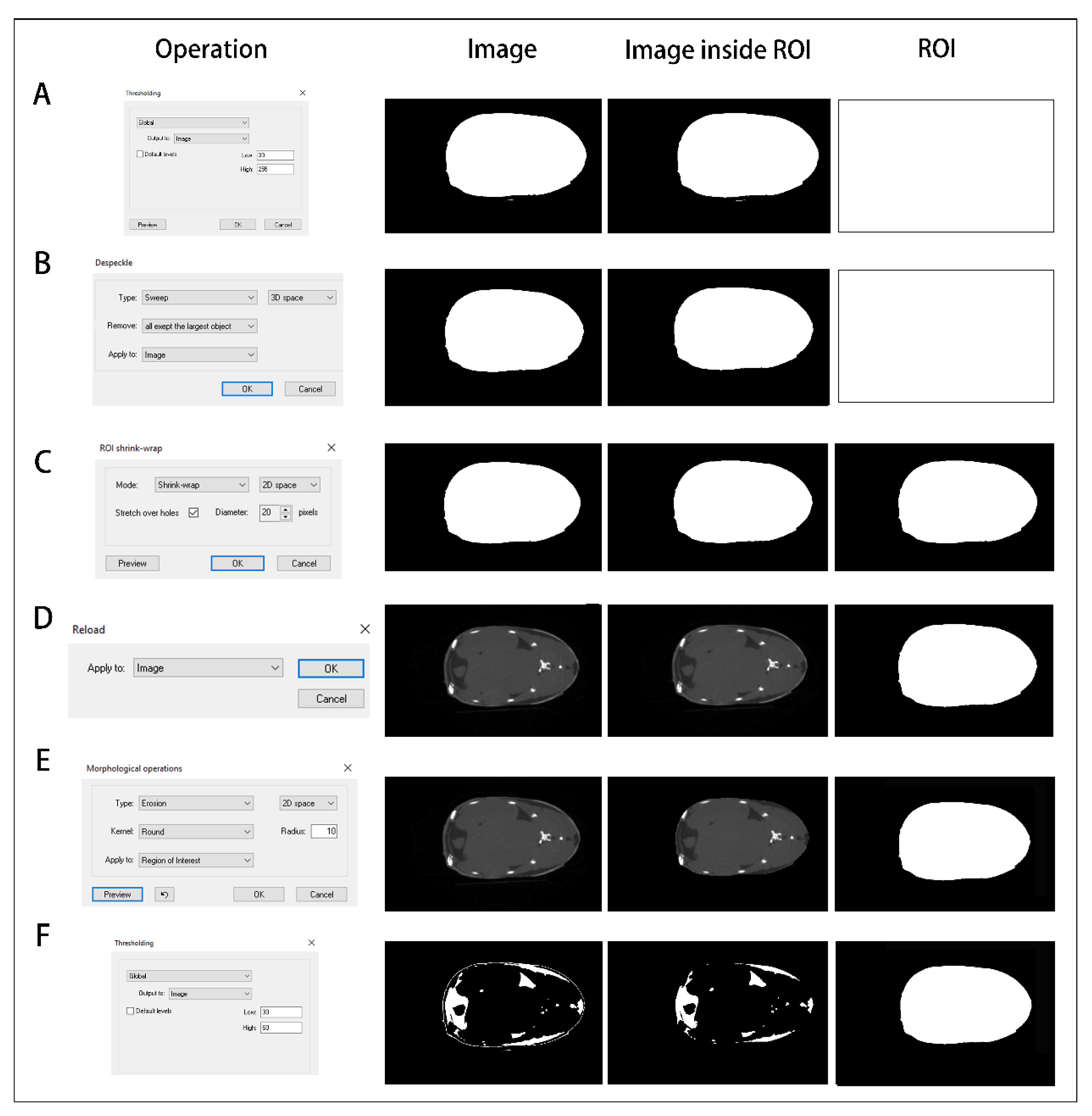
2.5. Data Processing
2.6. Construction of the 3D Model of Adipose Tissue
3. Results
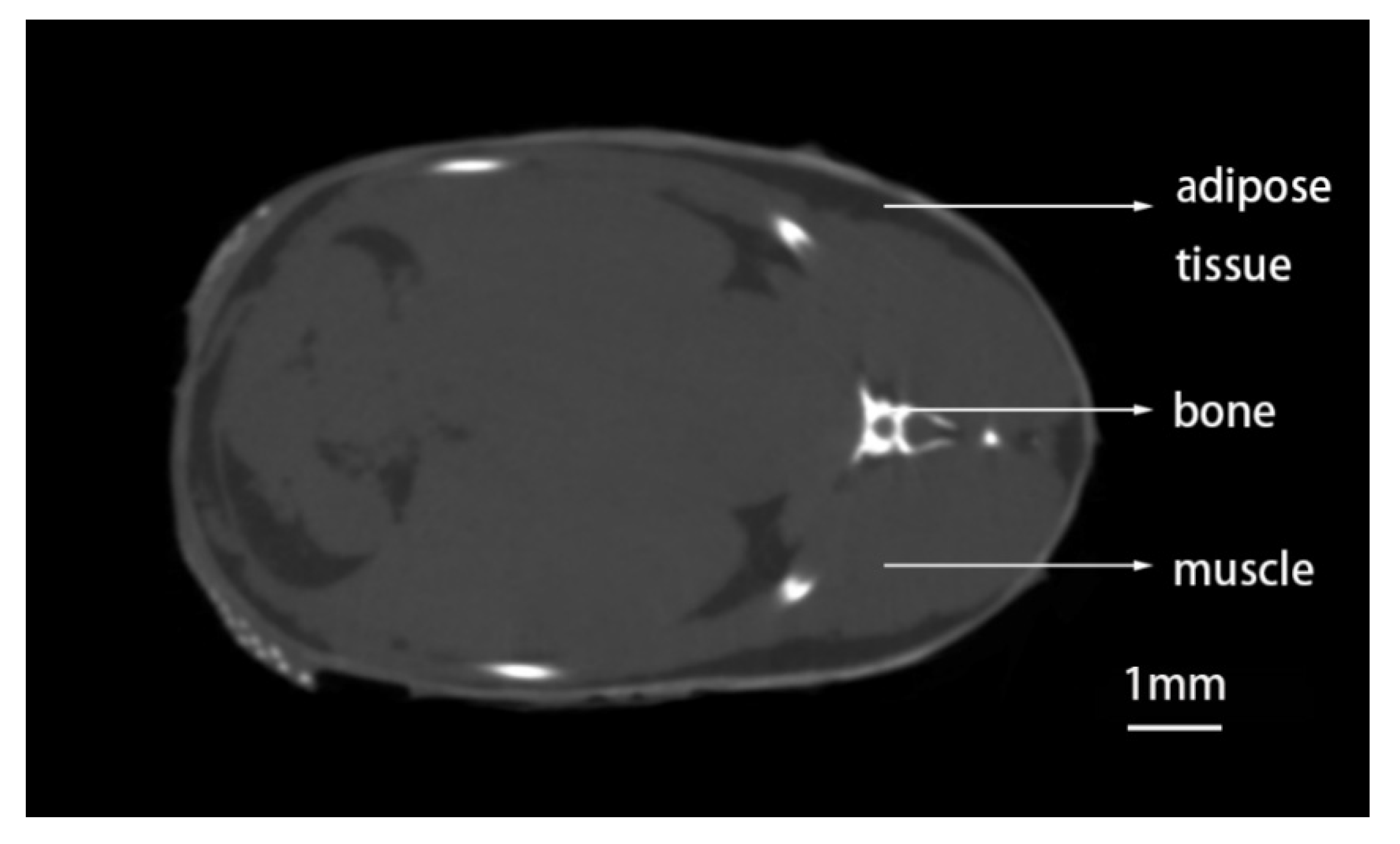
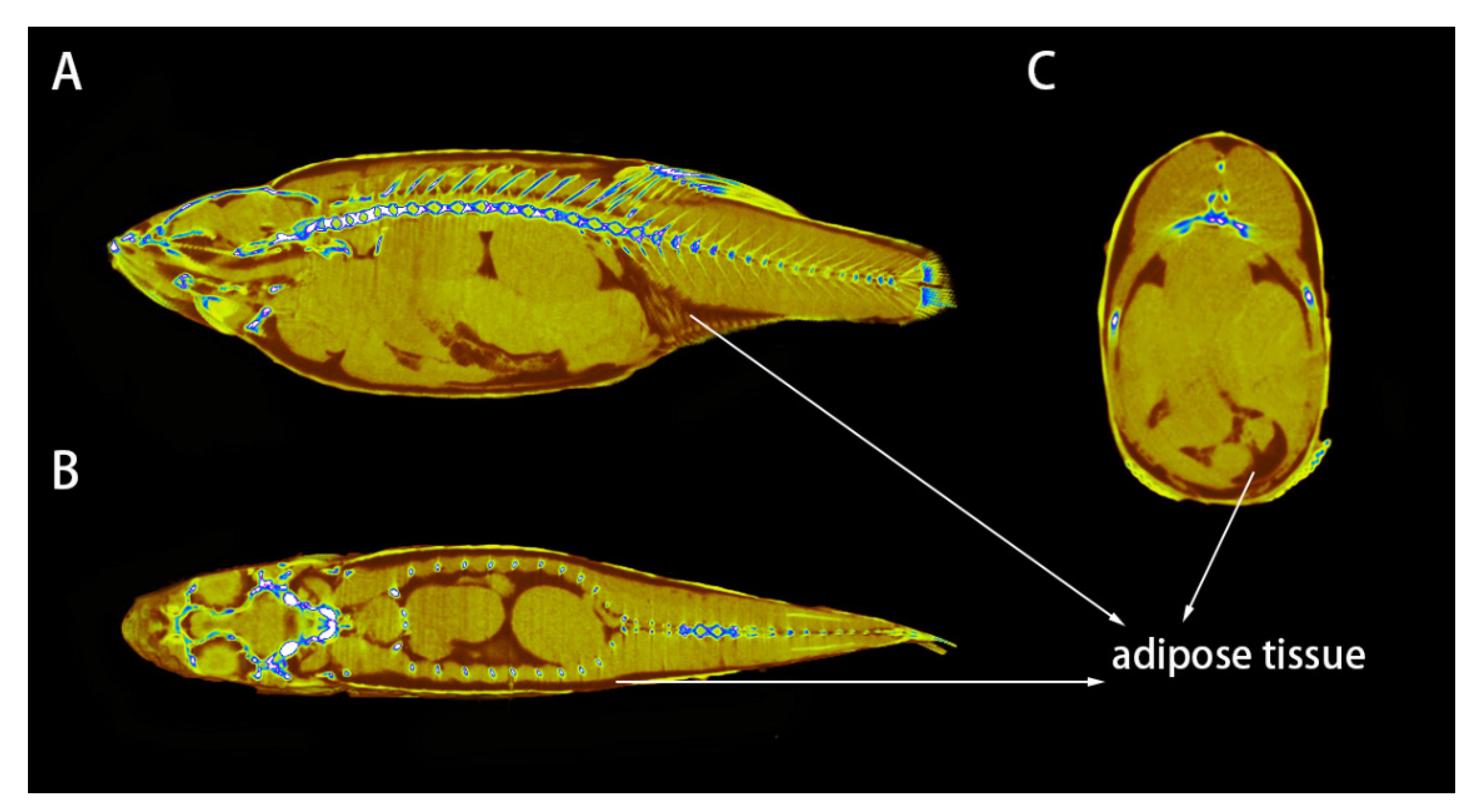
3.1. Method to Detect Adipose Tissue in Zebrafish by CT Scanning
3.2. Distribution of Adipose Tissue in Zebrafish
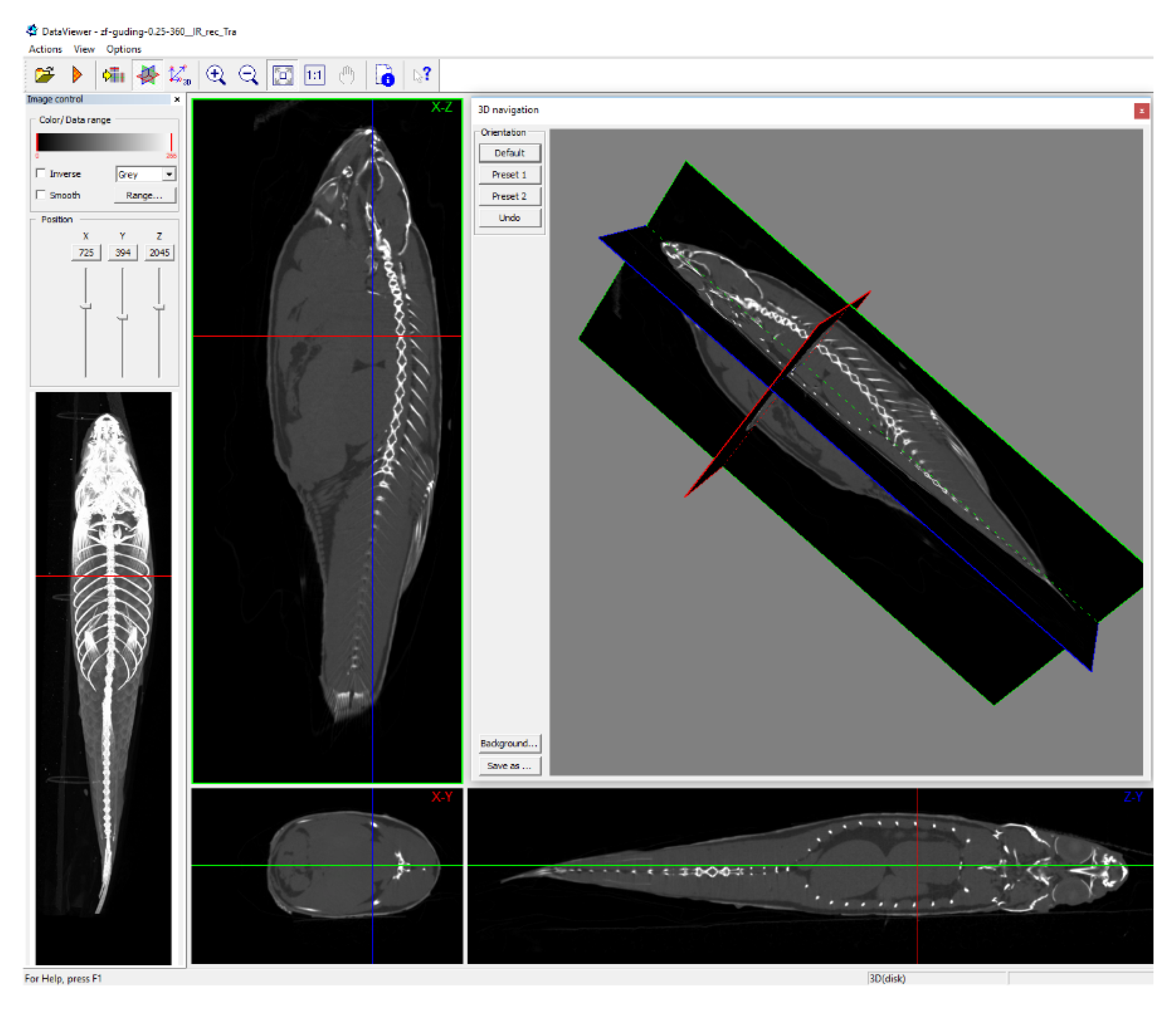
3.3. Scanning the Standard Plane of Trunk Adipose Tissue
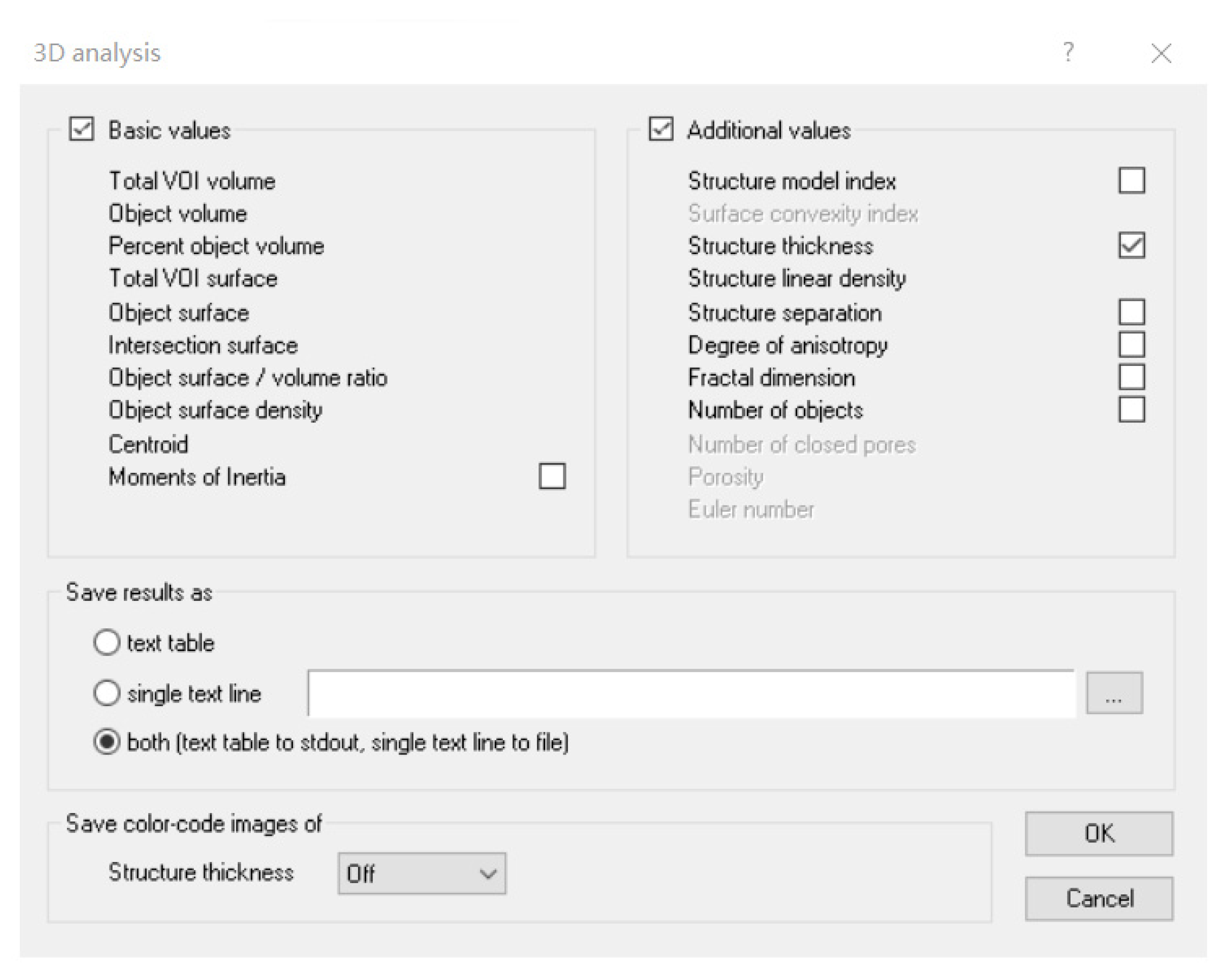
3.4. Quantitative Calculation of Trunk Adipose Tissue
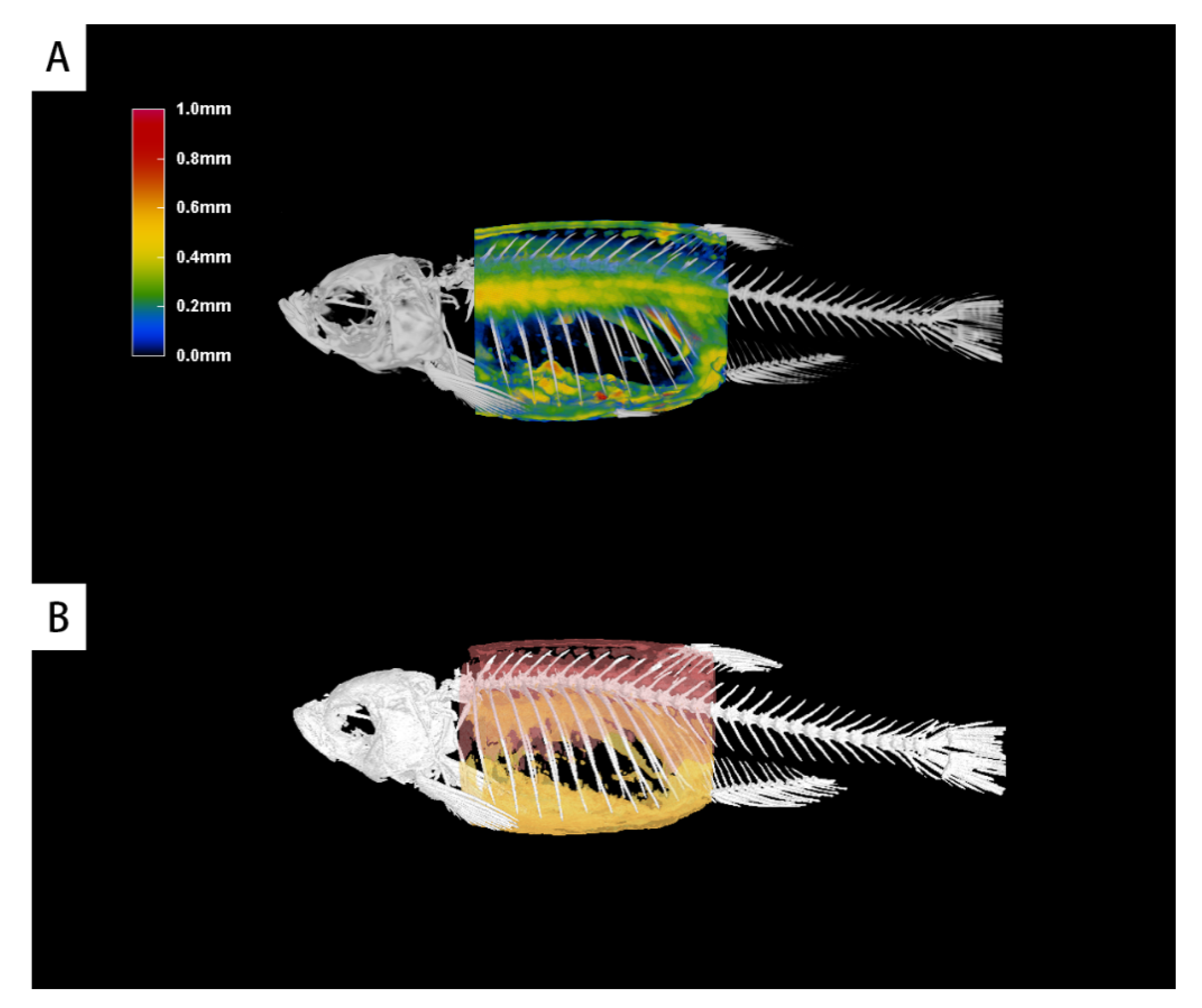
3.5. 3D Graphical Rendering
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niu, H.X.; Qi, F.; Jia, L. Fatty liver disease of fish under intensive culture and its control. Hebei Fish. 2007, 7, 47–48. [Google Scholar]
- Cheng, H.L.; Xia, D.Q.; Wu, T.T. Fatty Liver and Regulation of Lipids Metabolism in Fish. Chin. J. Anim. Nutr. 2006, 18, 294–298. [Google Scholar]
- Du, Z.Y.; Clouet, P.; Zheng, W.H.; Degrace, P.; Tian, L.X.; Liu, Y.J. Biochemical hepatic alterations and body lipid composition in the herbivorous grass carp (Ctenopharyngodon idella) fed high-fat diets. Br. J. Nutr. 2006, 95, 905–915. [Google Scholar] [CrossRef] [Green Version]
- Chatzifotis, S.; Panagiotidou, M.; Panagiotidou, N.; Pavlidis, M.; Nengas, L.; Mylonas, C.C. Effect of dietary lipid levels on growth, feed utilization, body composition and serμm metabolites of meagre (Argyrosomus regius) juveniles. Aquaculture 2010, 307, 65–70. [Google Scholar] [CrossRef]
- Yang, H.K.; Huang, K.; Ruan, D.J. Fatty livers of aquaculture fish species and research advances in the control methods. Reserv. Fish. 2007, 27, 4–5. [Google Scholar]
- Hölttä-Vuori, M.; Salo, V.; Nyberg, L.; Brackmann, C.; Enejder, A.; Panula, P.; Ikonen, E. Zebrafish: Gaining popularity in lipid research. Biochem. J. 2010, 429, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Nordlie, M.A.; Wold, L.E.; Kloner, R.A. Genetic contributors toward increased risk for ischemic heart disease. J. Mol. Cell. Cardiol. 2005, 39, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A. Fine-tuning the lipogenic/lipolytic balance to optimize the metabolic requirements of cancer cell growth: Molecular mechanisms and therapeutic perspectives. Biochim. Biophys. Acta 2010, 1801, 381–391. [Google Scholar] [CrossRef]
- Greene, D.; Selivonchick, D.P. Lipid metabolism in fish. Prog. Lipid Res. 1987, 26, 53–85. [Google Scholar] [CrossRef]
- Du, Z.Y.; Clouet, P.; Degrace, P.; Zheng, W.; Frøyland, L.; Tian, L.; Liu, Y. Hypolipidaemic effects of fenofibrate and fasting in the herbivorous grass carp (Ctenopharyngodon idella) fed a high-fat diet. Br. J. Nutr. 2008, 100, 1200–1212. [Google Scholar] [CrossRef] [Green Version]
- Grunwald, D.J.; Eisen, J.S. Headwaters of the zebrafish-emergence of a new model vertebrate. Nat. Rev. Genet. 2002, 3, 717–724. [Google Scholar] [CrossRef]
- Xin, S.C.; Zhao, Y.Q.; Li, S.; Lin, S.; Zhong, H. Application of zebrafish models in drug screening. Genetics 2012, 34, 1144–1152. [Google Scholar]
- Chen, F.L.; Zhang, S.L.; Li, Y.C. Advances in toxicology research on zebrafish embryos. Hubei Agr. Sci. 2010, 49, 1484–1487. [Google Scholar]
- Wang, M.Y.; Huang, G.D.; Wang, H. Advances in the zebrafish circadian clock mechanisms. Hereditas 2012, 34, 1133–1143. [Google Scholar]
- Wu, Y.P.; Xiong, Q.; Zhang, G.X.; Xu, A.L. The Research Progress of Zebrafish Gene Engineering. Acta Genet. Sin. 2004, 31, 1167–1174. [Google Scholar] [PubMed]
- Hu, Z.W. Research Advance of Nthinyl Estradiol and Phenanthrene Effect on the Zebrafish (Brachydanio rerio). J. Anhui Agr. Sci. 2014, 42, 12539–12541. [Google Scholar]
- Shimada, Y.; Kuninaga, S.; Ariyoshi, M.; Zhang, B.; Shiina, Y.; Takahashi, Y.; Umemoto, N.; Nishimura, Y.; Enari, H.; Tanaka, T. E2F8 promotes hepatic steatosis through FABP3 expression in diet-induced obesity in zebrafish. Nutr. Metab. 2015, 12, 17. [Google Scholar] [CrossRef] [Green Version]
- Meguro, S.; Hasumura, T.; Hase, T. Body fat accumulation in zebrafish is induced by a diet rich in fat and reduced by supplementation with green tea extract. PLoS ONE 2015, 10, e0120142. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Wang, K.; Zheng, W.; Chen, X.; Zhang, W.; Zheng, Y.; Hou, J.; Liu, L. High fat plus high cholesterol diet lead to hepatic steatosis in zebrafish larvae: A novel model for screening anti-hepatic steatosis drugs. Nutr. Metab. 2015, 12, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.H. Application of Hard X-ray Micro- and Nano-CT Techniques in Cell Imaging. Master’ Thesis, University of Science and Technology of China, Hefei, China, 2010. [Google Scholar]
- Available online: http://henke.lbl.gov/optical_constants/pert_form.html (accessed on 15 July 2021).
- Johns, H.E.; Cunningham, J.R. The Physics of Radiology, 4th ed.; Charles C Thomas: Springfield, IL, USA, 1983; ISBN 0-398-04669-7. [Google Scholar]
- Collewet, G.; Bugeon, J.; Idier, J.; Quellec, S.; Quittet, B.; Cambert, M.; Haffray, P. Rapid quantification of muscle fat content and subcutaneous adipose tissue in fish using MRI. Food Chem. 2013, 138, 2008–2015. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, C.; Fauconneau, B.; Médale, F.; Collewet, G.; Akoka, S.; Haffray, P.; Davenel, A. Description of the heterogeneity of lipid distribution in the flesh of brown trout (Salmo trutta) by MR imaging. Aquaculture 2005, 243, 255–267. [Google Scholar] [CrossRef]
- Yin, G.C. 30 nm resolution x-ray imaging at 8 keV using third order diffraction of a zone plate lens objective in a transmission microscope. Appl. Phys. Lett. 2006, 89, 221122. [Google Scholar] [CrossRef] [Green Version]
- Yin, G.C.; Tang, M.T.; Song, Y.F.; Chen, F.R.; Liang, K.S.; Duewer, F.W.; Yun, W.; Ko, C.; Shieh, H.D. Energy-tunable transmission x-ray microscope for differential contrast imaging with near 60 nm resolution tomography. Appl. Phys. Lett. 2006, 88, 241115. [Google Scholar] [CrossRef] [Green Version]
- Chu, Y.S.; Yi, J.M.; Cario, F.D.; Shen, Q.; Lee, W.; Wu, H.J.; Wang, C.L.; Wang, J.Y.; Liu, C.J.; Wang, C.H.; et al. Hard-x-ray microscopy with Fresnel zone plates reaches 40 nm Rayleigh resolution. Appl. Phys. Lett. 2008, 92, 103119. [Google Scholar] [CrossRef] [Green Version]
- Gui, J.B.; Hu, Z.L.; Zhou, Y.; Zheng, H.R. Technology development of micro-CT with high spatial resolution. Comput. Tomo. Theory Appl. 2009, 18, 106–116. [Google Scholar]
- Wang, T.; Xia, W.J.; Zhao, Y.S.; Zhang, Y. Review of metal artifact reduction in computed tomography. Chin. J. Stereol. Image Anal. 2020, 25, 5–21. [Google Scholar]
- Cejas, J.R.; Almansa, E.; Jérez, S.; Bolaños, A.; Samper, M.; Lorenzo, A. Lipid and fatty acid composition of muscle and liver from wild and captive mature female broodstocks of white seabream, Diplodus sargus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004, 138, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Zou, Q.; Wang, H.; Huang, F.; Liu, X.; Chen, L.; Yang, C.; Yang, Y. Conjugated linoleic acid differentially modulates growth, tissue lipid deposition, and gene expression involved in the lipid metabolism of grass carp. Aquaculture 2014, 432, 181–191. [Google Scholar] [CrossRef]
- Wang, Y.; Rimm, E.B.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am. J. Clin. Nutr. 2005, 81, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Du, Z.Y.; Tao, M.; Lock, E.J.; Hao, Q.; Kristiansen, K.; Frøyland, L.; Madsen, L. Depot-dependent effects of adipose tissue explants on co-cultured hepatocytes. PLoS ONE 2011, 6, e20917. [Google Scholar] [CrossRef] [Green Version]
- Misra, A.; Garg, A.; Abate, N.; Peshock, R.M.; Stray-Gundersen, J.; Grundy, S.M. Relationship of anterior and posterior subcutaneous abdominal fat to insulin sensitivity in nondiabetic men. Obes. Res. 1997, 5, 93–99. [Google Scholar] [CrossRef]
- Horrocks, L.A.; Sun, G.Y.; D’Amato, R. Changes in brain lipids during aging. Neurobiol. Aging 1975, 16, 359–367. [Google Scholar]
- Lyu, X.D.; Zhou, Y.M. The role of B-ultrasound measurement of subcutaneous fat thicknesses at different sites in detecting malnutrition. Zhejiang Med. J. 2021, 43, 781–782. [Google Scholar]
- Jiao, Y.B.; Deng, L.; Du, P.; Guo, H.; Wang, R.Y.; Fan, X.Y. Study on correlation between body composition and subcutaneous fatty thickness by ultrasonography in adult males. Clin. Med Equip. 2013, 34, 59–60. [Google Scholar]
- Wang, Q.A.; Tao, C.; Gupta RKScherer, P.E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 2013, 19, 1338–1344. [Google Scholar] [CrossRef]
- Le, J.C.; Wu, Q.P.; Zhou, N.; Zhang, K.; Zheng, D.; Lv, G.L.; Cheng, J.D. Correlation between abdominal wall subcutaneous fat thickness and heart weight in southern Chinese population. J. Forensic Med. 2021, 37, 351–357. [Google Scholar]
- Zhao, L. Diagnostic Significance of BMI (abdominal fat thickness) and Common Tumor Markers on Endometrial Cancer. Master’ Thesis, Dalian Medical University, Dalian, China, 2020. [Google Scholar]
- Huang, Z.D.; Li, S.C.; Zhang, A.Z.; Wang, X.; Ding, Z.; Zhang, X.F. Correlation between upper abdominal fat volume and body mass index in patients with non-alcoholic fatty liver disease based on magnetic resonance imaging. Chin. J. Magn. Reson. Imaging 2021, 12, 80–83. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 495–509. [Google Scholar] [CrossRef]





| Element | Atomic Number | Absorption Area | f1 | f2 |
|---|---|---|---|---|
| H | 1 | 5.881 × 10−3 | 1.000 | 0.1127 × 10−5 |
| C | 6 | 4.260 | 6.019 | 0.9728 × 10−2 |
| N | 7 | 6.984 | 7.033 | 0.1860 × 10−1 |
| O | 8 | 11.22 | 8.053 | 0.3414 × 10−1 |
| Na | 11 | 28.31 | 11.14 | 0.1237 |
| Mg | 12 | 39.67 | 12.18 | 0.1833 |
| P | 15 | 74.94 | 15.31 | 0.4413 |
| S | 16 | 91.31 | 16.34 | 0.5567 |
| K | 19 | 150.2 | 19.42 | 1.116 |
| Ca | 20 | 174.1 | 20.38 | 1.327 |
| H | C | N | O | Na | Mg | P | S | K | Ca | Mass-Weighted Average of Atomic Number | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Atomic number | 1 | 6 | 7 | 8 | 11 | 12 | 15 | 16 | 19 | 20 | |
| Water | 11.2 | - | - | 88.8 | - | - | - | - | - | - | 7.2 |
| Muscle | 10.2 | 12.3 | 3.5 | 72.9 | 0.08 | 0.02 | 0.2 | 0.5 | 0.3 | 0.007 | 7.1 |
| Fat | 11.2 | 57.3 | 1.1 | 30.3 | - | - | - | 0.006 | - | - | 6.1 |
| Skeleton | 6.4 | 27.8 | 2.7 | 41 | - | 0.2 | 7 | 0.2 | - | 14.7 | 9.1 |
| Camera Resolution Setting (Pixel Field Width) | Image Pixel Size (μm) | X-ray Voltage (KV) | X-ray Current (μA) | Tomographic Rotation | Rotation Step | Filter (mm) | |
|---|---|---|---|---|---|---|---|
| Experiment 1 | 2000 | 13 | 55 | 200 | 180° | 0.5° | Al 0.25 |
| 4000 | 10 | 55 | 200 | 180° | 0.5° | Al 0.25 | |
| Experiment 2 | 2000 | 13 | 55 | 200 | 180° | 0.5° | Al 0.25 |
| 2000 | 13 | 55 | 200 | 360° | 0.5° | Al 0.25 |
| Description | Tissue Volume (TV) | Object Volume (Obj.V) | Percent Object Volume (Obj.V/TV) | Structure Thickness Distribution (St.Th) |
|---|---|---|---|---|
| Unit | mm3 | mm3 | % | mm |
| 385.62 | 55.91 | 14.49 | 0.3092 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, G.; Xiao, Y.; Zuo, Y.; Zhou, F. A Nondestructive Method of Measuring Zebrafish Adipose Tissue Based on Micro-Computed Tomography (Micro-CT). Appl. Sci. 2021, 11, 10510. https://doi.org/10.3390/app112210510
Wang X, Wang G, Xiao Y, Zuo Y, Zhou F. A Nondestructive Method of Measuring Zebrafish Adipose Tissue Based on Micro-Computed Tomography (Micro-CT). Applied Sciences. 2021; 11(22):10510. https://doi.org/10.3390/app112210510
Chicago/Turabian StyleWang, Xin, Guangxin Wang, Yuan Xiao, Yanxia Zuo, and Fang Zhou. 2021. "A Nondestructive Method of Measuring Zebrafish Adipose Tissue Based on Micro-Computed Tomography (Micro-CT)" Applied Sciences 11, no. 22: 10510. https://doi.org/10.3390/app112210510






