Anti-Inflammatory Effects of Jakyakgamcho-Tang in IL-4- and TNF-α-Stimulated Lung Epithelial Cells and Lipopolysaccharide-Stimulated Macrophages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Preparation of Extract
2.2. Preparation of Sample and Standard Solutions for UPLC-DAD-MS/MS Analysis
2.3. UPLC and MS Conditions
2.4. Cell Culture
2.5. Cytotoxicity Assay
2.6. Measurement of Chemokine Production, MMP Activity, and Adhesion Molecule Expression in BEAS-2B Cells
2.7. Measurement of NO in LPS-Stimulated RAW264.7 Cells
2.8. Western Blot Analysis
2.9. OVA-Induced Asthmatic Mice
2.10. Measurement of IL-5, IL-13 and IgE Levels in BALF and Plasma
2.11. Statistical Analyses
3. Results
3.1. UPLC-DAD-MS/MS Analysis of JGTW
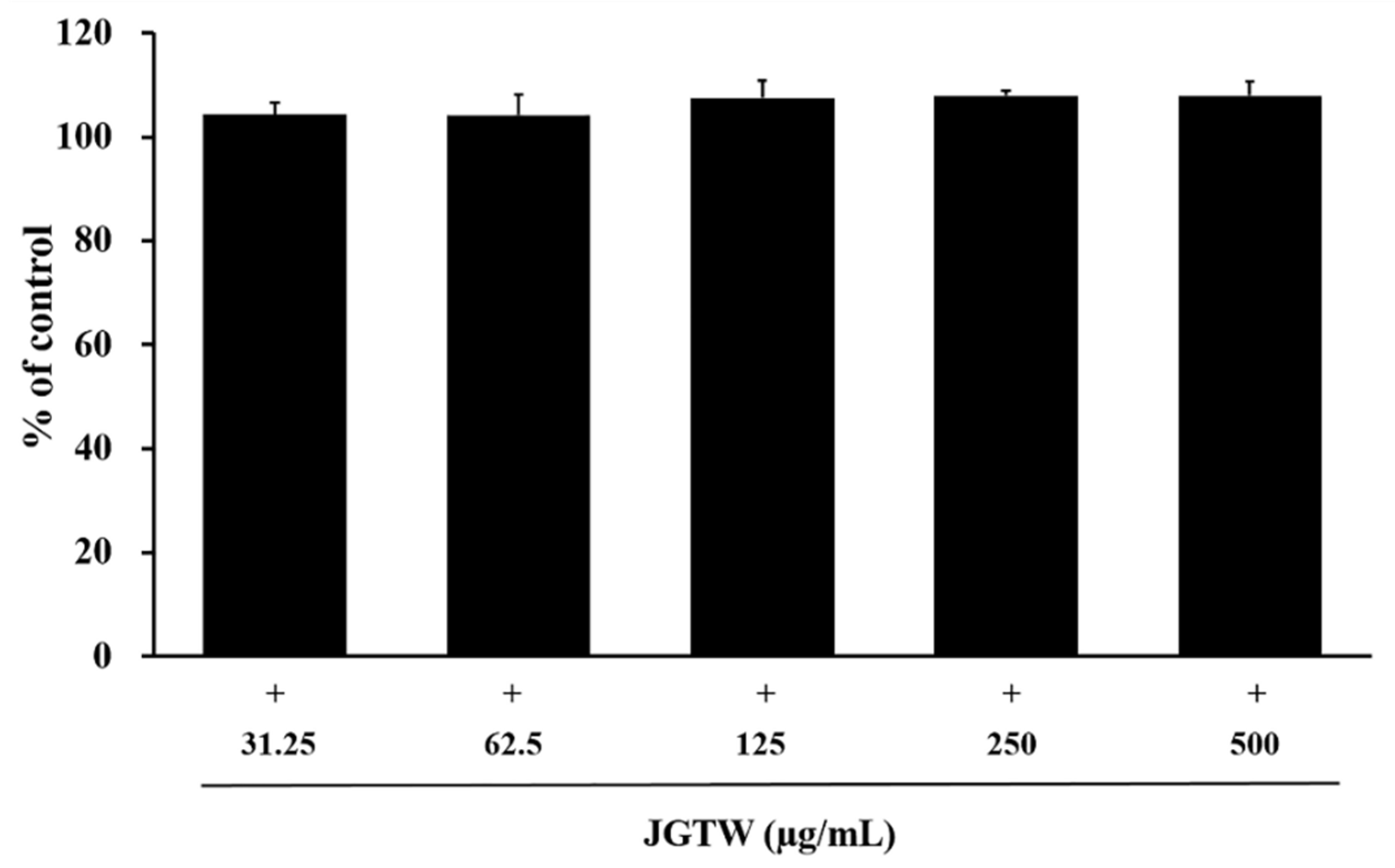
3.2. Cytotoxicity of JGTW in BEAS-2B and RAW264.7 Cells
3.3. Effect of JGTW on CHEMOKINE Production
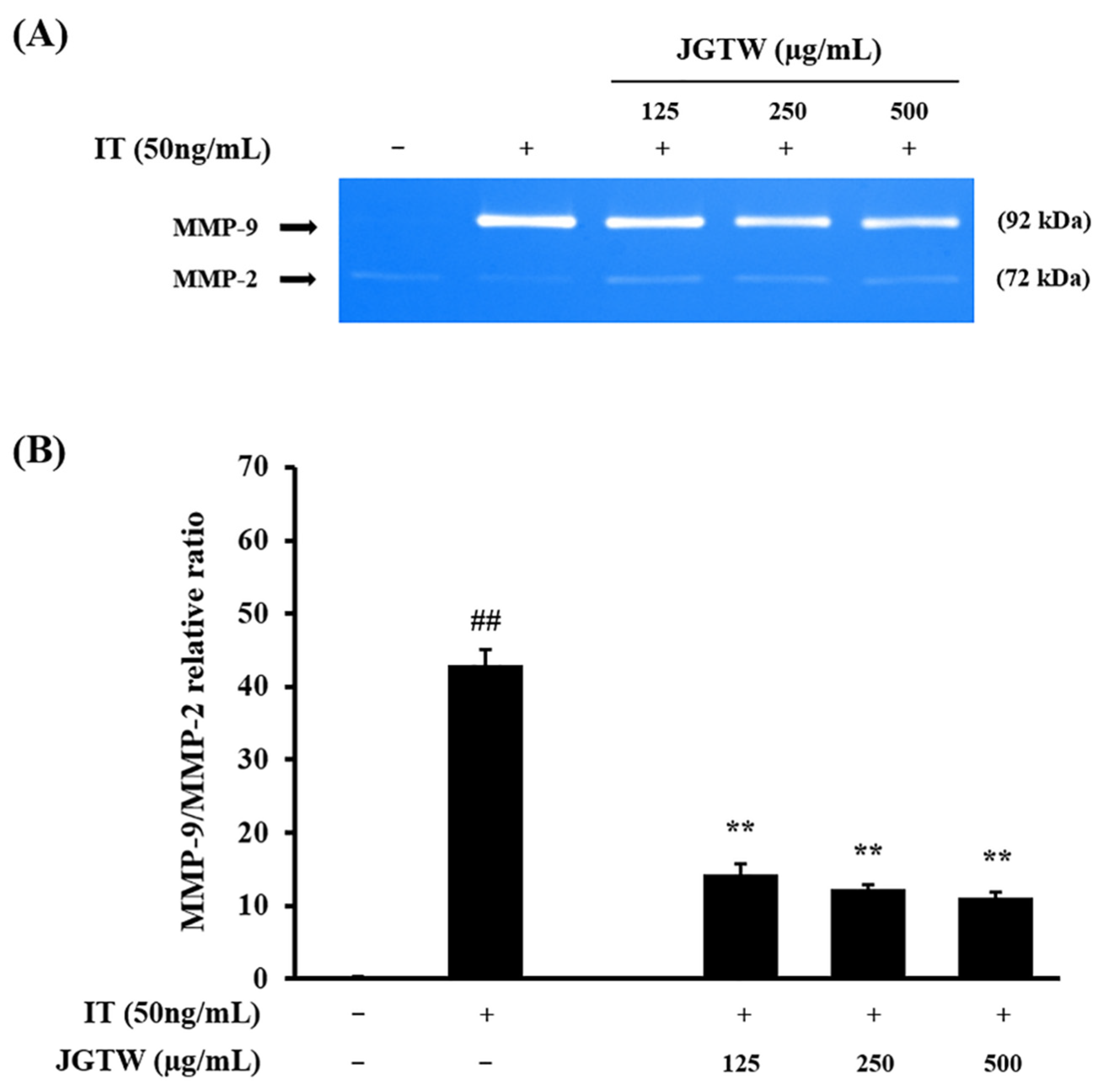
3.4. Effect of JGTW on MMP-9 Activity
3.5. Effect of JGTW on Adhesion Molecules Expression
3.6. Effect of JGTW on the iNOS Expression and NO Production
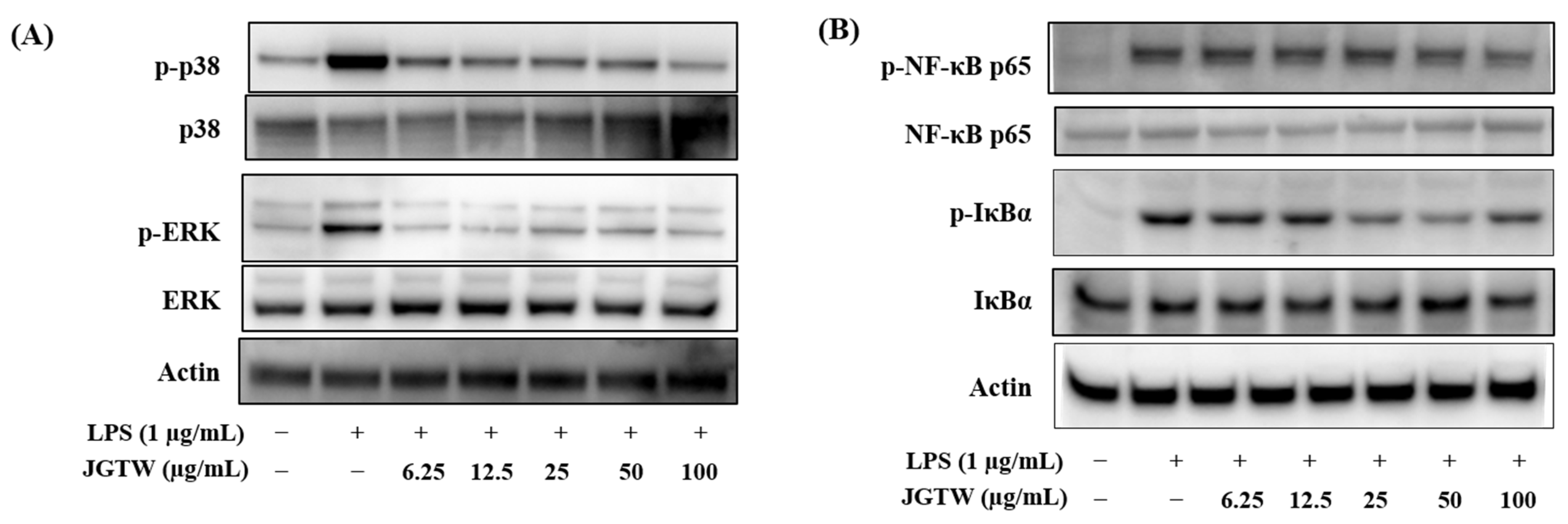
3.7. Effect of JGTW on the LPS-Induced MAPK Activation and NF-κB Signaling
3.8. Effect of JGTW on Number of Inflammatory Cells
3.9. Effect of JGTW on Th2 Cytokines and IgE Levels
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| JGT | Jakyakgamcho-tang |
| JGTW | Jakyakgamcho-tang water extract |
| OVA | ovalbumin |
| BALF | bronchoalveolar lavage fluid |
| RANTES | regulated on activation normal T-cell expressed and secreted |
| NO | nitric oxide |
| iNOS | inducible nitric oxide synthase |
| LPS | lipopolysaccharide |
| TNF-α | tumor necrosis factor-α |
| MMP-9 | matrix metalloproteinase-9 |
| ICAM-1 | intracellular adhesion molecule-1 |
| VCAM-1 | vascular cell adhesion molecule-1 |
| UPLC-DAD-MS/MS | ultra-performance liquid chromatography-diode array detector-tandem mass spectrometry |
| EIC | extracted ion chromatogram |
References
- Lee, N.H.; Lee, M.Y.; Lee, J.A.; Jung, D.Y.; Seo, C.S.; Kim, J.H.; Shin, H.K. Anti-asthmatic effect of Sanguisorba officinalis L. and potential role of heme oxygenase-1 in an ovalbumin-induced murine asthma model. Int. J. Mol. Med. 2010, 26, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Lee, J.A.; Seo, C.S.; Ha, H.; Lee, N.H.; Shin, H.K. Protective effects of Mentha haplocalyx ethanol extract (MH) in a mouse model of allergic asthma. Phytother. Res. 2011, 25, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Ricciardolo, F.L.; Sterk, P.J.; Gaston, B.; Folkerts, G. Nitric oxide in health and disease of the respiratory system. Physiol. Rev. 2004, 84, 731–765. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.M.; Galli, S.J. Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. J. Exp. Med. 2000, 192, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Corbel, M.; Boichot, E.; Lagente, V. Role of gelatinases MMP-2 and MMP-9 in tissue remodeling following acute lung injury. Braz. J. Med. Biol. Res. 2000, 33, 749–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosseau, S.; Selhorst, J.; Wiechmann, K.; Leissner, K.; Maus, U.; Mayer, K.; Grimminger, F.; Seeger, W.; Lohmeyer, J. Monocyte migration through the alveolar epithelial barrier: Adhesion molecule mechanisms and impact of chemokines. J. Immunol. 2000, 164, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.S.; Jin, S.M.; Kim, H.J.; Lee, Y.C. Matrix metalloproteinase inhibitor regulates inflammatory cell migration by reducing ICAM-1 and VCAM-1 expression in a murine model of toluene diisocyanate-induced asthma. J. Allergy Clin. Immunol. 2003, 111, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Fehervari, Z. Alveolar macrophages in asthma. Nat. Immunol. 2015, 16, 64. [Google Scholar] [CrossRef]
- Lee, Y.G.; Jeong, J.J.; Nyenhuis, S.; Berdyshev, E.; Chung, S.; Ranjan, R.; Karpurapu, M.; Deng, J.; Qian, F.; Kelly, E.A.; et al. Recruited alveolar macrophages, in response to airway epithelial-derived monocyte chemoattractant protein 1/CCl2, regulate airway inflammation and remodeling in allergic asthma. Am. J. Respir. Cell Mol. Biol. 2015, 52, 772–784. [Google Scholar] [CrossRef] [Green Version]
- Hinoshita, F.; Ogura, Y.; Suzuki, Y.; Hara, S.; Yamada, A.; Tanaka, N.; Yamashita, A.; Marumo, F. Effect of orally administered shao-yao-gan-cao-tang (Shakuyaku-kanzo-to) on muscle cramps in maintenance hemodialysis patients: A preliminary study. Am. J. Chin. Med. 2003, 31, 445–453. [Google Scholar] [CrossRef]
- Hyodo, T.; Taira, T.; Takemura, T.; Yamamoto, S.; Tsuchida, M.; Yoshida, K.; Uchida, T.; Sakai, T.; Hidai, H.; Baba, S. Immediate effect of Shakuyaku-kanzo-to on muscle cramp in hemodialysis patients. Nephron. Clin. Pract. 2006, 104, c28–c32. [Google Scholar] [CrossRef]
- Tsuji, S.; Yasuda, K.; Sumi, G.; Cho, H.; Tsuzuki, T.; Okada, H.; Kanzaki, H. Shakuyaku-kanzo-to inhibits smooth muscle contractions of human pregnant uterine tissue in vitro. J. Obstet. Gynaecol. Res. 2012, 38, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Kim, D.S. The Extraction Solvent Influences the Anti-Inflammatory Effects of Jakyakgamcho-Tang in Lipopolysaccharide-Stimulated Macrophages and Mice with Gouty Arthritis. Int. J. Mol. Sci. 2020, 21, 9748. [Google Scholar] [CrossRef]
- Seo, S.H.; Unno, T.; Park, S.E.; Kim, E.J.; Lee, Y.M.; Na, C.S.; Son, H.S. Korean Traditional Medicine (Jakyakgamcho-tang) Ameliorates Colitis by Regulating Gut Microbiota. Metabolites 2019, 9, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidaka, T.; Shima, T.; Nagira, K.; Ieki, M.; Nakamura, T.; Aono, Y.; Kuraishi, Y.; Arai, T.; Saito, S. Herbal medicine Shakuyaku-kanzo-to reduces paclitaxel-induced painful peripheral neuropathy in mice. Eur. J. Pain 2009, 13, 22–27. [Google Scholar] [CrossRef]
- Kim, J.; Kim, C.S.; Kim, Y.S.; Lee, I.S.; Kim, J.S. Jakyakgamcho-tang and Its Major Component, Paeonia Lactiflora, Exhibit Potent Anti-glycation Properties. J. Exerc. Nutr. Biochem. 2016, 20, 60–64. [Google Scholar] [CrossRef]
- Han, K.; Kwon, O.; Jung, S.Y.; Park, I.H.; Hwang, M.S.; Park, S.Y.; Hwang, E.H.; Lee, J.H. Jakyakgamcho-tang in the relief of delayed-onset muscle soreness in healthy adults: Study protocol for a randomized, double-blind, placebo-controlled, crossover design clinical trial. Trials 2020, 21, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, F.; Xu, J.; Yang, M.H.; Ru, C.H.; Shen, X.Q.; Wu, B.; Cai, W.R. Influence of Shaoyao Gancao Decoction on ratio of Treg/Th17 in rats with asthma. Chin. J. Tradit. Chin. Med. Pharm. 2016, 31, 4477–4479. [Google Scholar]
- Jeong, S.J.; Lim, H.S.; Seo, C.S.; Kim, J.H.; Jin, S.E.; Yoo, S.R.; Shin, H.K. Traditional herbal formula Jakyakgamcho-tang (Paeonia lactiflora and Glycyrrhiza uralensis) impairs inflammatory chemokine production by inhibiting activation of STAT1 and NF-κB in HaCaT cells. Phytomedicine 2015, 22, 326–332. [Google Scholar] [CrossRef]
- Zhu, G.-W.; Guo, J.; Li, Y.-J.; Luo, L.; Sugita, T.; Tomoda, T. The effect of peony and licorice decoction on the voltage-gated sodium channel subtype 1.4 based on standard decoction. World J. Tradit. Chin. Med. 2018, 4, 69–76. [Google Scholar] [CrossRef]
- Kim, Y.J.; Jeon, W.Y.; Hwang, Y.H.; Lee, M.Y. Inhibitory Effects of Gyeji-Tang on MMP-9 Activity and the Expression of Adhesion Molecules in IL-4- and TNF-α-Stimulated BEAS-2B Cells. Plants 2021, 10, 951. [Google Scholar] [CrossRef]
- Zhou, J.X.; Braun, M.S.; Wetterauer, P.; Wetterauer, B.; Wink, M. Antioxidant, Cytotoxic, and Antimicrobial Activities of Glycyrrhiza glabra L., Paeonia lactiflora Pall., and Eriobotrya japonica (Thunb.) Lindl. Extracts. Medicines 2019, 6, 43. [Google Scholar] [CrossRef] [Green Version]
- Choung, M.-G. Variation of Bioactive Component Contents in Plant Parts of Paeonia lactiflora Pall. Korean J. Med. Crop. Sci. 2002, 10, 392–398. [Google Scholar]
- Matsukura, S.; Osakabe, Y.; Sekiguchi, A.; Inoue, D.; Kakiuchi, Y.; Funaki, T.; Yamazaki, Y.; Takayasu, H.; Tateno, H.; Kato, E.; et al. Overexpression of microRNA-155 suppresses chemokine expression induced by Interleukin-13 in BEAS-2B human bronchial epithelial cells. Allergol. Int. 2016, 65, 17–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jose, P.J.; Griffiths-Johnson, D.A.; Collins, P.D.; Walsh, D.T.; Moqbel, R.; Totty, N.F.; Truong, O.; Hsuan, J.J.; Williams, T.J. Eotaxin: A potent eosinophil chemoattractant cytokine detected in a guinea pig model of allergic airways inflammation. J. Exp. Med. 1994, 179, 881–887. [Google Scholar] [CrossRef] [Green Version]
- Forssmann, U.; Uguccioni, M.; Loetscher, P.; Dahinden, C.A.; Langen, H.; Thelen, M.; Baggiolini, M. Eotaxin-2, a novel CC chemokine that is selective for the chemokine receptor CCR3, and acts like eotaxin on human eosinophil and basophil leukocytes. J. Exp. Med. 1997, 185, 2171–2176. [Google Scholar] [CrossRef] [Green Version]
- Kitaura, M.; Suzuki, N.; Imai, T.; Takagi, S.; Suzuki, R.; Nakajima, T.; Hirai, K.; Nomiyama, H.; Yoshie, O. Molecular cloning of a novel human CC chemokine (Eotaxin-3) that is a functional ligand of CC chemokine receptor 3. J. Biol. Chem. 1999, 274, 27975–27980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provost, V.; Larose, M.C.; Langlois, A.; Rola-Pleszczynski, M.; Flamand, N.; Laviolette, M. CCL26/eotaxin-3 is more effective to induce the migration of eosinophils of asthmatics than CCL11/eotaxin-1 and CCL24/eotaxin-2. J. Leukoc. Biol. 2013, 94, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Alam, R.; Stafford, S.; Forsythe, P.; Harrison, R.; Faubion, D.; Lett-Brown, M.A.; Grant, J.A. RANTES is a chemotactic and activating factor for human eosinophils. J. Immunol. 1993, 150, 3442–3448. [Google Scholar] [PubMed]
- Brand, K.H.; Ahout, I.M.; de Groot, R.; Warris, A.; Ferwerda, G.; Hermans, P.W. Use of MMP-8 and MMP-9 to assess disease severity in children with viral lower respiratory tract infections. J. Med. Virol. 2012, 84, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Malla, N.; Sjøli, S.; Winberg, J.O.; Hadler-Olsen, E.; Uhlin-Hansen, L. Biological and pathobiological functions of gelatinase dimers and complexes. Connect. Tissue Res. 2008, 49, 180–184. [Google Scholar] [CrossRef]
- Nakajima, H.; Sano, H.; Nishimura, T.; Yoshida, S.; Iwamoto, I. Role of vascular cell adhesion molecule 1/very late activation antigen 4 and intercellular adhesion molecule 1/lymphocyte function-associated antigen 1 interactions in antigen-induced eosinophil and T cell recruitment into the tissue. J. Exp. Med. 1994, 179, 1145–1154. [Google Scholar] [CrossRef]
- van der Veen, T.A.; de Groot, L.E.S.; Melgert, B.N. The different faces of the macrophage in asthma. Curr. Opin. Pulm. Med. 2020, 26, 62–68. [Google Scholar] [CrossRef]
- Lee, J.W.; Min, J.H.; Kim, M.G.; Kim, S.M.; Kwon, O.K.; Oh, T.K.; Lee, J.K.; Kim, T.Y.; Lee, S.W.; Choi, S.; et al. Pistacia weinmannifolia root exerts a protective role in ovalbumin-induced lung inflammation in a mouse allergic asthma model. Int. J. Mol. Med. 2019, 44, 2171–2180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.H.; Maltby, S.; Simpson, J.L.; Eyers, F.; Baines, K.J.; Gibson, P.G.; Foster, P.S.; Yang, M. TNF-α and Macrophages Are Critical for Respiratory Syncytial Virus-Induced Exacerbations in a Mouse Model of Allergic Airways Disease. J. Immunol. 2016, 196, 3547–3558. [Google Scholar] [CrossRef] [Green Version]
- Yuk, H.J.; Lee, J.W.; Park, H.A.; Kwon, O.-K.; Seo, K.-H.; Ahn, K.-S.; Oh, S.-R.; Ryu, H.W. Protective effects of coumestrol on lipopolysaccharide-induced acute lung injury via the inhibition of proinflammatory mediators and NF-κB activation. J. Funct. Foods 2017, 34, 181–188. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Z.; Yang, S.; Du, J.; Wang, S. Immunoregulatory Effects of Paeoniflorin Exerts Anti-asthmatic Effects via Modulation of the Th1/Th2 Equilibrium. Inflammation 2015, 38, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wu, Q.; Wei, L.; Peng, S. Paeoniflorin inhibits PDGF-BB-induced human airway smooth muscle cell growth and migration. Mol. Med. Rep. 2018, 17, 2660–2664. [Google Scholar] [CrossRef] [Green Version]
- Shou, Q.; Lang, J.; Jin, L.; Fang, M.; Cao, B.; Cai, Y.; Ni, Z.; Qiu, F.; Li, C.; Cao, G.; et al. Total glucosides of peony improve ovalbumin-induced allergic asthma by inhibiting mast cell degranulation. J. Ethnopharmacol. 2019, 244, 112136. [Google Scholar] [CrossRef]
- Ram, A.; Mabalirajan, U.; Das, M.; Bhattacharya, I.; Dinda, A.K.; Gangal, S.V.; Ghosh, B. Glycyrrhizin alleviates experimental allergic asthma in mice. Int. Immunopharmacol. 2006, 6, 1468–1477. [Google Scholar] [CrossRef]
- Hocaoglu, A.B.; Karaman, O.; Erge, D.O.; Erbil, G.; Yilmaz, O.; Bagriyanik, A.; Uzuner, N. Glycyrrhizin and long-term histopathologic changes in a murine model of asthma. Curr. Ther. Res. Clin. Exp. 2011, 72, 250–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.; Ma, Z.; Liao, X.L.; Liu, J.; Fu, Q.; Ma, S. Immunoregulatory effects of glycyrrhizic acid exerts anti-asthmatic effects via modulation of Th1/Th2 cytokines and enhancement of CD4(+)CD25(+)Foxp3+ regulatory T cells in ovalbumin-sensitized mice. J. Ethnopharmacol. 2013, 148, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yang, J.; Wen, Q.; Li, Y. Isoliquiritigenin, a flavonoid from licorice, relaxes guinea-pig tracheal smooth muscle in vitro and in vivo: Role of cGMP/PKG pathway. Eur. J. Pharmacol. 2008, 587, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Liu, C.Y.; Shen, S.C.; Chen, L.C.; Yeh, K.W.; Liu, S.H.; Liou, C.J. Protective Effects of Licochalcone A Improve Airway Hyper-Responsiveness and Oxidative Stress in a Mouse Model of Asthma. Cells 2019, 8, 617. [Google Scholar] [CrossRef] [Green Version]
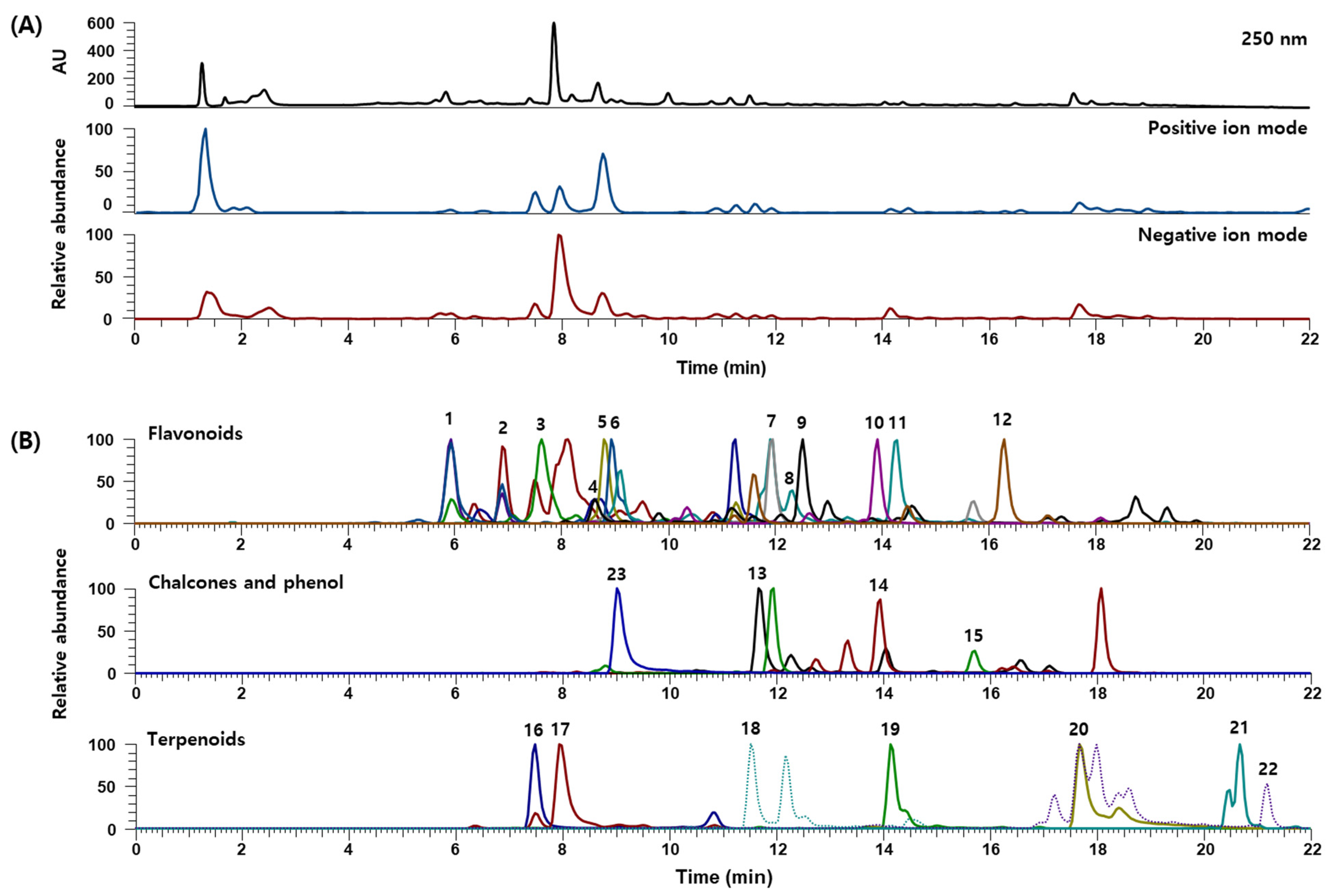





| No. | RT (min) | Calculated (m/z) | Estimated (m/z) | Adduct | Error (ppm) | Formula | MS/MS Fragments (m/z) | Identifications | Contents (mg/g) |
|---|---|---|---|---|---|---|---|---|---|
| Flavonoids | |||||||||
| 1 | 5.94 | 289.0718 | 289.0717 | [M−H]− | 0.377 | C15H14O6 | 289.0707, 245.0808, 205.0492, 179.0334, 125.0225 | Catechin [22] | 0.833 |
| 2 | 6.91 | 593.1512 | 593.1516 | [M−H]− | 1.019 | C27H30O15 | 593.1500, 503.1207, 473.1066, 413.0872, 383.0770, 354.0663 | Vicenin-2 [22] | 0.102 |
| 3 | 7.63 | 563.1406 | 563.1406 | [M−H]− | 0.316 | C26H28O14 | 473.1069, 443.0968, 383.0760, 353.0656 | Isoschaftoside or Schaftoside [22] | - |
| 4 | 8.65 | 419.1337 | 419.1338 | [M+H]+ | 0.308 | C21H22O9 | 257.0808 | Neoliquiritin [22] | - |
| 5 | 8.80 | 417.1191 | 417.1192 | [M−H]− | 0.512 | C21H22O9 | 417.1173, 255.0651, 135.0069, 119.0481 | Liquiritin [22] | - |
| 6 | 8.93 | 303.0510 | 303.0509 | [M−H]− | 0.287 | C15H12O7 | 285.0392, 275.0558, 259.0611, 217.0493, 177.0177, 125.0224 | Taxifolin [23] | 0.005 |
| 7 | 11.92 | 255.0663 | 255.0660 | [M−H]− | 0.136 | C15H12O4 | 255.0652, 135.0069, 119.0483 | Liquiritigenin [22] | 0.173 |
| 8 | 12.29 | 285.0405 | 285.0405 | [M−H]− | 0.022 | C15H10O6 | 285.0393, 241.0500, 217.0493, 151.0022, 135.0433 | Luteolin [22] | 0.001 |
| 9 | 12.53 | 285.0758 | 285.0760 | [M+H]+ | 0.731 | C16H12O5 | 285.0757, 270.0525 | Calycosin [22] | 0.007 |
| 10 | 13.91 | 271.0612 | 271.0612 | [M−H]− | −0.158 | C15H12O5 | 271.0604, 177.0177, 165.0181, 151.0019 | Naringenin [22] | 0.018 |
| 11 | 14.23 | 285.0405 | 285.0403 | [M−H]− | 0.343 | C15H10O6 | 285.0396, 167.0332, 109.0275 | Kaempferol [22] | 0.002 |
| 12 | 16.28 | 269.0808 | 269.0809 | [M+H]+ | 0.344 | C16H12O4 | 269.0807, 254.0577, 237.0548 | Formononetin [22] | 0.024 |
| Chalcones | |||||||||
| 13 | 11.67 | 285.0768 | 285.0768 | [M−H]− | −0.131 | C16H14O5 | 285.0758, 270.0524 | Licochalcone B [22] | 0.024 |
| 14 | 13.95 | 271.0965 | 271.0965 | [M+H]+ | 0.024 | C16H14O4 | 271.0962, 121.0287 | Echinatin [22] | 0.021 |
| 15 | 15.67 | 255.0663 | 255.0661 | [M−H]− | 0.495 | C15H12O4 | 255.0653, 135.0070, 119.0483 | Isoliquiritigenin [22] | 0.022 |
| Terpenoids | |||||||||
| 16 | 7.51 | 481.1704 | 481.1705 | [M+H]+ | 0.509 | C23H28O11 | 197.0808, 151.0754, 133.0650 | Albiflorin [22] | 4.323 |
| 17 | 7.95 | 525.1614 | 525.1614 | [M+HCO2]− | −0.200 | C23H28O11 | 328.1104, 165.0539, 121.0275 | Paeoniflorin [22] | 63.971 |
| 18 | 11.53 | 599.1770 | 599.1774 | [M−H]− | 0.700 | C30H32O13 | 477.1277, 429.1172, 137.0226, 121.0276 | Mudanpioside C [22] | 0.029 |
| 19 | 14.13 | 629.1876 | 629.1876 | [M+HCO2]− | 0.455 | C30H32O12 | 165.0544, 121.0276 | Benzoylpaeoniflorin [22] | 1.464 |
| 20 | 17.69 | 821.3965 | 821.3967 | [M−H]− | 0.563 | C42H62O16 | 821.3934, 351.0559, 193.0337 | Glycyrrhizin [22] | 11.853 |
| 21 | 20.67 | 471.3480 | 471.3480 | [M−H]− | −0.039 | C30H48O4 | - | Hederagenin [22] | 0.005 |
| 22 | 21.19 | 471.3469 | 471.3471 | [M+H]+ | 0.072 | C30H46O4 | 471.3470, 454.3427, 407.3318, 189.1641 | Glycyrrhetinic acid [22] | 0.007 |
| Phenol | |||||||||
| 23 | 9.02 | 939.1109 | 939.1111 | [M−H]− | 1.236 | C41H32O26 | 769.0868, 617.0763, 447.0572, 295.0449, 169.0125 | 1,2,3,4,6-O-Pentagalloylglucose [22] | 2.634 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.J.; Jeon, W.-Y.; Lee, M.-Y.; Hwang, Y.-H.; Kim, J. Anti-Inflammatory Effects of Jakyakgamcho-Tang in IL-4- and TNF-α-Stimulated Lung Epithelial Cells and Lipopolysaccharide-Stimulated Macrophages. Appl. Sci. 2021, 11, 10569. https://doi.org/10.3390/app112210569
Kim YJ, Jeon W-Y, Lee M-Y, Hwang Y-H, Kim J. Anti-Inflammatory Effects of Jakyakgamcho-Tang in IL-4- and TNF-α-Stimulated Lung Epithelial Cells and Lipopolysaccharide-Stimulated Macrophages. Applied Sciences. 2021; 11(22):10569. https://doi.org/10.3390/app112210569
Chicago/Turabian StyleKim, Yu Jin, Woo-Young Jeon, Mee-Young Lee, Youn-Hwan Hwang, and Jinhee Kim. 2021. "Anti-Inflammatory Effects of Jakyakgamcho-Tang in IL-4- and TNF-α-Stimulated Lung Epithelial Cells and Lipopolysaccharide-Stimulated Macrophages" Applied Sciences 11, no. 22: 10569. https://doi.org/10.3390/app112210569
APA StyleKim, Y. J., Jeon, W.-Y., Lee, M.-Y., Hwang, Y.-H., & Kim, J. (2021). Anti-Inflammatory Effects of Jakyakgamcho-Tang in IL-4- and TNF-α-Stimulated Lung Epithelial Cells and Lipopolysaccharide-Stimulated Macrophages. Applied Sciences, 11(22), 10569. https://doi.org/10.3390/app112210569






