Alleviation of Osteoarthritis-Induced Pain and Motor Deficits in Rats by a Novel Device for the Intramuscular Insertion of Cog Polydioxanone Filament
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Induction of OA and MEST Application
2.3. Behavioral Tests
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Western Blotting
2.6. Histology
2.7. Gross Morphology
2.8. Micro-CT
2.9. Statistical Analysis
3. Results
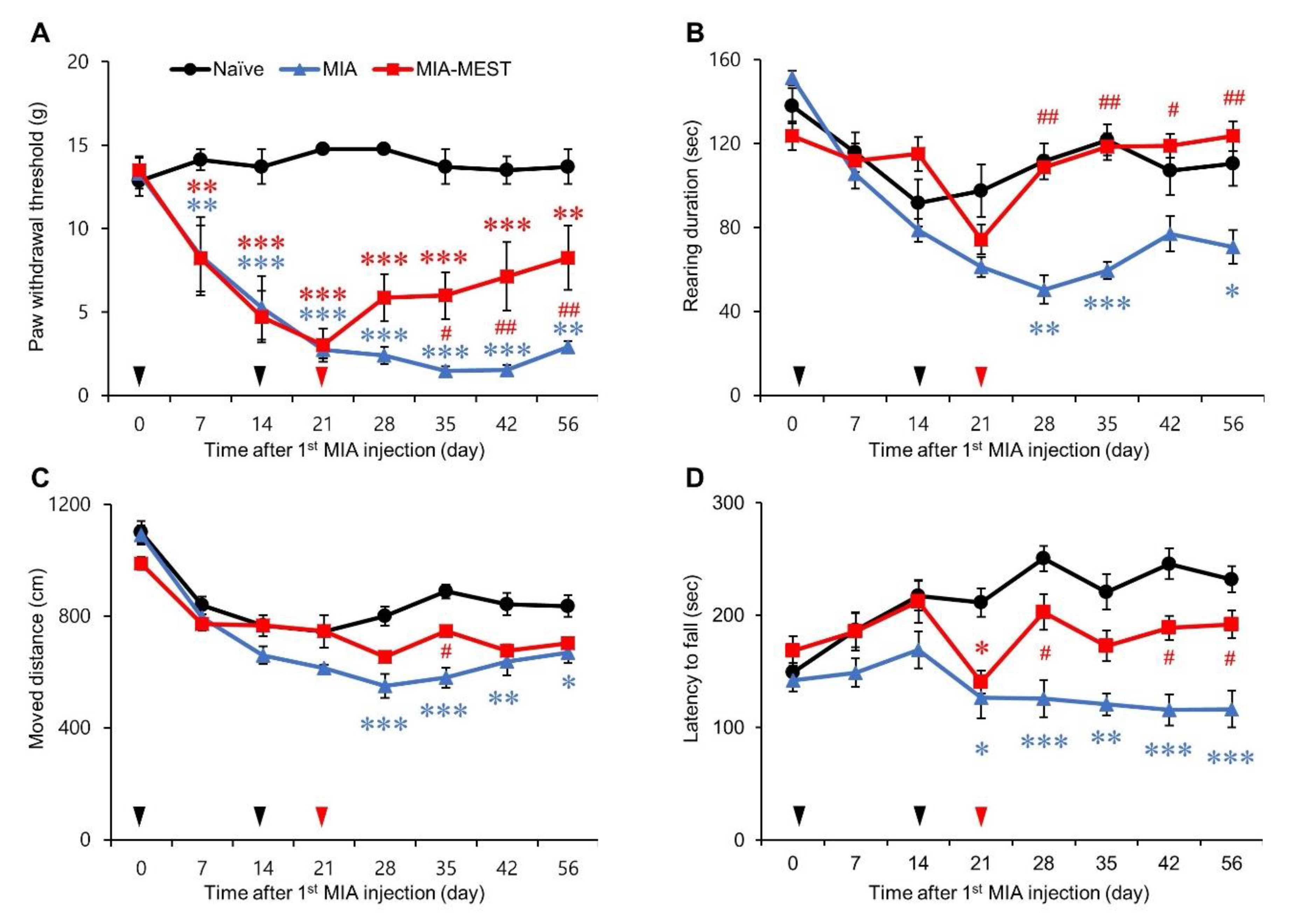
3.1. Pain Relief and Motor Deficit Recovery Using MEST
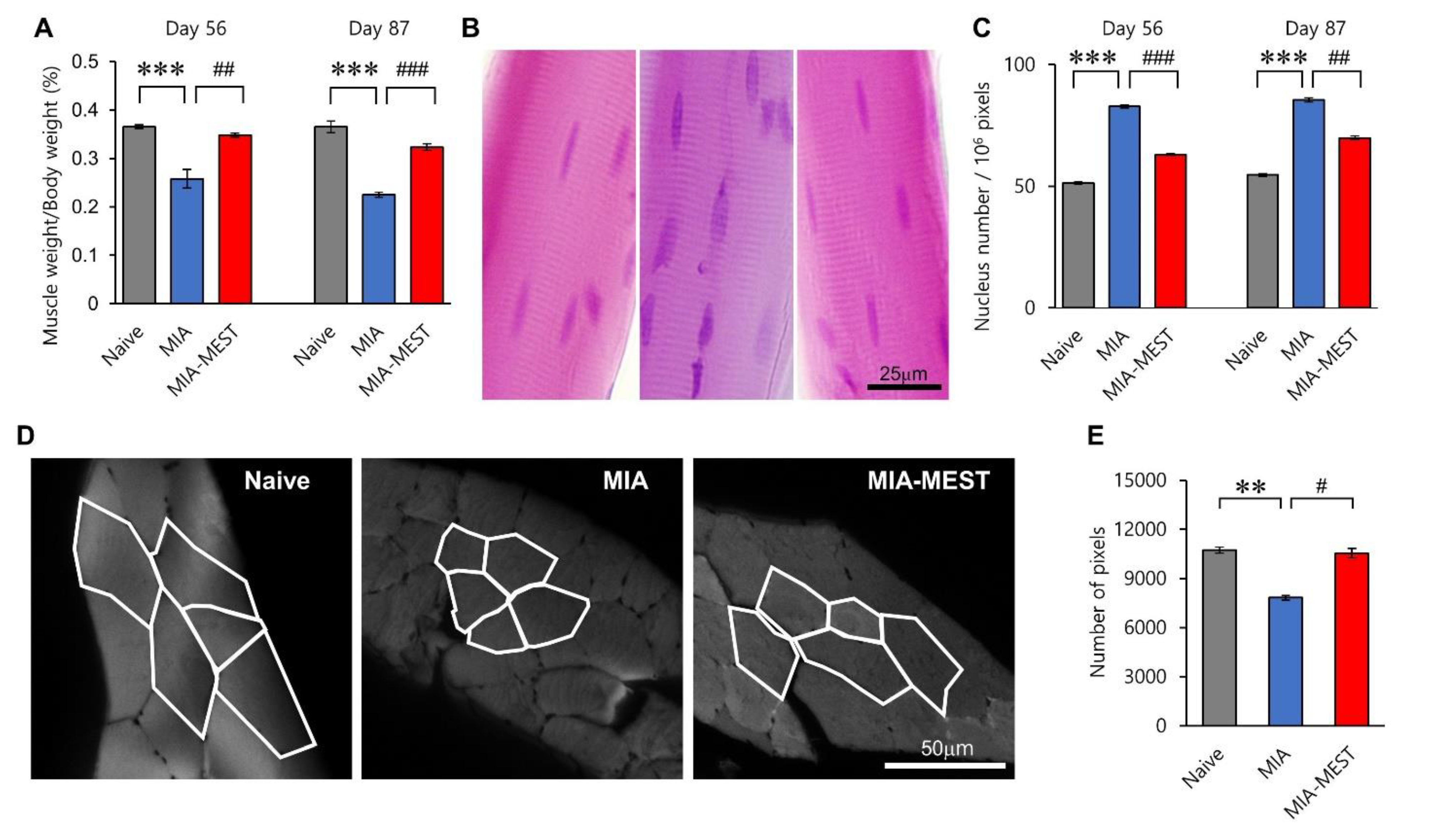
3.2. Restoration of Muscle Atrophy Using MEST
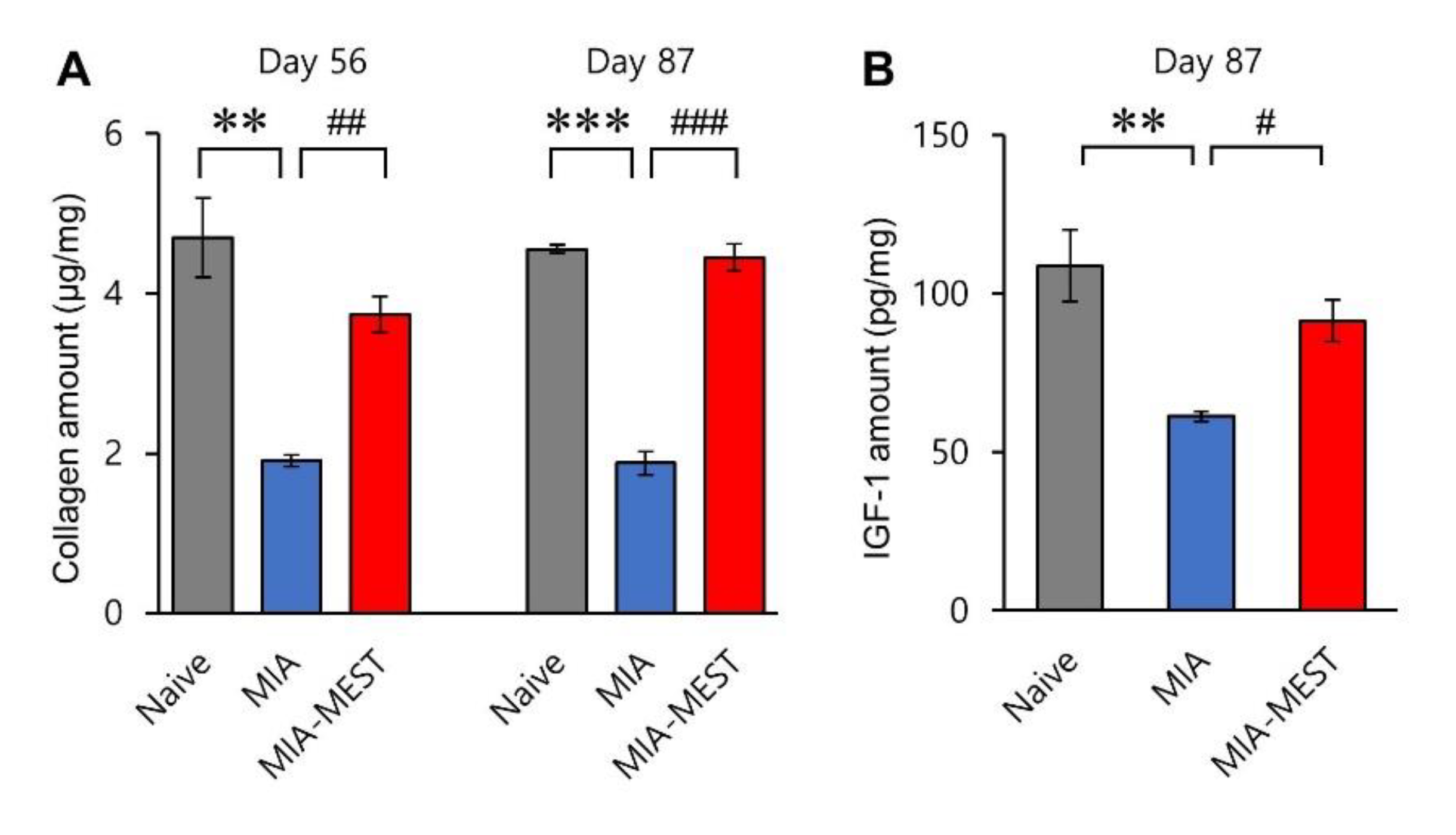
3.3. Recovery of Collagen and IGF-1 Levels Using MEST
3.4. No Inflammatory Response Using MEST
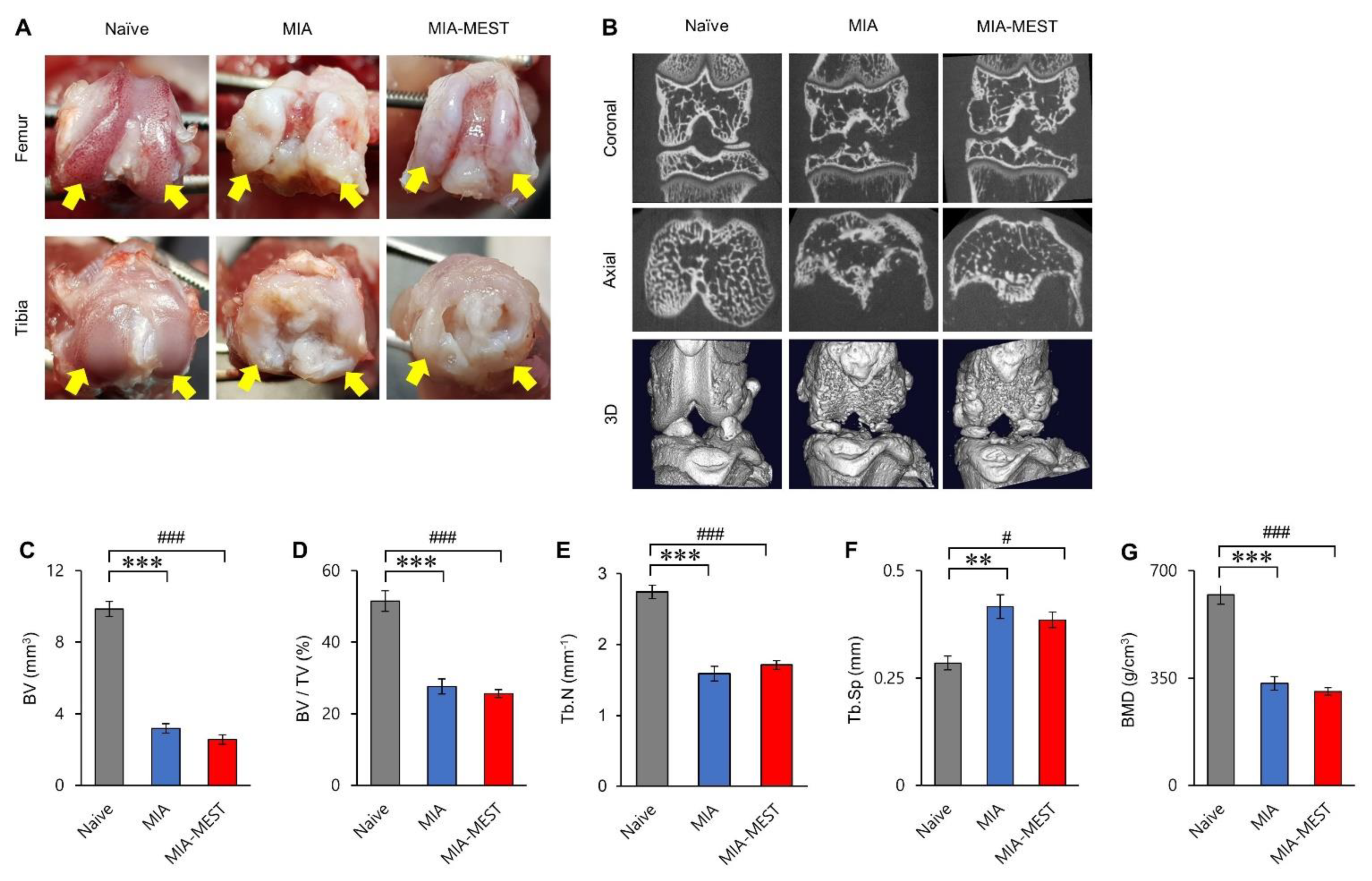
3.5. Effect of MEST on the Joint Structure
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Healey, E.L.; Main, C.J.; Ryan, S.; McHugh, G.A.; Porcheret, M.; Finney, A.G.; Morden, A.; Dziedzic, K.S. A nurse-led clinic for patients consulting with osteoarthritis in general practice: Development and impact of training in a cluster randomised controlled trial. BMC Fam. Pract. 2016, 17, 173. [Google Scholar] [CrossRef] [Green Version]
- Nelson, A. Osteoarthritis year in review 2017: Clinical. Osteoarthr. Cartil. 2018, 26, 319–325. [Google Scholar] [CrossRef] [Green Version]
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Barr, A.; Cicuttini, F.; Conaghan, P.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.-P. Osteoarthritis. Nat. Rev. Dis. Prim. 2016, 2, 16072. [Google Scholar] [CrossRef] [Green Version]
- Shorter, E.; Sannicandro, A.J.; Poulet, B.; Goljanek-Whysall, K. Skeletal Muscle Wasting and Its Relationship with Osteoarthritis: A Mini-Review of Mechanisms and Current Interventions. Curr. Rheumatol. Rep. 2019, 21, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Krishnasamy, P.; Hall, M.; Robbins, S. The role of skeletal muscle in the pathophysiology and management of knee osteoarthritis. Rheumatology 2018, 57, iv124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Øiestad, B.; Juhl, C.; Eitzen, I.; Thorlund, J. Knee extensor muscle weakness is a risk factor for development of knee osteoarthritis. A systematic review and meta-analysis. Osteoarthr. Cartil. 2015, 23, 171–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segal, N.A.; Glass, N. Is Quadriceps Muscle Weakness a Risk Factor for Incident or Progressive Knee Osteoarthritis? Physician Sportsmed. 2011, 39, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.; Hagen, K.B.; Bijlsma, J.W.J.; Andreassen, O.; Christensen, P.; Conaghan, P.; Doherty, M.; Geenen, R.; Hammond, A.; Kjeken, I.; et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann. Rheum. Dis. 2013, 72, 1125–1135. [Google Scholar] [CrossRef] [Green Version]
- Imoto, A.M.; Peccin, M.S.; Trevisani, V. Exercícios de fortalecimento de quadríceps são efetivos na melhora da dor, função e qualidade de vida de pacientes com osteoartrite do joelho. Acta Ortopédica Bras. 2012, 20, 174–179. [Google Scholar] [CrossRef] [Green Version]
- Zacharias, A.; Green, R.; Semciw, A.; Kingsley, M.; Pizzari, T. Efficacy of rehabilitation programs for improving muscle strength in people with hip or knee osteoarthritis: A systematic review with meta-analysis. Osteoarthr. Cartil. 2014, 22, 1752–1773. [Google Scholar] [CrossRef] [Green Version]
- McAlindon, T.E.; Bannuru, R.R.; Sullivan, M.C.; Arden, N.K.; Berenbaum, F.; Bierma-Zeinstra, S.M.; Hawker, G.A.; Henrotin, Y.; Hunter, D.J.; Kawaguchi, H.; et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 363–388. [Google Scholar] [CrossRef] [Green Version]
- Hochberg, M.C.; Altman, R.D.; April, K.T.; Benkhalti, M.; Guyatt, G.; McGowan, J.; Towheed, T.; Welch, V.; Wells, G.; Tugwell, P. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Rheum. 2012, 64, 465–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Moskowitz, R.W.; Nuki, G.; Abramson, S.; Altman, R.D.; Arden, N.; Bierma-Zeinstra, S.; Brandt, K.D.; Croft, P.; Doherty, M.; et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthr. Cartil. 2008, 16, 137–162. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.J.; Park, T.J.; Kim, B.Y.; Kim, C.M.; Suh, D.H.; Lee, S.J.; Moon, H.-R.; Ryu, H.J. Comparative effects of various absorbable threads in a rat model. J. Cosmet. Laser Ther. 2018, 21, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Abhari, R.E.; Mouthuy, P.-A.; Zargar, N.; Brown, C.; Carr, A. Effect of annealing on the mechanical properties and the degradation of electrospun polydioxanone filaments. J. Mech. Behav. Biomed. Mater. 2017, 67, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Dudhia, J.; Dakin, S.G.; Snelling, S.; Lach, A.; De Godoy, R.; Mouthuy, P.-A.; Smith, R.; Morrey, M.; Carr, A.J. Histological evaluation of cellular response to a multifilament electrospun suture for tendon repair. PLoS ONE 2020, 15, e0234982. [Google Scholar] [CrossRef]
- Peterson, L.; Ernest, C. 39-Augmented Grafts: Synthetic/Allograft/Autograft. In The Anterior Cruciate Ligament, 2nd ed.; Prodromos, C.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 160–162.e161. [Google Scholar]
- Tong, L.X.; Rieder, E. Thread-Lifts: A Double-Edged Suture? A Comprehensive Review of the Literature. Dermatol. Surg. 2019, 45, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, G.W.; Mercer, H.; Cormier, J.; Dunbar, C.; Benoit, L.; Adams, C.; Jezierski, J.; Luginbuhl, A.; Bilsky, E. Monosodium iodoacetate-induced osteoarthritis produces pain-depressed wheel running in rats: Implications for preclinical behavioral assessment of chronic pain. Pharmacol. Biochem. Behav. 2011, 98, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, V.D.O.N.; Filippin, L.I.; Viacava, P.R.; de Oliveira, P.G.; Xavier, R.M. Muscle wasting in collagen-induced arthritis and disuse atrophy. Exp. Biol. Med. 2013, 238, 1421–1430. [Google Scholar] [CrossRef]
- Lampropoulou-Adamidou, K.; Lelovas, P.; Karadimas, E.V.; Liakou, C.; Triantafillopoulos, I.K.; Dontas, I.; Papaioannou, N.A. Useful animal models for the research of osteoarthritis. Eur. J. Orthop. Surg. Traumatol. 2014, 24, 263–271. [Google Scholar] [CrossRef]
- Bonin, R.P.; Bories, C.; De Koninck, Y. A Simplified Up-Down Method (SUDO) for Measuring Mechanical Nociception in Rodents Using von Frey Filaments. Mol. Pain 2014, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Kalra, R. Use of barbed threads in facial rejuvenation. Indian J. Plast. Surg. 2008, 41, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Myung, Y.; Jung, C. Mini-midface Lift Using Polydioxanone Cog Threads. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2920. [Google Scholar] [CrossRef] [PubMed]
- Tavares, J.D.P.; Oliveira, C.A.C.P.; Torres, R.P.; Bahmad, F. Facial thread lifting with suture suspension. Braz. J. Otorhinolaryngol. 2017, 83, 712–719. [Google Scholar] [CrossRef]
- Sardesai, M.G.; Zakhary, K.; Ellis, D.A.F. Thread-lifts: The Good, the Bad, and the Ugly. Arch. Facial Plast. Surg. 2008, 10, 284–285. [Google Scholar] [CrossRef]
- Piel, M.J.; Kroin, J.S.; van Wijnen, A.J.; Kc, R.; Im, H.-J. Pain assessment in animal models of osteoarthritis. Gene 2014, 537, 184–188. [Google Scholar] [CrossRef] [Green Version]
- Brooks, S.P.; Dunnett, S. Tests to assess motor phenotype in mice: A user’s guide. Nat. Rev. Neurosci. 2009, 10, 519–529. [Google Scholar] [CrossRef]
- McAlindon, T.E.; Cooper, C.; Kirwan, J.R.; Dieppe, P.A. Determinants of disability in osteoarthritis of the knee. Ann. Rheum. Dis. 1993, 52, 258–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurley, M.V. The Role of Muscle Weakness in the Pathogenesis of Osteoarthritis. Rheum. Dis. Clin. North Am. 1999, 25, 283–298. [Google Scholar] [CrossRef]
- Andriacchi, T.P.; Mündermann, A.; Smith, R.L.; Alexander, E.J.; Dyrby, C.O.; Koo, S. A Framework for the in Vivo Pathomechanics of Osteoarthritis at the Knee. Ann. Biomed. Eng. 2004, 32, 447–457. [Google Scholar] [CrossRef]
- Shaktivesh; Malekipour, F.; Lee, P.V. Shock absorbing ability in healthy and damaged cartilage-bone under high-rate compression. J. Mech. Behav. Biomed. Mater. 2018, 90, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Macri, E.; Stefanik, J.J.; Khan, K.K.; Crossley, K.M. Is Tibiofemoral or Patellofemoral Alignment or Trochlear Morphology Associated With Patellofemoral Osteoarthritis? A Systematic Review. Arthritis Rheum. 2016, 68, 1453–1470. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Eckstein, F. Exercise and osteoarthritis. J. Anat. 2009, 214, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Juhl, C.; Christensen, R.; Roos, E.; Zhang, W.; Lund, H. Impact of Exercise Type and Dose on Pain and Disability in Knee Osteoarthritis: A Systematic Review and Meta-Regression Analysis of Randomized Controlled Trials. Arthritis Rheumatol. 2014, 66, 622–636. [Google Scholar] [CrossRef]
- Luc-Harkey, B.A.; Safran-Norton, C.E.; Mandl, L.A.; Katz, J.N.; Losina, E. Associations among knee muscle strength, structural damage, and pain and mobility in individuals with osteoarthritis and symptomatic meniscal tear. BMC Musculoskelet. Disord. 2018, 19, 258. [Google Scholar] [CrossRef]
- Hurley, M.; Dickson, K.; Hallett, R.; Grant, R.; Hauari, H.; Walsh, N.; Stansfield, C.; Oliver, S. Exercise interventions and patient beliefs for people with hip, knee or hip and knee osteoarthritis: A mixed methods review. Cochrane Database Syst. Rev. 2018, 4, CD010842. [Google Scholar] [CrossRef] [PubMed]
- Schiphof, D.; Driest, J.J.V.D.; Runhaar, J. Osteoarthritis year in review 2017: Rehabilitation and outcomes. Osteoarthr. Cartil. 2018, 26, 326–340. [Google Scholar] [CrossRef] [Green Version]
- Fransen, M.; McConnell, S.; Harmer, A.R.; Van Der Esch, M.; Simic, M.; Bennell, K. Exercise for osteoarthritis of the knee: A Cochrane systematic review. Br. J. Sports Med. 2015, 49, 1554–1557. [Google Scholar] [CrossRef]
- Goh, S.-L.; Persson, M.S.M.; Stocks, J.; Hou, Y.; Welton, N.; Lin, J.; Hall, M.C.; Doherty, M.; Zhang, W. Relative Efficacy of Different Exercises for Pain, Function, Performance and Quality of Life in Knee and Hip Osteoarthritis: Systematic Review and Network Meta-Analysis. Sports Med. 2019, 49, 743–761. [Google Scholar] [CrossRef] [Green Version]
- Tidball, J.G. Mechanical signal transduction in skeletal muscle growth and adaptation. J. Appl. Physiol. 2005, 98, 1900–1908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinac, B.; Cox, C.D. Mechanosensory Transduction: Focus on Ion Channels☆. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-0-12-809633-8. [Google Scholar] [CrossRef]
- Vandebrouck, C.; Martin, D.; Schoor, M.C.-V.; Debaix, H.; Gailly, P. Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers. J. Cell Biol. 2002, 158, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Krüger, J.; Kunert-Keil, C.; Bisping, F.; Brinkmeier, H. Transient receptor potential cation channels in normal and dystrophic mdx muscle. Neuromuscul. Disord. 2008, 18, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.B. Mechanotransduction in skeletal muscle. Front. Biosci. 2007, 12, 174–191. [Google Scholar] [CrossRef] [Green Version]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef] [Green Version]
- Perrone, C.E.; Fenwick-Smith, D.; Vandenburgh, H.H. Collagen and Stretch Modulate Autocrine Secretion of Insulin-like Growth Factor-1 and Insulin-like Growth Factor Binding Proteins from Differentiated Skeletal Muscle Cells. J. Biol. Chem. 1995, 270, 2099–2106. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.S.; Ahmad, K.; Lee, E.J.; Lee, Y.-H.; Choi, I. Implications of Insulin-Like Growth Factor-1 in Skeletal Muscle and Various Diseases. Cells 2020, 9, 1773. [Google Scholar] [CrossRef]
- Sandri, M. Signaling in Muscle Atrophy and Hypertrophy. Physiology 2008, 23, 160–170. [Google Scholar] [CrossRef] [Green Version]
- Frost, R.A.; Lang, C.H. Multifaceted Role of Insulin-Like Growth Factors and Mammalian Target of Rapamycin in Skeletal Muscle. Endocrinol. Metab. Clin. North Am. 2012, 41, 297–322. [Google Scholar] [CrossRef] [Green Version]
- Coleman, M.E.; DeMayo, F.; Yin, K.C.; Lee, H.M.; Geske, R.; Montgomery, C.; Schwartz, R.J. Myogenic Vector Expression of Insulin-like Growth Factor I Stimulates Muscle Cell Differentiation and Myofiber Hypertrophy in Transgenic Mice. J. Biol. Chem. 1995, 270, 12109–12116. [Google Scholar] [CrossRef] [Green Version]
- Gillery, P.; Leperre, A.; Borel, J.-P. Insulin-like growth factor-I (IGF-i) stimulates protein synthesis and collagen gene expression in monolayer and lattice cultures of fibroblasts. J. Cell. Physiol. 1992, 152, 389–396. [Google Scholar] [CrossRef]
- Swasdison, S.; Mayne, R. Formation of highly organized skeletal muscle fibers in vitro. Comparison with muscle development in vivo. J. Cell Sci. 1992, 102, 643–652. [Google Scholar] [CrossRef] [PubMed]
- VanDenburgh, H.H.; Karlisch, P.; Farr, L. Maintenance of highly contractile tissue-cultured avian skeletal myotubes in collagen gel. Vitr. Cell. Dev. Biol.-Anim. 1988, 24, 166–174. [Google Scholar] [CrossRef]
- Zdzieblik, D.; Oesser, S.; Baumstark, M.W.; Gollhofer, A.; König, D. Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: A randomised controlled trial. Br. J. Nutr. 2015, 114, 1237–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Yi, C.-W.; Lee, S. The effects of kinesiology taping therapy on degenerative knee arthritis patients’ pain, function, and joint range of motion. J. Phys. Ther. Sci. 2016, 28, 63–66. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-S.; Yoon, T.; Lee, S.-H.; Hwang, N.-K.; Lee, J.-H.; Jung, Y.-J.; Lee, G. Immediate effects of kinesiology tape on the pain and gait function in older adults with knee osteoarthritis. Medicine 2019, 98, e17880. [Google Scholar] [CrossRef] [PubMed]
- Ostrander, R.V.; Leddon, C.E.; Hackel, J.G.; O’Grady, C.P.; Roth, C.A. Efficacy of Unloader Bracing in Reducing Symptoms of Knee Osteoarthritis. Am. J. Orthop. 2016, 45, 306–311. [Google Scholar]
- Thoumie, P.; Marty, M.; Avouac, B.; Pallez, A.; Vaumousse, A.; Pipet, L.P.T.; Monroche, A.; Graveleau, N.; Bonnin, A.; Ben Amor, C.; et al. Effect of unloading brace treatment on pain and function in patients with symptomatic knee osteoarthritis: The ROTOR randomized clinical trial. Sci. Rep. 2018, 8, 10519. [Google Scholar] [CrossRef]
- Kuyinu, E.L.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Animal models of osteoarthritis: Classification, update, and measurement of outcomes. J. Orthop. Surg. Res. 2016, 11, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Franke, T.F.; Hornik, C.P.; Segev, L.; Shostak, G.A.; Sugimoto, C. PI3K/Akt and apoptosis: Size matters. Oncogene 2003, 22, 8983–8998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strzalka, W.; Ziemienowicz, A. Proliferating cell nuclear antigen (PCNA): A key factor in DNA replication and cell cycle regulation. Ann. Bot. 2010, 107, 1127–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.; Gang, G.G.; Kang, Y.G.; Jung, S.S.; Park, H.-G.; Jang, J.H. Alleviation of Osteoarthritis-Induced Pain and Motor Deficits in Rats by a Novel Device for the Intramuscular Insertion of Cog Polydioxanone Filament. Appl. Sci. 2021, 11, 10534. https://doi.org/10.3390/app112210534
Lee K, Gang GG, Kang YG, Jung SS, Park H-G, Jang JH. Alleviation of Osteoarthritis-Induced Pain and Motor Deficits in Rats by a Novel Device for the Intramuscular Insertion of Cog Polydioxanone Filament. Applied Sciences. 2021; 11(22):10534. https://doi.org/10.3390/app112210534
Chicago/Turabian StyleLee, Kilyong, Geung Gyu Gang, Yun Gyu Kang, Sung Sam Jung, Hee-Gon Park, and Jun Ho Jang. 2021. "Alleviation of Osteoarthritis-Induced Pain and Motor Deficits in Rats by a Novel Device for the Intramuscular Insertion of Cog Polydioxanone Filament" Applied Sciences 11, no. 22: 10534. https://doi.org/10.3390/app112210534





