Image-Free Ultrasound Blood-Flow Monitoring Circuit System with Automatic Range-Gate Positioning Scheme: A Pilot Study
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Overall Structure of Image-Free Ultrasound Blood-Flow-Monitoring Circuit System
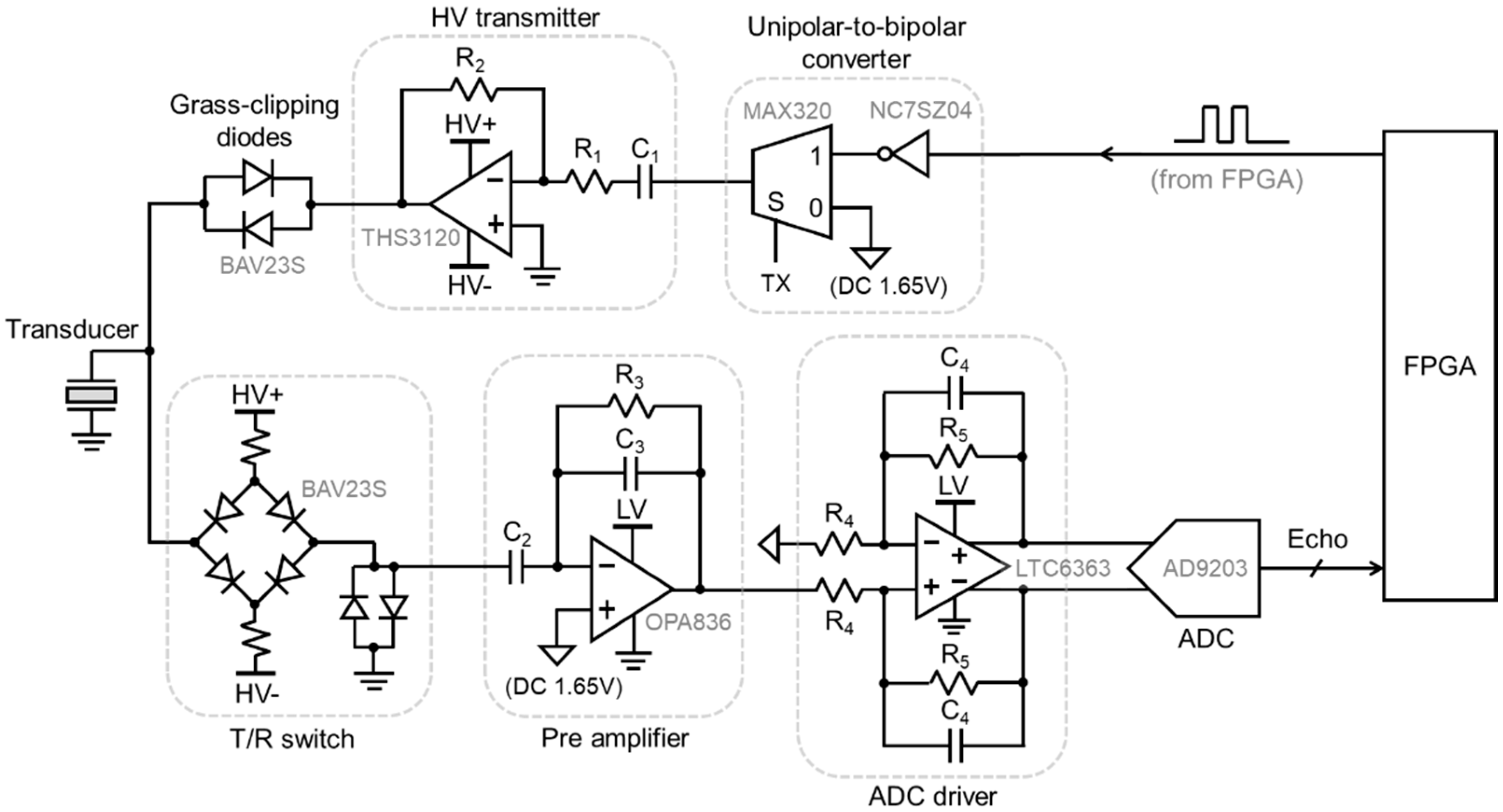
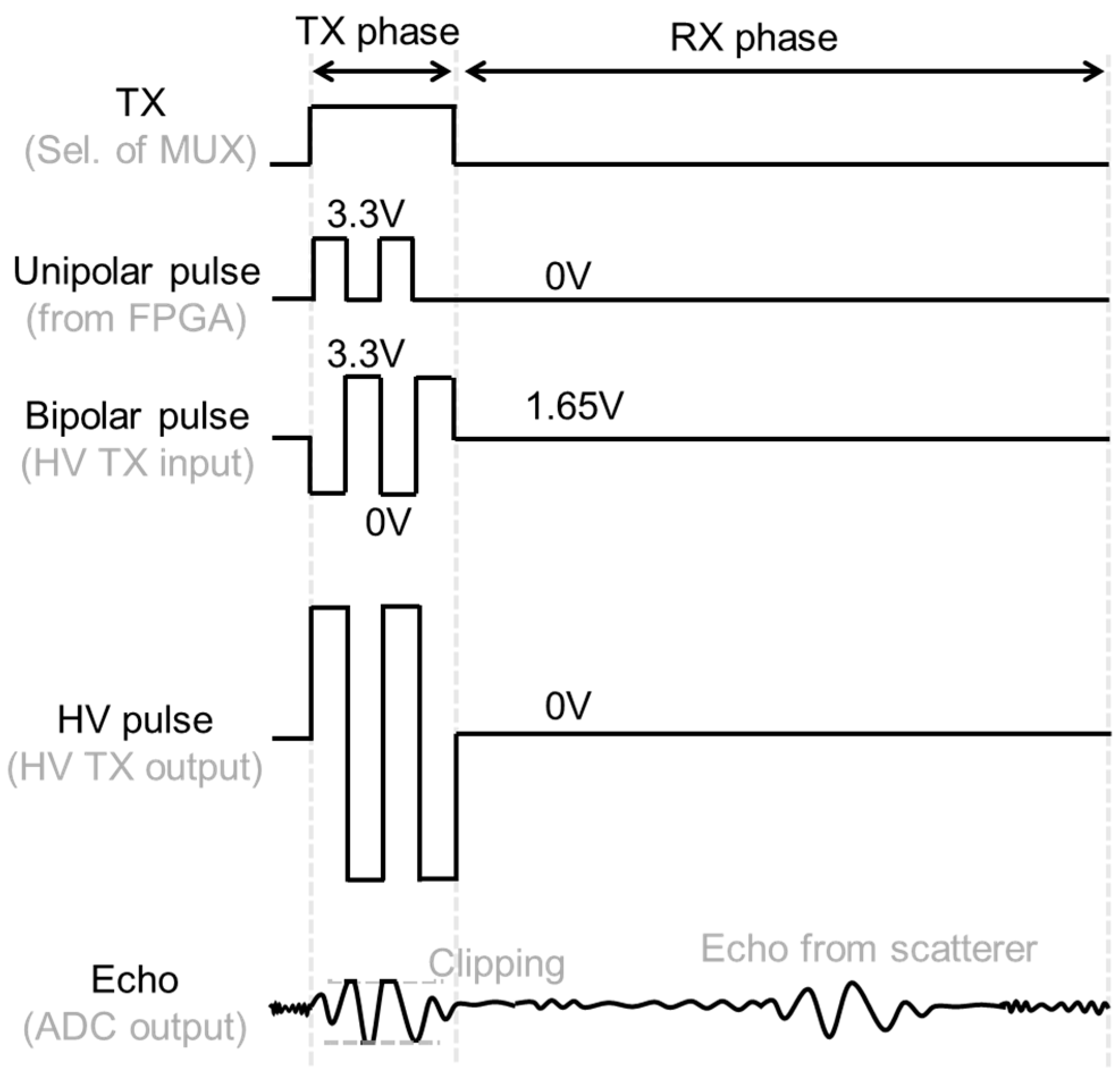
2.2. Analog Interface Circuit
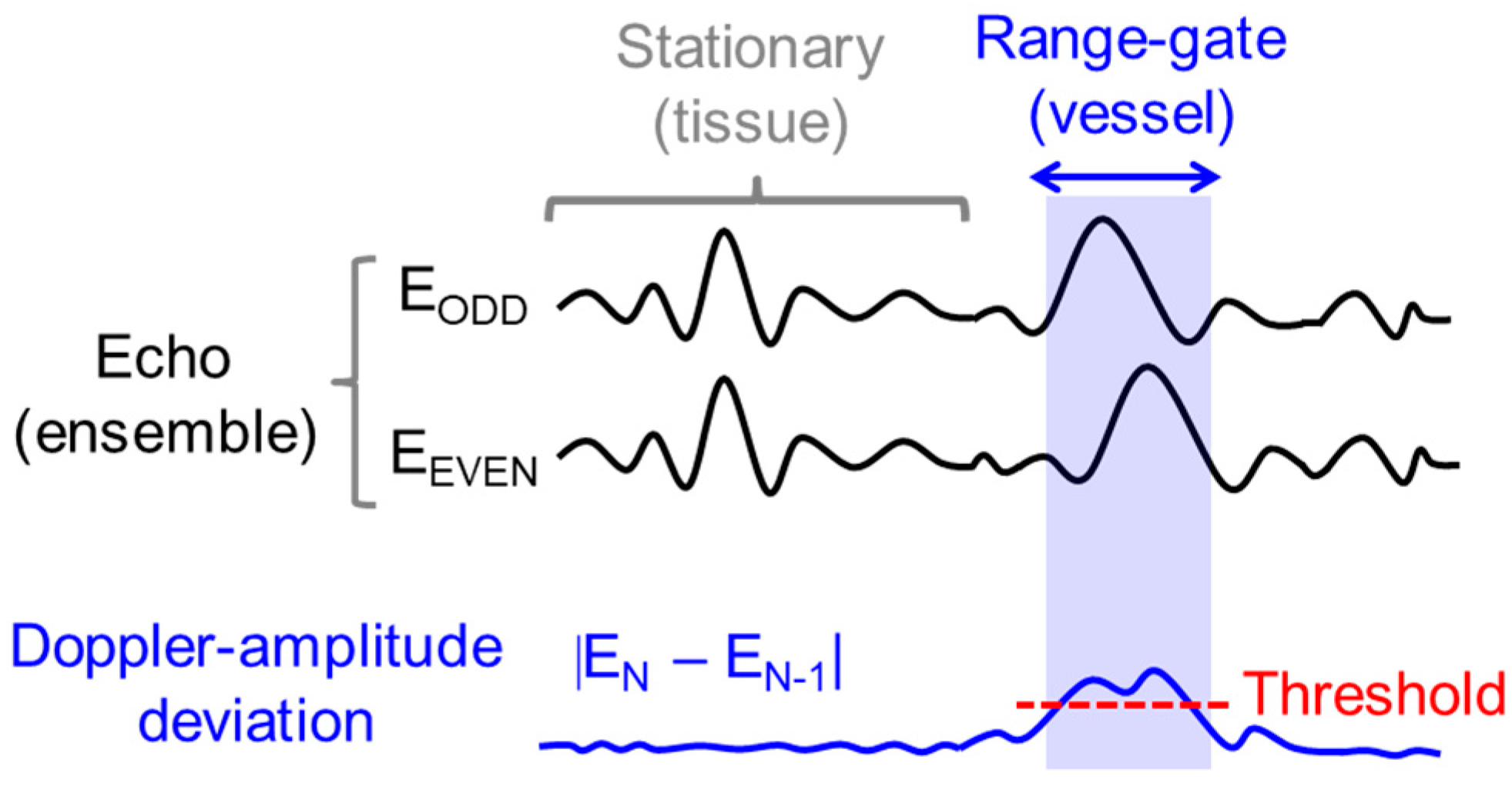
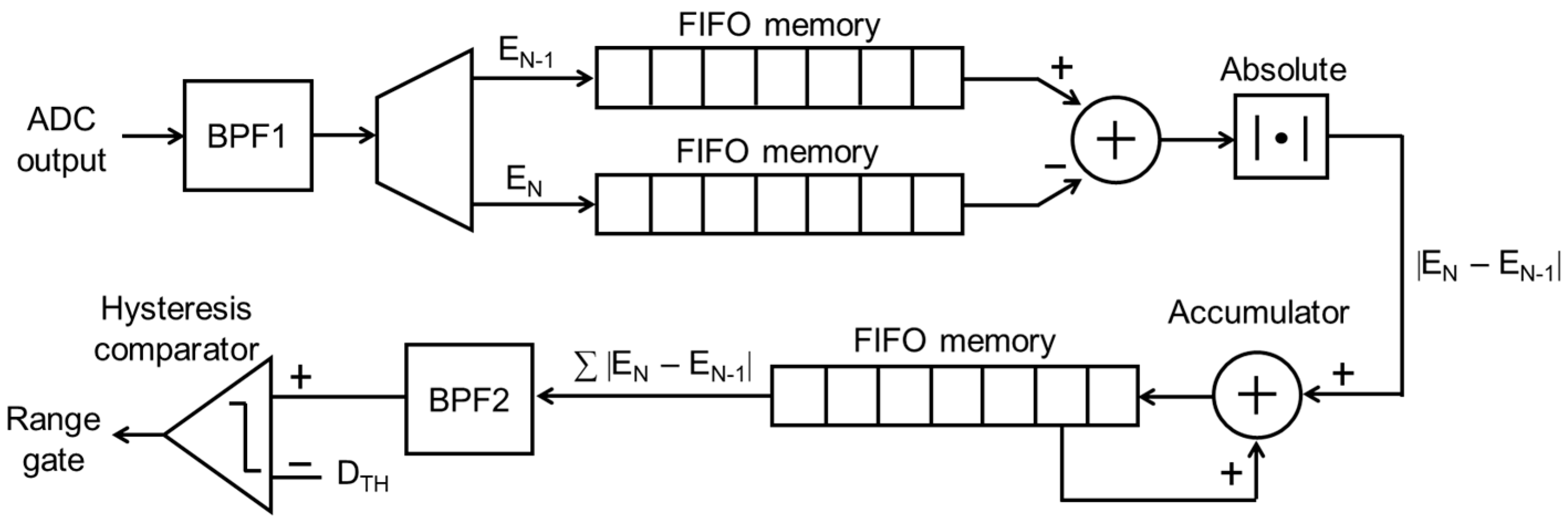
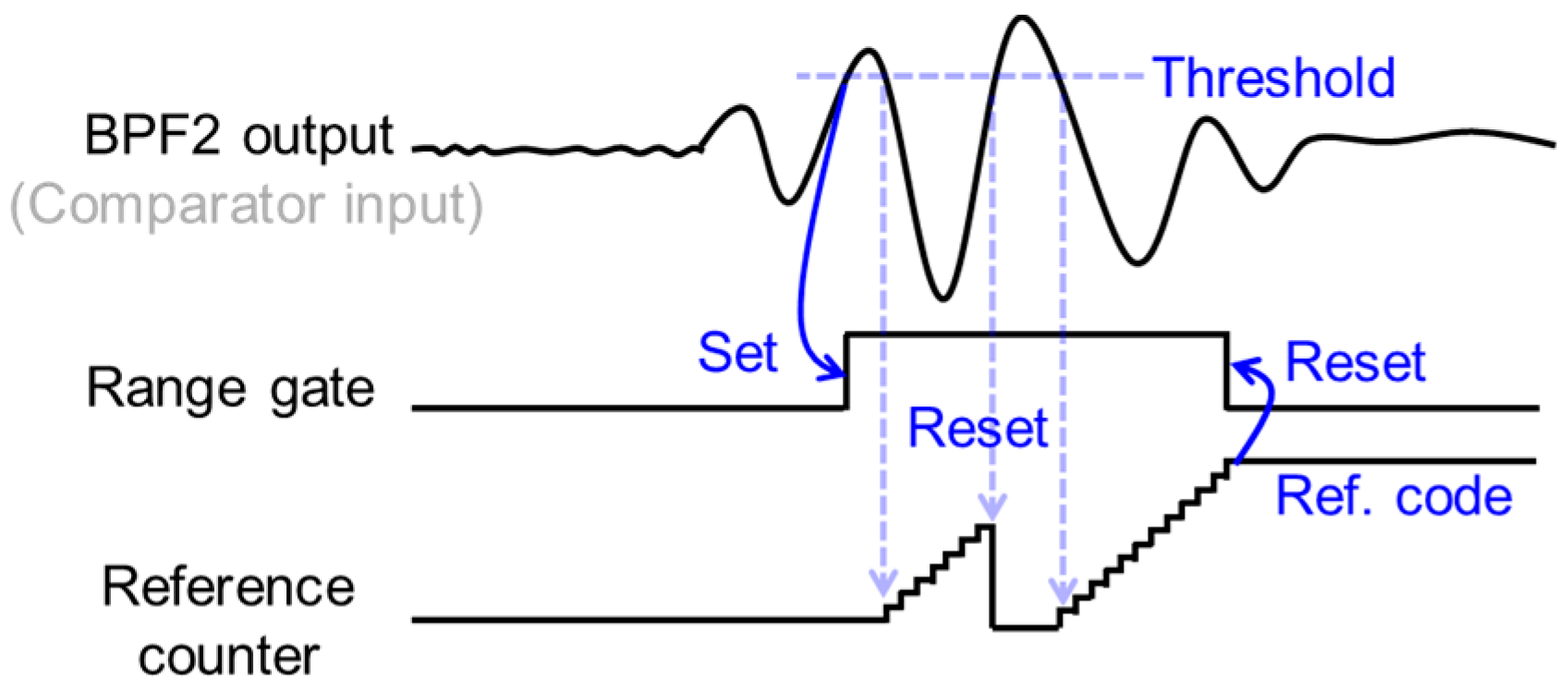
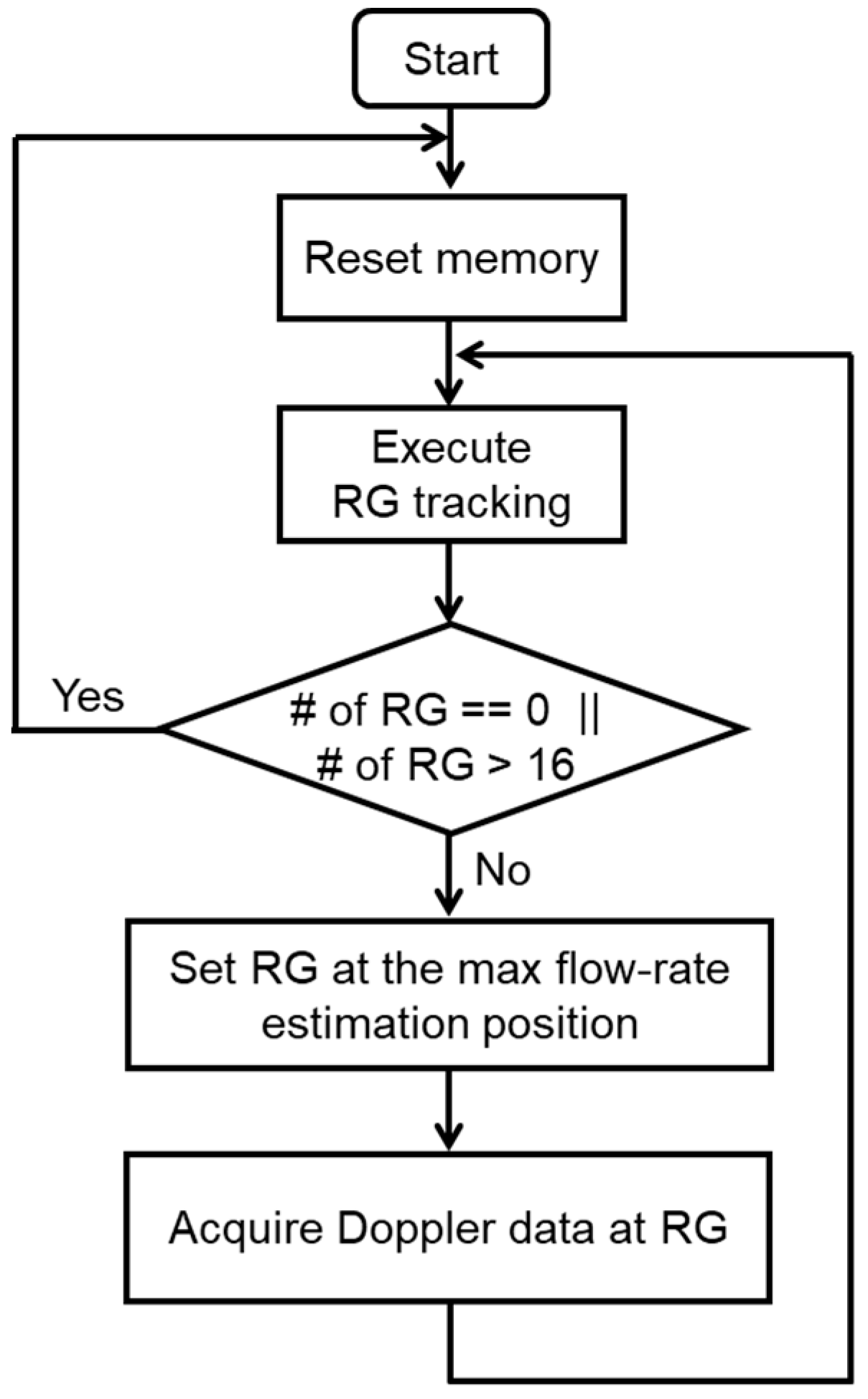
2.3. Automatic Range-Gate-Tracking Scheme in FPGA
2.4. Stabilization of Range-Gate Position and Doppler Raw Data Acquisition
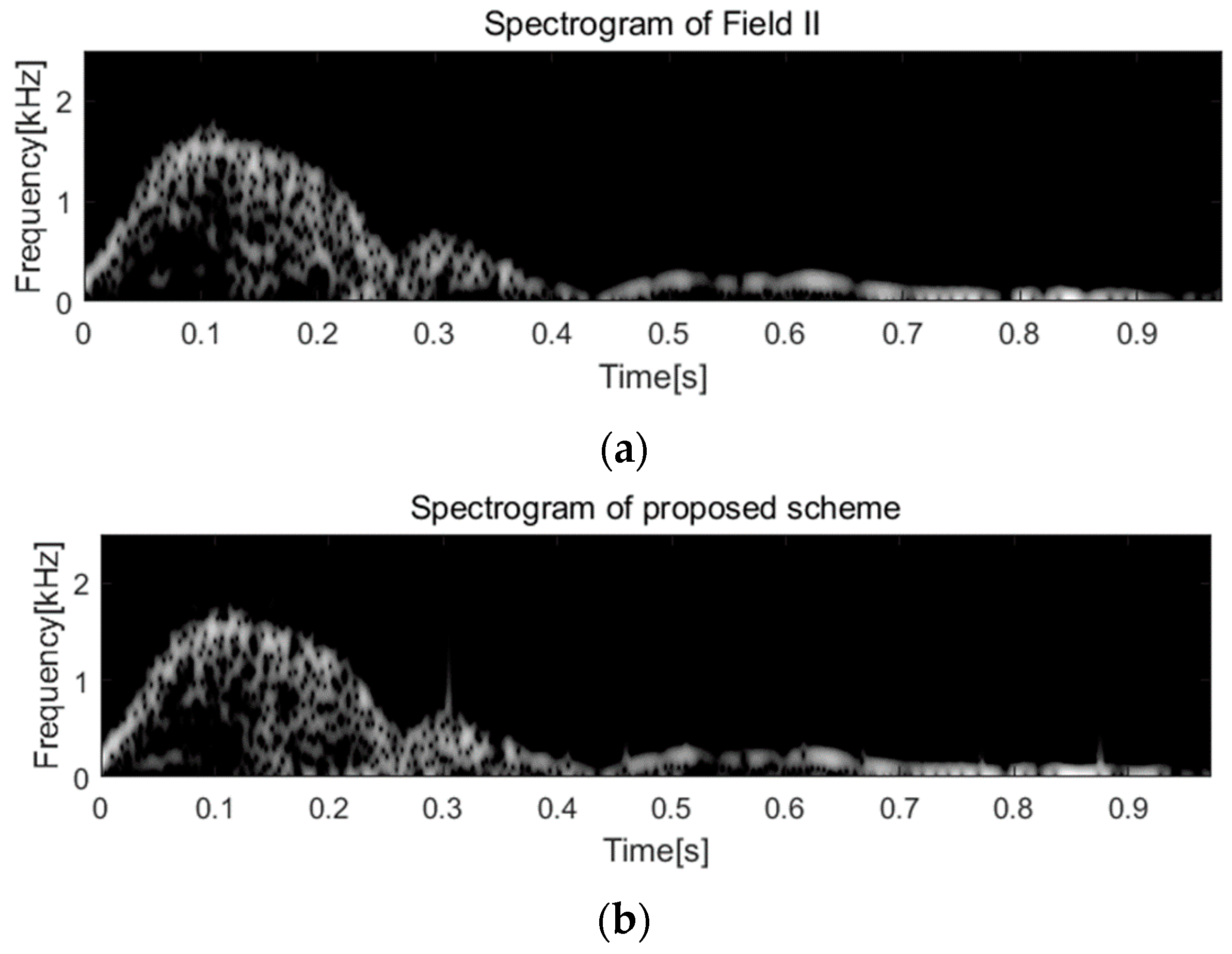
2.5. Behavioral Simulation Using Field II Program
3. Results and Discussion
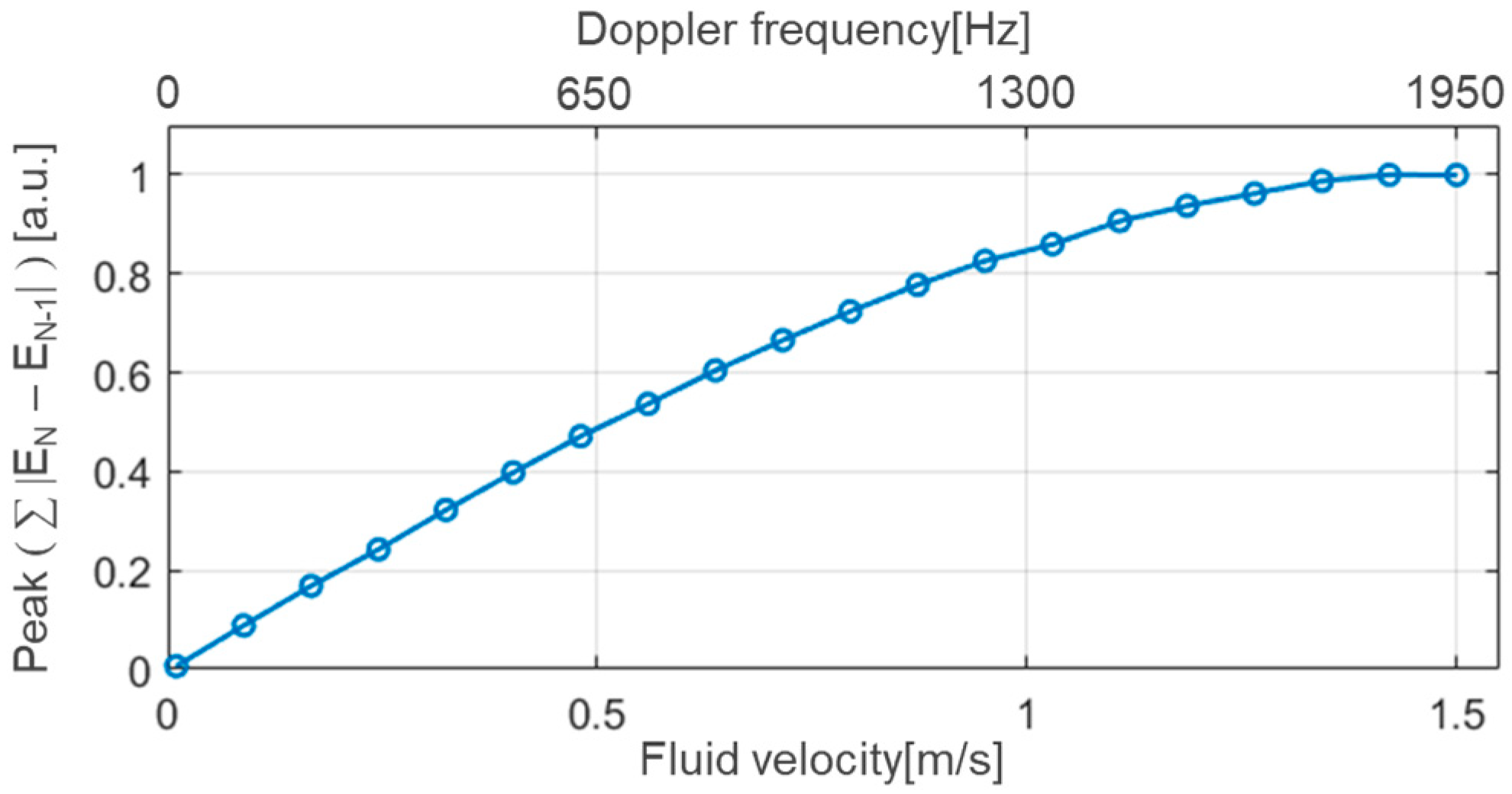
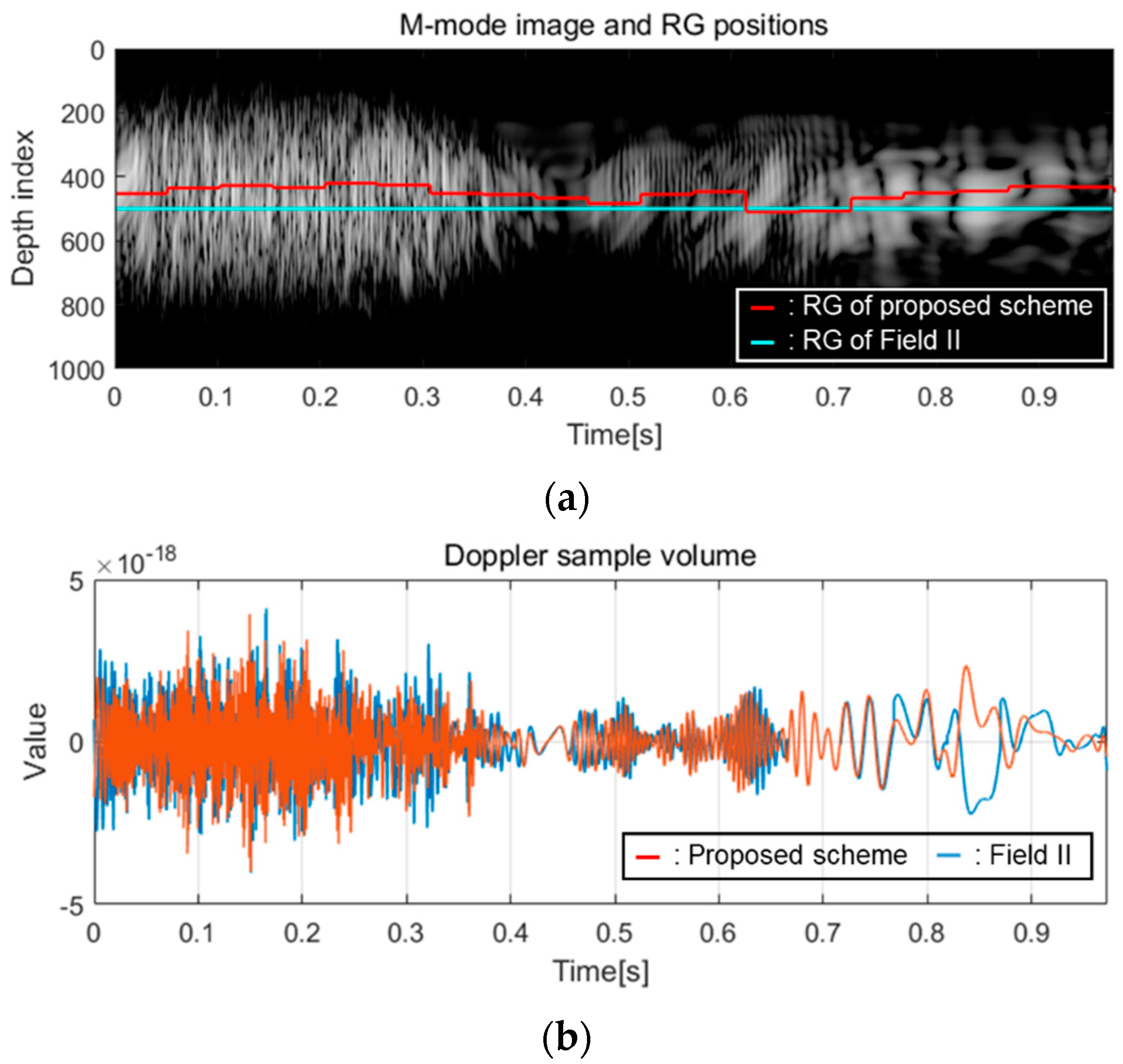
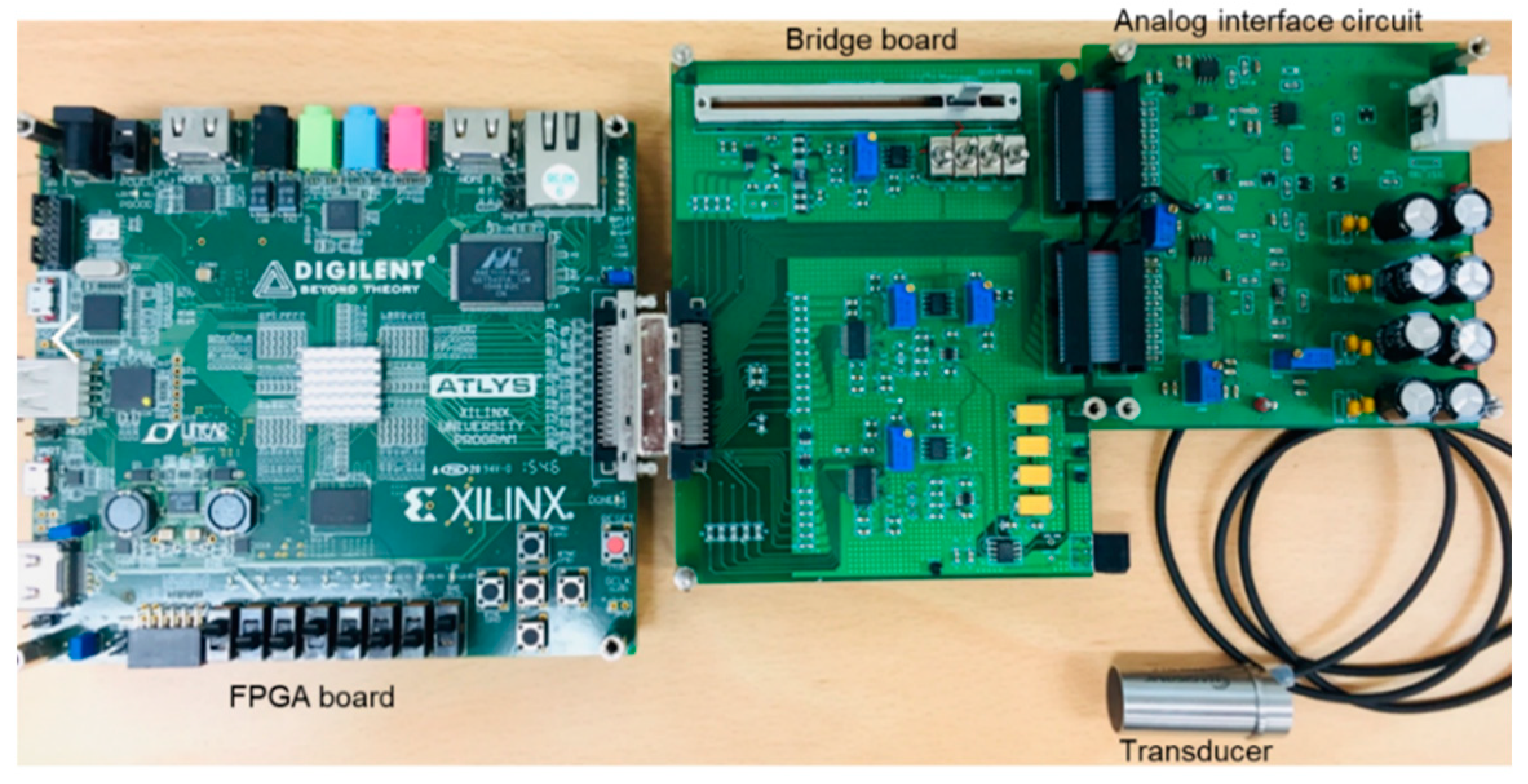
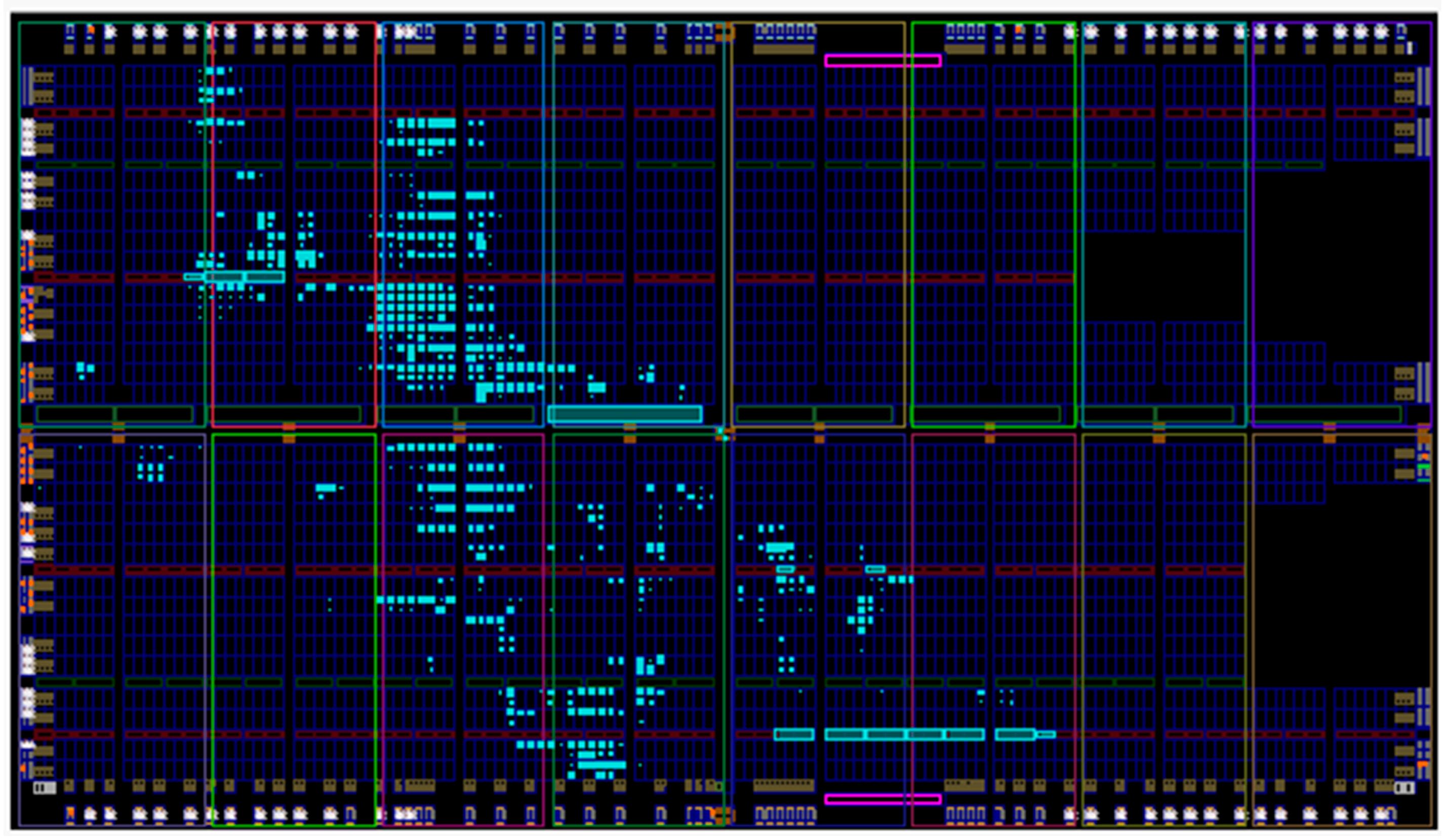
3.1. Implementation Results
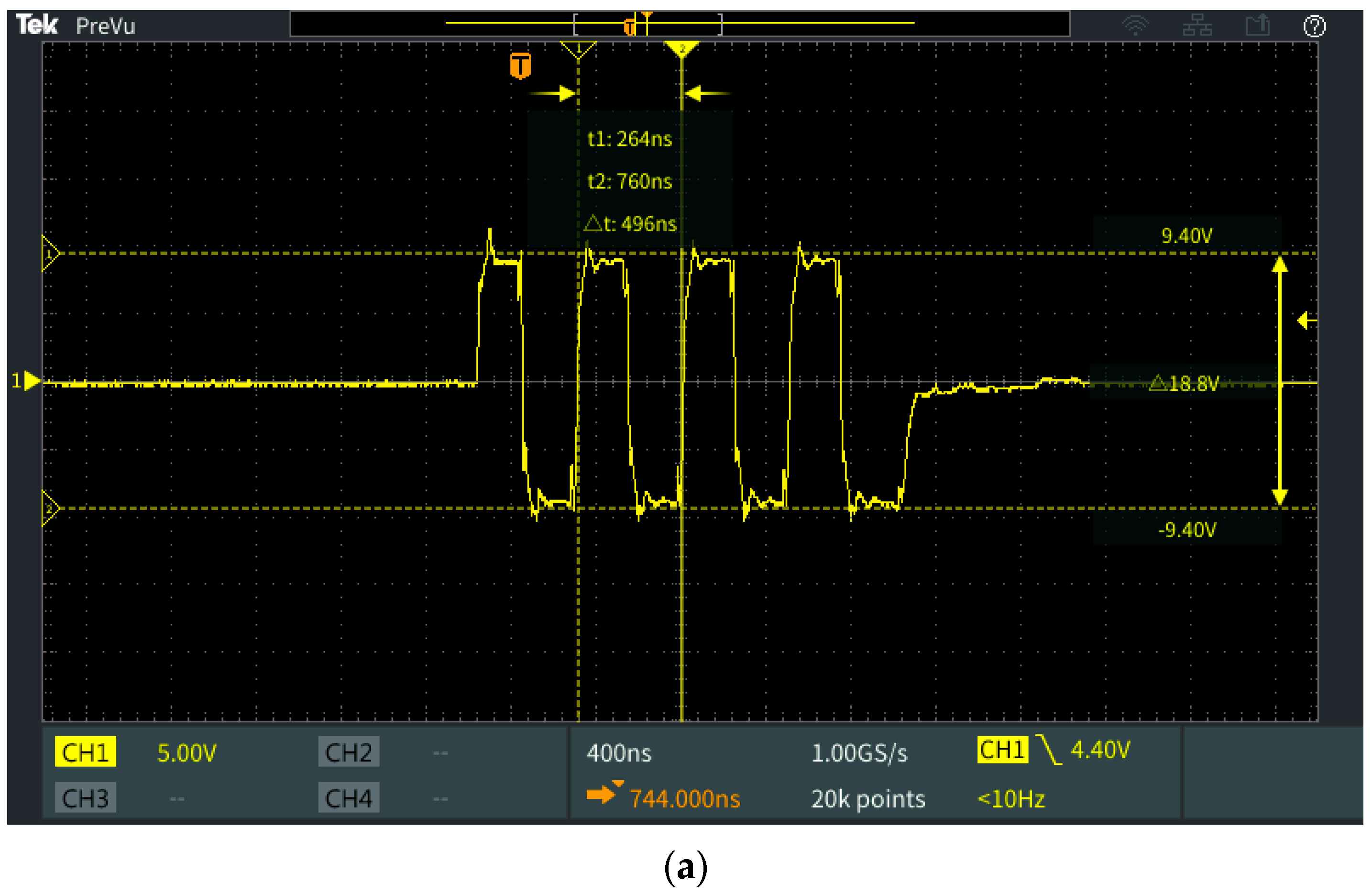
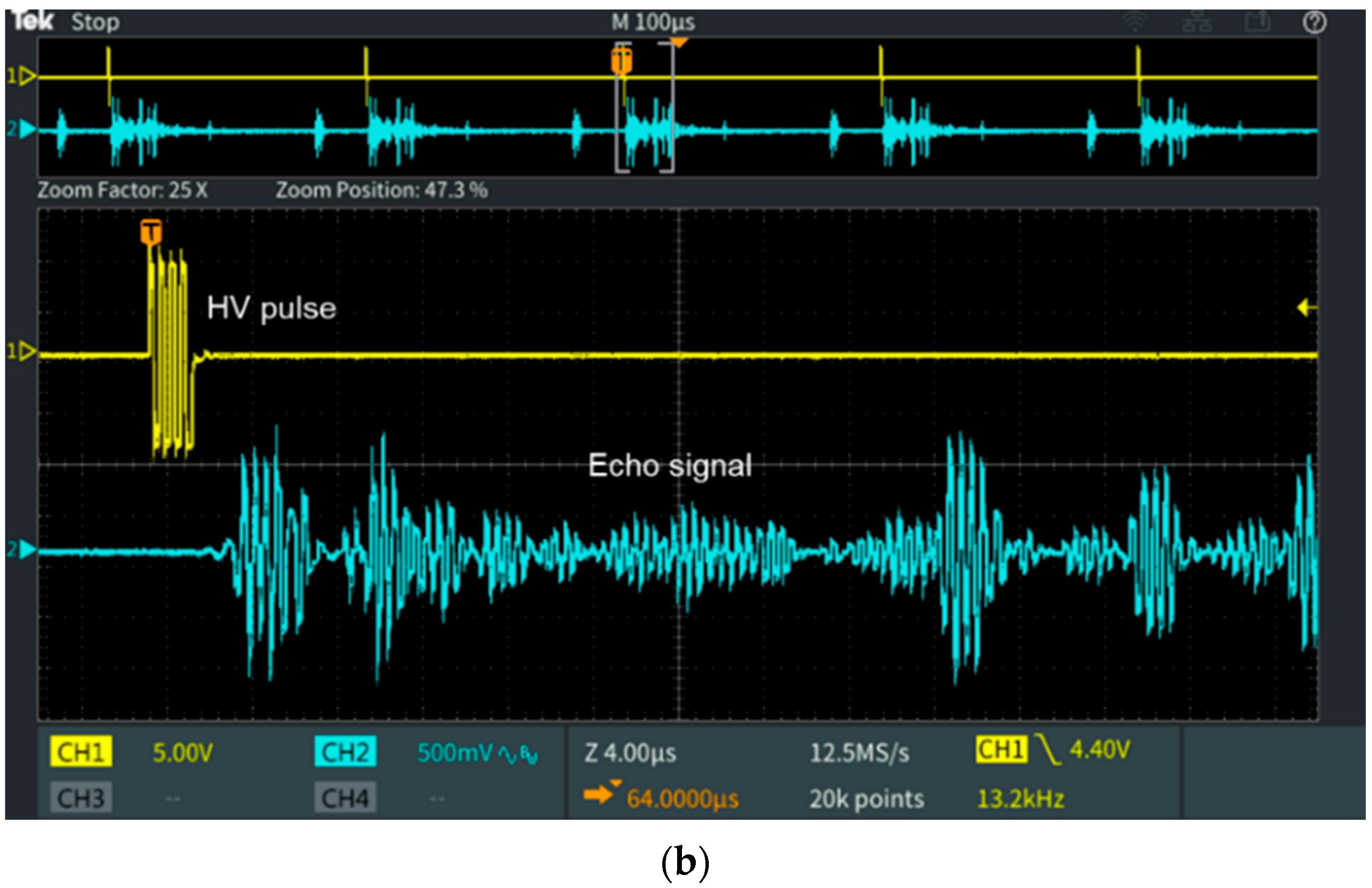
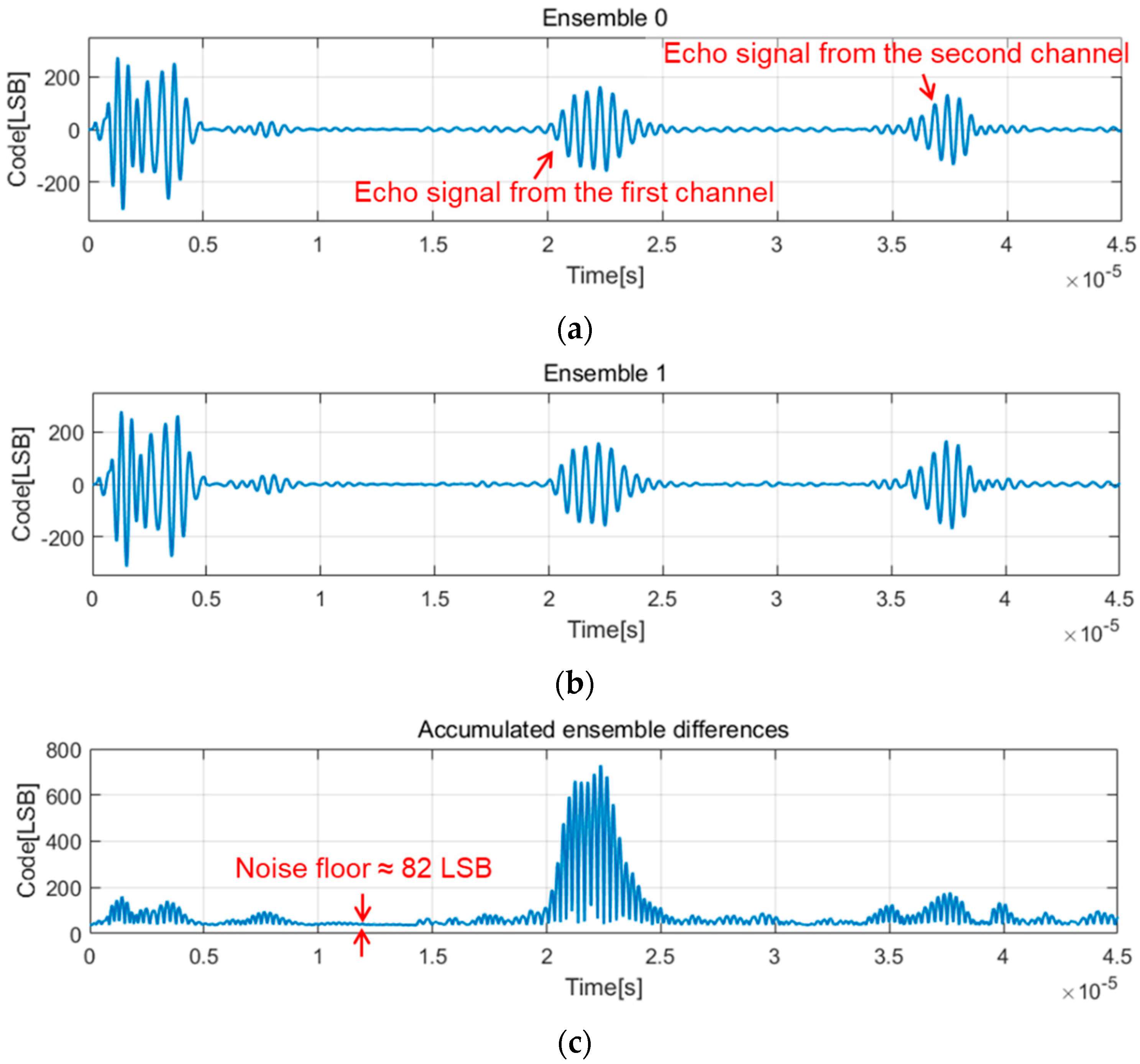
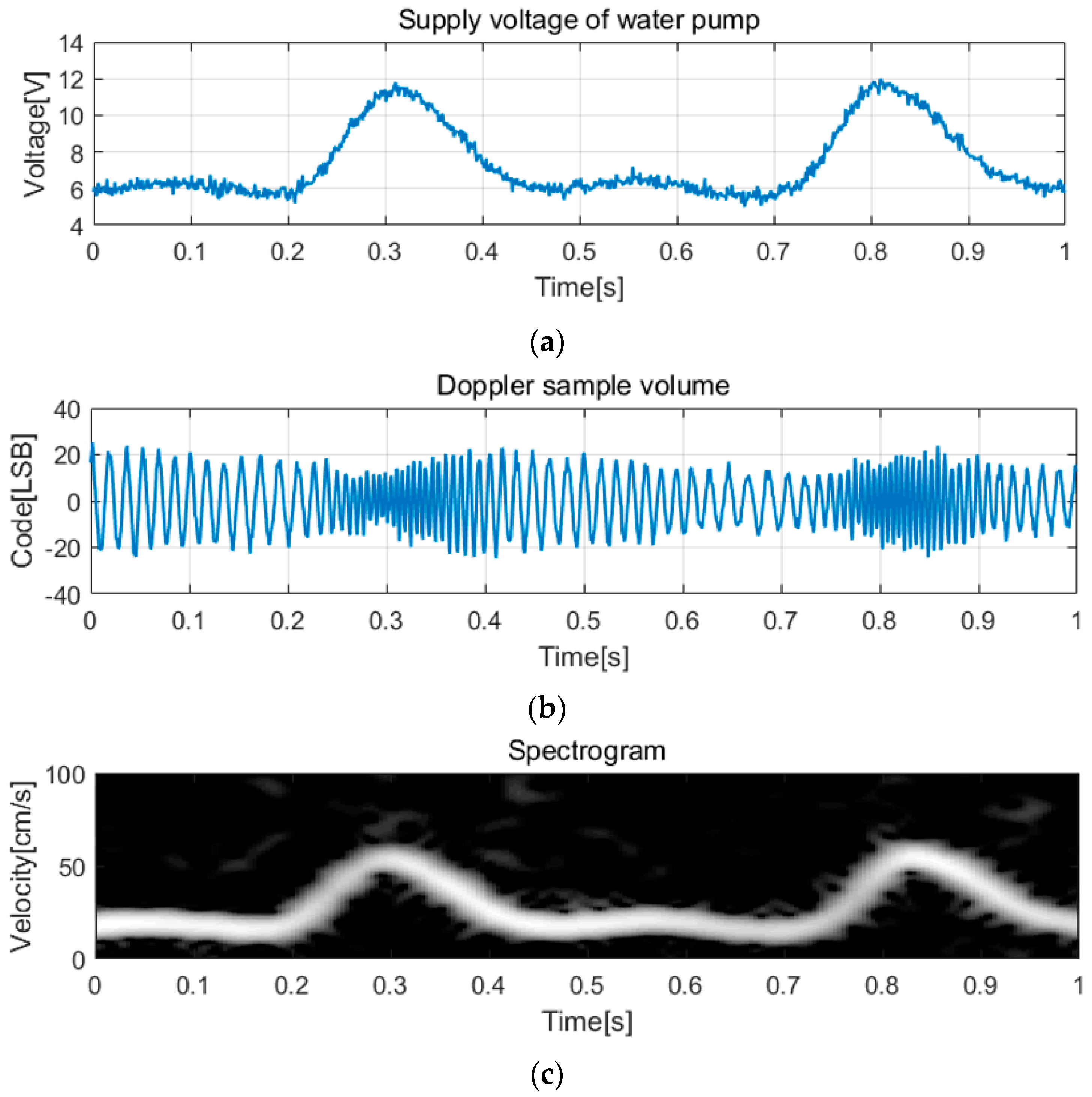
3.2. Measurements and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agnew, C.E.; McCann, A.J.; Lockhart, C.J.; Hamilton, P.K.; McVeigh, G.E.; McGivern, R.C. Comparison of rootMUSIC and discrete wavelet transform analysis of Doppler ultrasound blood flow waveforms to detect microvascular abnormalities in Type I diabetes. IEEE Trans. Biomed. Eng. 2011, 58, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zhang, D.; Xu, Y.; Chen, Y.; Wu, Z.; Cui, Y. H-scan subtraction Doppler imaging: A novel ultrasound small blood vessel flow characterization with scattering and reflection identification. Appl. Sci. 2020, 10, 7604. [Google Scholar] [CrossRef]
- Osmanski, B.F.; Bercoff, J.; Montaldo, G.; Loupas, T.; Fink, M.; Tanter, M. Cancellation of Doppler intrinsic spectral broadening using ultrafast Doppler imaging. IEEE Trans. Ultrason. Ferroelect. Freq. Control 2014, 61, 1396–1408. [Google Scholar] [CrossRef]
- Jannewein, L.; Theissen, S.; Pfeifenberger, H.R.; Zander, N.; Fischer, K.; Eichbaum, C.; Louwen, F. Differences in biometric fetal weight estimation accuracy and Doppler examination results in uncomplicated term singleton pregnancies between vertex and breech presentation. J. Clin. Med. 2021, 10, 3252. [Google Scholar] [CrossRef] [PubMed]
- Ovali, G.Y.; Ersoy, B.; Urk, O.T.V.; Ozkol, M.; Ozhan, B.; Baser, E.; Pabuscu, P. Doppler ultrasonography imaging of hemodynamic alteration of retrobulbar circulation in type 1 diabetic children and adolescents without retinopathy. Diabetes Res. Clin. Pract. 2008, 79, 243–248. [Google Scholar] [CrossRef]
- Michelson, G.; Harazny, J.; Schmieder, R.E.; Berendes, R.; Fiermann, T.; Wärntges, S. Fourier analysis of the envelope of the ophthalmic artery blood flow velocity: Age-and blood pressure related impact. Hypertension 2007, 50, 964–969. [Google Scholar] [CrossRef]
- David, J.Y.; Jones, S.A.; Giddens, D.P. Modern spectral analysis techniques for blood flow velocity and spectral measurements with pulsed Doppler ultrasound. IEEE Trans. Biomed. Eng. 1991, 38, 589–596. [Google Scholar] [CrossRef]
- Staub, D.; Meyehans, A.; Bundi, B.; Schmid, H.P.; Frauchiger, B. Prediction of cardiovascular morbidity and mortality: Comparison of the internal carotid artery resistive index with the common carotid artery intima-media thickness. Stroke 2006, 37, 800–805. [Google Scholar] [CrossRef]
- Um, J.-Y.; Kim, Y.-J.; Cho, S.-E.; Chae, M.-K.; Song, J.; Kim, B.; Lee, S.; Bang, J.; Kim, Y.; Cho, K.; et al. An analog-digital-hybrid RX beamformer chip with non-uniform sampling for ultrasound imaging with 2D CMUT array. IEEE Trans. Biomed. Circuits Syst. 2015, 9, 138–151. [Google Scholar] [CrossRef]
- Kim, G.-D.; Yoon, C.; Kye, S.-B.; Lee, Y.; Kang, J.; Yoo, Y.; Song, T.-K. A single FPGA-based portable ultrasound imaging system for point-of-care applications. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 59, 1386–1394. [Google Scholar]
- Lee, H.; Sohn, H.-Y.; Yoon, C.; Yoo, Y.; Song, T.-K. Software-based hand-held ultrasound color Doppler imaging system. In Proceedings of the IEEE International Ultrasonics Symposium, Rome, Italy, 20–23 September 2009; pp. 1844–1847. [Google Scholar]
- Hu, C.-H.; Zou, Q.; Shung, K.K. Design and implementation of high frequency ultrasound pulsed-wave Doppler using FPGA. IEEE Trans. Ultrason. Ferroelect. Freq. Control 2008, 55, 2109–2111. [Google Scholar]
- Jana, B.; Biswas, B.; Nath, P.K.; Saha, G.; Banerjee, S. Smartphone based point-of-care system using continuous wave portable Doppler. IEEE Trans. Instrum. Meas. 2020, 69, 8352–8361. [Google Scholar] [CrossRef]
- Huang, C.-C.; Lee, P.-Y.; Chen, P.-Y.; Liu, T.-Y. Design and implementation of a smartphone-based portable ultrasound pulsed-wave Doppler device for blood flow measurement. IEEE Trans. Ultrason. Ferroelect. Freq. Control 2012, 59, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Song, I.; Yoon, J.; Kang, J.; Kim, M.; Jang, W.S.; Shin, N.-Y.; Yoo, Y. Design and implementation of a new wireless carotid neckband Doppler system with wearable ultrasound sensors: Preliminary results. Appl. Sci. 2019, 9, 2202. [Google Scholar] [CrossRef]
- Sahani, A.K.; Shah, M.I.; Radhakrishnan, R.; Joseph, J.; Sivaprakasam, M. An imageless ultrasound device to measure local and regional arterial stiffness. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 200–208. [Google Scholar] [CrossRef]
- Tan, M.; Kang, E.; An, J.-S.; Chang, Z.-Y.; Vince, P.; Matéo, T.; Sénégond, N.; Pertijs, M.A.P. A 64-channel transmit beamformer with ±30-V bipolar high-voltage pulsers for catheter-based ultrasound probes. IEEE J. Solid State Circuits 2020, 55, 1796–1806. [Google Scholar] [CrossRef]
- Qiu, W.; Yu, Y.; Tsang, F.K.; Sun, L. A multifunctional, reconfigurable pulse generator for high-frequency ultrasound imaging. IEEE Trans. Ultrason. Ferroelect. Freq. Control 2012, 59, 1558–1567. [Google Scholar]
- Xu, X.; Sun, L.; Cannata, J.M.; Yen, J.T.; Shung, K.K. High frequency ultrasound Doppler system for biomedical applications with a 30-MHz linear array. Ultrasound Med. Biol. 2008, 34, 638–646. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, X.; Yen, J.T.; Shung, K.K. A low-cost bipolar pulse generator for high-frequency ultrasound application. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2007, 54, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Ultra Compact Octal 3L/Quad 5L Pulser with T/R Switches and Beamforming Capability, Maxim Integrated; MSDS No. MAX14813 Data Sheet; San Jose, CA, USA. November 2019. Available online: http://maximintegrated.com (accessed on 1 September 2021).
- 8-Channel, Low Jitter Ultrasound Transmit Beamformer with Programmable Duty-Cycle and High Resolution; MSDS No. LM96570 Data Sheet; Texas Instruments: Dallas, TX, USA, 2013; Available online: http://ti.com (accessed on 1 September 2021).
- Shung, K.K. Diagnostic Ultrasound: Imaging and Blood Flow Measurements, 1st ed.; CRC Press: New York, NY, USA, 2006; pp. 113–115. [Google Scholar]
- Jensen, J.A. Acoustical Imaging, 1st ed.; Springer: Boston, MA, USA, 1996; pp. 377–384. [Google Scholar]
- Field II Simulation Program. PW Example. Available online: https://field-ii.dk (accessed on 1 September 2021).
- Feneck, R.; Kneeshaw, J.; Ranucci, M. Core Topics in Transesophageal Echocardiography, 1st ed.; Cambridge University Press: Cambridge, UK, 2010; pp. 13–33. [Google Scholar]
- Pater, L.A.; Kindt, J.T. Simulations of NaCl aggregation from solution: Solvent determines topography of free energy landscape. J. Comput. Chem. 2019, 40, 135–147. [Google Scholar]
- Pal, B.; Kundu, S. Anomalous ultrasonic attenuation in aqueous NaCl solutions. Univers. J. Chem. 2013, 1, 96–101. [Google Scholar] [CrossRef]
- Matera, R.; Ricci, S. Automatic measurement of the carotid blood flow for wearable sensors: A pilot study. Sensors 2021, 21, 5877. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Zhou, S.K.; Simopoulos, C.; Comaniciu, D. AutoGate: Fast and Automatic Doppler Gate Localization in B-Mode Echocardiogram. Medical Image Computing and Computer-Assisted Intervention—Lecture Note in Computer Science; Springer: Berlin, Germany, 2008; pp. 230–237. [Google Scholar] [CrossRef]
- Evans, D.H.; McDicken, W.N. Doppler Ultrasound, Physics, Instrumentation and Signal Processing, 2nd ed.; Wiley Press: New York, NY, USA, 2000; pp. 200–222. [Google Scholar]






| Logic Utilization | Used | Utilization Percentage |
|---|---|---|
| Slice registers | 382 | 0.7% |
| Slice LUTs | 1535 | 5.6% |
| Fully used LUT–FF pairs | 336 | 21.2% |
| Bonded IOBs | 25 | 11.5% |
| Method | Concept | Strengths | Limitations |
|---|---|---|---|
| This work | Monitoring Doppler amplitude deviation | Single transducer Detection of low-resistance arbitrary vessels | Lack of in vivo test Manual angle correction |
| [16] | Recognition of featured A-mode pattern | Single transducer Detection of separate vessel walls | High center frequency Specific target vessel |
| [29] | Recognition of featured shape in B-mode image | Non-expert-user-friendly Automatic tracking of carotid artery | Two transducer probes Specific target vessel |
| [30] | Learning-based segmentation in B-mode image | Robust RG positioning Operation in cardiac application | Intensive computing Complicated algorithm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-T.; Um, J.-Y. Image-Free Ultrasound Blood-Flow Monitoring Circuit System with Automatic Range-Gate Positioning Scheme: A Pilot Study. Appl. Sci. 2021, 11, 10617. https://doi.org/10.3390/app112210617
Park H-T, Um J-Y. Image-Free Ultrasound Blood-Flow Monitoring Circuit System with Automatic Range-Gate Positioning Scheme: A Pilot Study. Applied Sciences. 2021; 11(22):10617. https://doi.org/10.3390/app112210617
Chicago/Turabian StylePark, Hyun-Tae, and Ji-Yong Um. 2021. "Image-Free Ultrasound Blood-Flow Monitoring Circuit System with Automatic Range-Gate Positioning Scheme: A Pilot Study" Applied Sciences 11, no. 22: 10617. https://doi.org/10.3390/app112210617
APA StylePark, H.-T., & Um, J.-Y. (2021). Image-Free Ultrasound Blood-Flow Monitoring Circuit System with Automatic Range-Gate Positioning Scheme: A Pilot Study. Applied Sciences, 11(22), 10617. https://doi.org/10.3390/app112210617





