The Effectiveness of Far-Ultraviolet (UVC) Light Prototype Devices with Different Wavelengths on Disinfecting SARS-CoV-2
Abstract
:1. Introduction
2. Materials and Methods
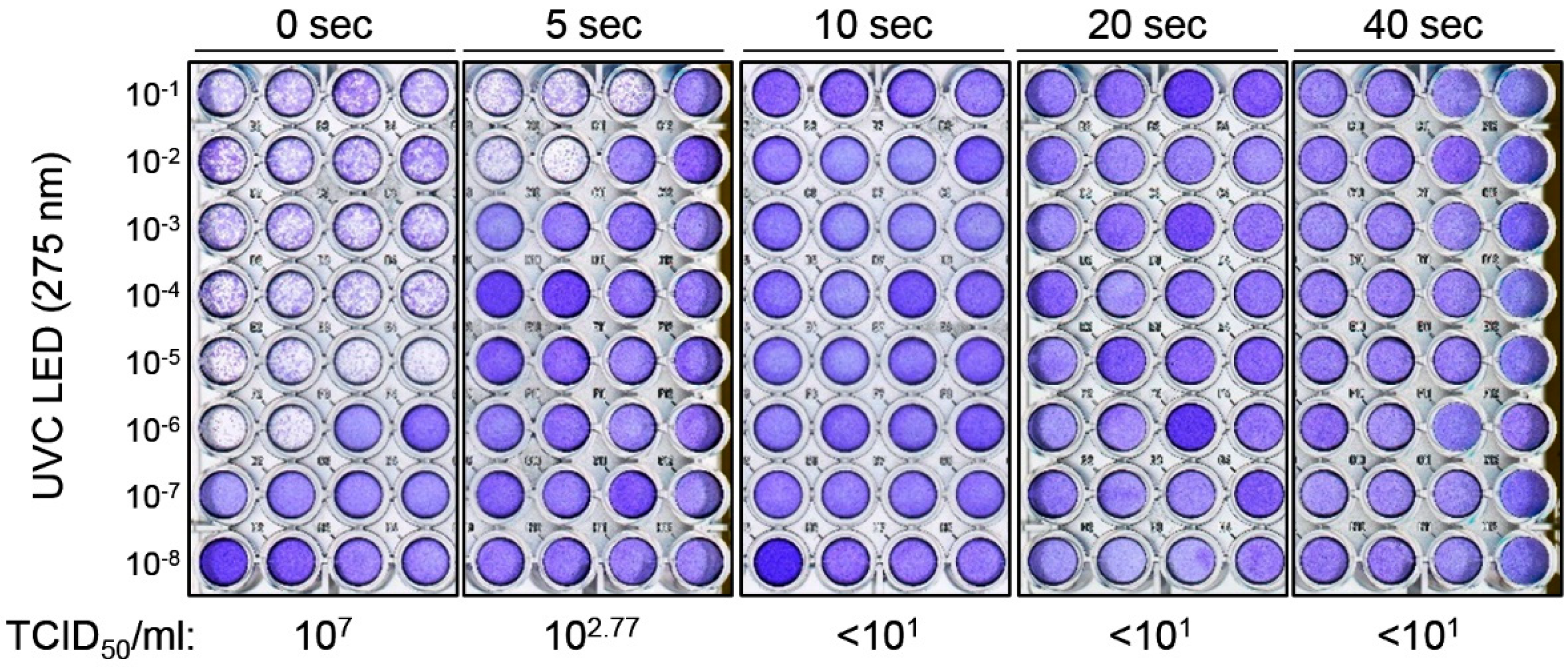
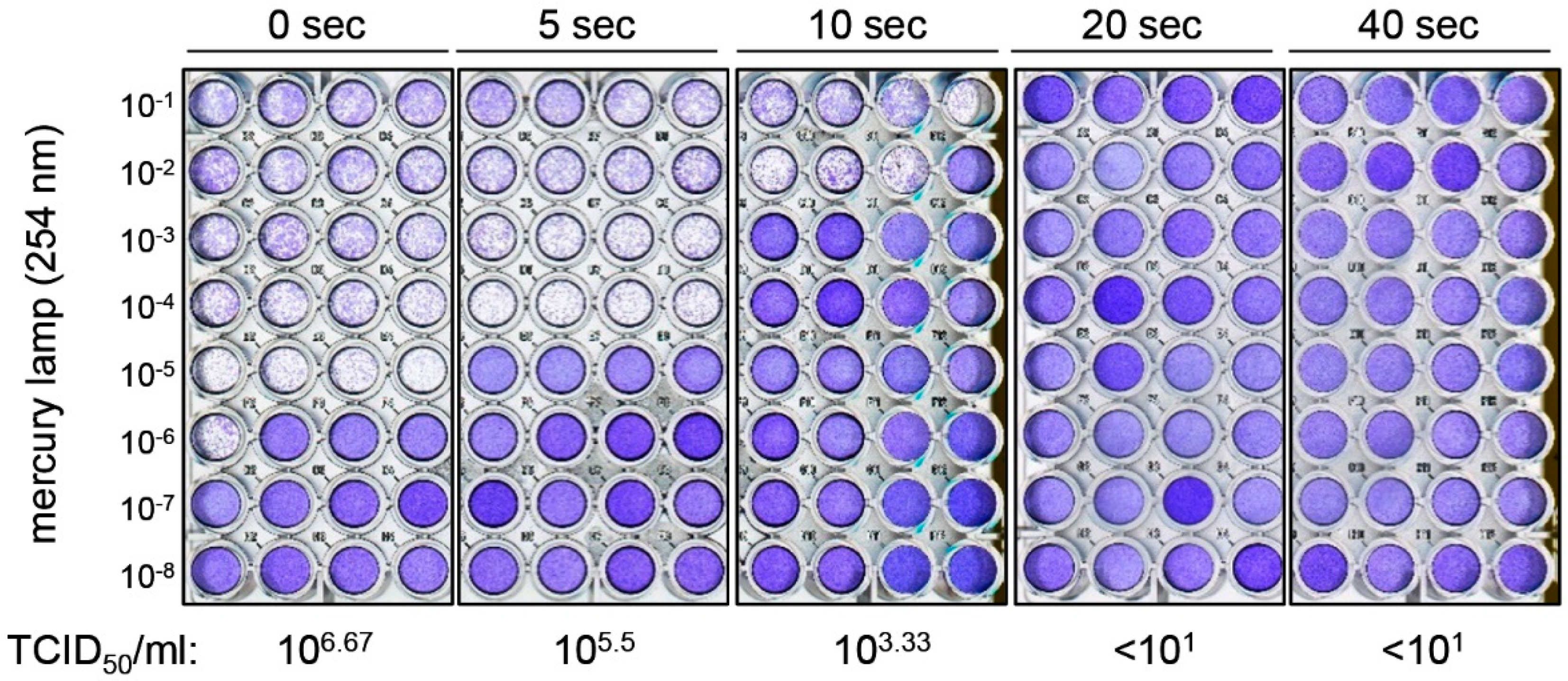
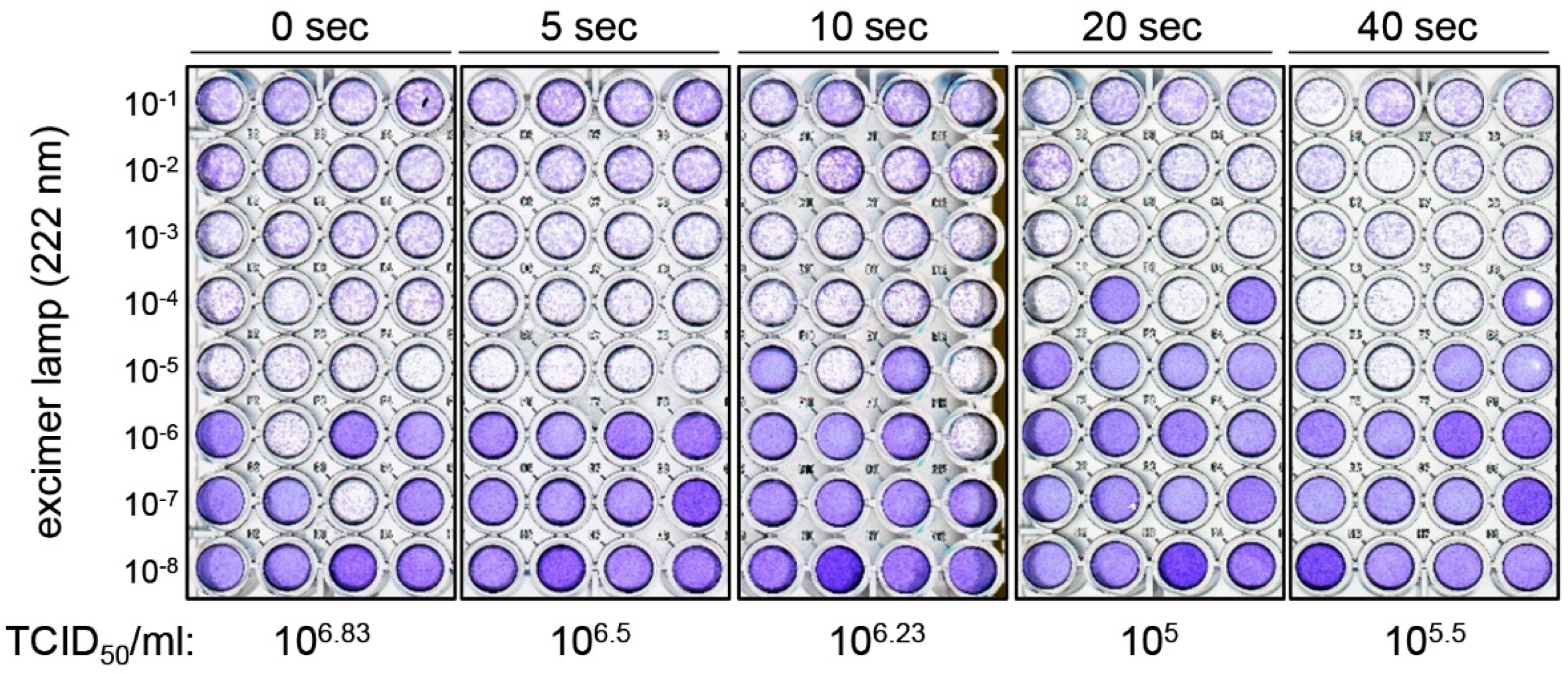
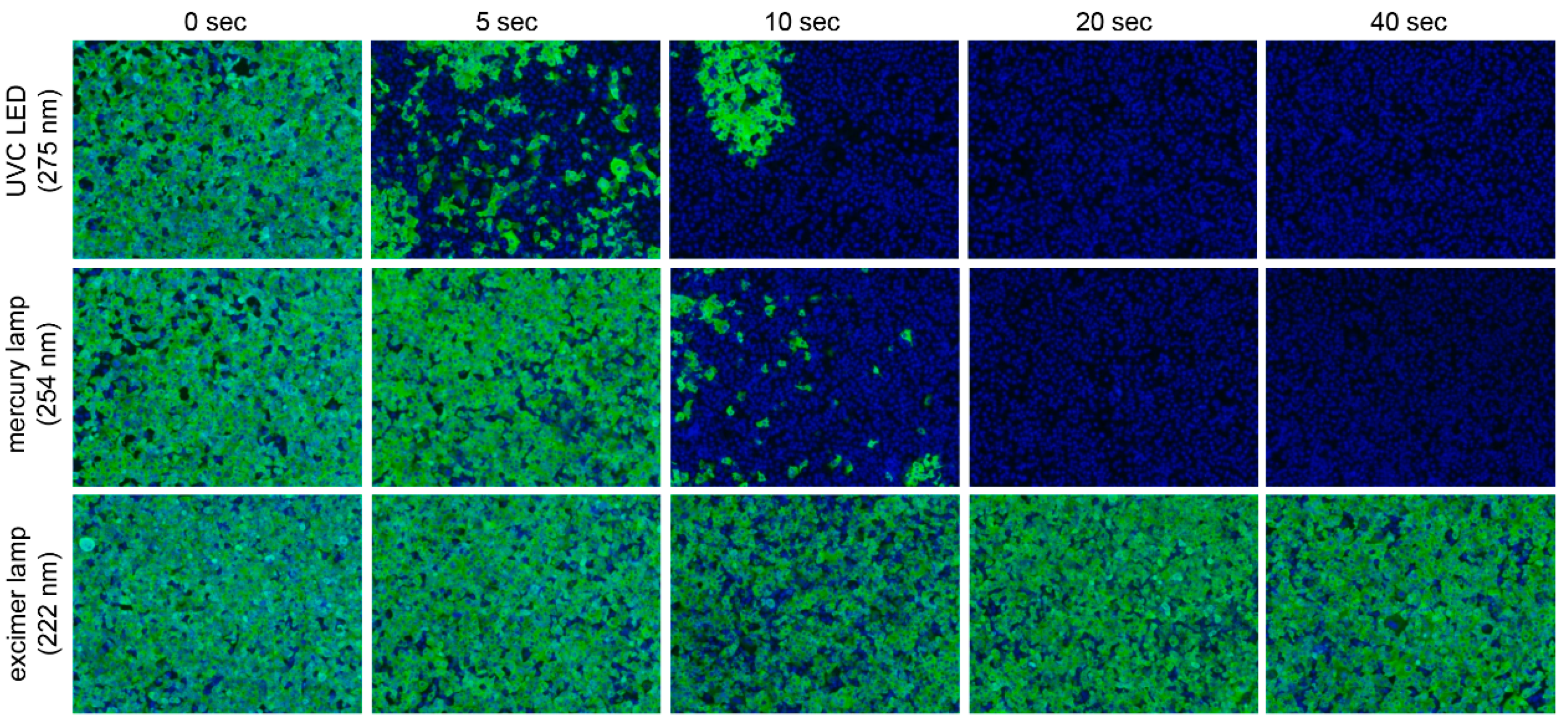
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ahidjo, B.; Loe, M.W.C.; Ng, Y.L.; Mok, C.-K.; Chu, J.J.H. Current Perspective of Antiviral Strategies against COVID-19. ACS Infect. Dis. 2020, 6, 1624–1634. [Google Scholar] [CrossRef] [PubMed]
- Osman, E.E.; Toogood, P.L.; Neamati, N. COVID-19: Living through Another Pandemic. ACS Infect. Dis. 2020, 6, 1548–1552. [Google Scholar] [CrossRef] [PubMed]
- Sahin, A.R.; Erdogan, A.; Agaoglu, P.M.; Dineri, Y.; Cakirci, A.Y.; Senel, M.E.; Okyay, R.A.; Tasdogan, A.M. 2019 Novel Coronavirus (COVID-19) Outbreak: A Review of the Current Literature. Eurasian J. Med. Oncol. 2020, 4, 1–7. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raeiszadeh, M.; Adeli, B. A Critical Review on Ultraviolet Disinfection Systems against COVID-19 Outbreak: Applicability, Validation, and Safety Considerations. ACS Photonics 2020, 7, 2941–2951. [Google Scholar] [CrossRef]
- Kowalski, W. Ultraviolet Germicidal Irradiation Handbook: UVGI for Air and Surface Disinfection; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Dai, T.; Vrahas, M.S.; Murray, C.K.; Hamblin, M.R. Ultraviolet C irradiation: An alternative antimicrobial approach to localized infections? Expert Rev. Anti-Infect. Ther. 2012, 10, 185–195. [Google Scholar] [CrossRef] [Green Version]
- Shining a light on COVID-19. Nat. Photonics 2020, 14, 337. [CrossRef]
- Darnell, M.E.; Subbarao, K.; Feinstone, S.M.; Taylor, D.R. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J. Virol. Methods 2004, 121, 85–91. [Google Scholar] [CrossRef]
- Heilingloh, C.S.; Aufderhorst, U.W.; Schipper, L.; Dittmer, U.; Witzke, O.; Yang, D.; Zheng, X.; Sutter, K.; Trilling, M.; Alt, M.; et al. Susceptibility of SARS-CoV-2 to UV irradiation. Am. J. Infect. Control 2020, 48, 1273–1275. [Google Scholar] [CrossRef]
- Kitagawa, H.; Nomura, T.; Nazmul, T.; Omori, K.; Shigemoto, N.; Sakaguchi, T.; Ohge, H. Effectiveness of 222-nm ultraviolet light on disinfecting SARS-CoV-2 surface contamination. Am. J. Infect. Control 2021, 49, 299–301. [Google Scholar] [CrossRef]
- Buonanno, M.; Welch, D.; Shuryak, I.; Brenner, D.J. Far-UVC light (222 nm) efficiently and safely inactivates airborne human coronaviruses. Sci. Rep. 2020, 10, 10285. [Google Scholar] [CrossRef]
- Song, K.; Taghipour, F.; Mohseni, M. Microorganisms inactivation by wavelength combinations of ultraviolet light-emitting diodes (UV-LEDs). Sci. Total. Environ. 2019, 665, 1103–1110. [Google Scholar] [CrossRef]
- Craik, S.A.; Weldon, D.; Finch, G.R.; Bolton, J.R.; Belosevic, M. Inactivation of cryptosporidium parvum oocysts using medium- and low-pressure ultraviolet radiation. Water Res. 2001, 35, 1387–1398. [Google Scholar] [CrossRef]
- Raeiszadeh, M.; Taghipour, F. Microplasma UV lamp as a new source for UV-induced water treatment: Protocols for characterization and kinetic study. Water Res. 2019, 164, 114959. [Google Scholar] [CrossRef] [PubMed]
- Health Quality Ontario. Portable Ultraviolet Light Surface-Disinfecting Devices for Prevention of Hospital-Acquired Infections: A Health Technology Assessment. Ont. Health Technol. Assess. Ser. 2018, 18, 1–73. Available online: https://pubmed.ncbi.nlm.nih.gov/29487629 (accessed on 8 November 2021).
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Jinadatha, C.; Simmons, S.; Dale, C.; Ganachari-Mallappa, N.; Villamaria, F.C.; Goulding, N.; Tanner, B.; Stachowiak, J.; Stibich, M. Disinfecting personal protective equipment with pulsed xenon ultraviolet as a risk mitigation strategy for health care workers. Am. J. Infect. Control 2015, 43, 412–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanley Electric Co., Ltd. How UV-C Effect for Disinfection? Available online: https://www.stanley.co.jp/e/product/uvc_product/effect/ (accessed on 8 November 2021).
- Trivellin, N.; Buffolo, M.; Onelia, F.; Pizzolato, A.; Barbato, M.; Orlandi, V.; Del Vecchio, C.; Dughiero, F.; Zanoni, E.; Meneghesso, G.; et al. Inactivating SARS-CoV-2 Using 275 nm UV-C LEDs through a Spherical Irradiation Box: Design, Characterization and Validation. Materials 2021, 14, 2315. [Google Scholar] [CrossRef]
- Biasin, M.; Bianco, A.; Pareschi, G.; Cavalleri, A.; Cavatorta, C.; Fenizia, C.; Galli, P.; Lessio, L.; Lualdi, M.; Tombetti, E.; et al. UV-C irradiation is highly effective in inactivating SARS-CoV-2 replication. Sci. Rep. 2021, 11, 6260. [Google Scholar] [CrossRef]
- Buonanno, M.; Ponnaiya, B.; Welch, D.; Stanislauskas, M.; Randers-Pehrson, G.; Smilenov, L.; Lowy, F.D.; Owens, D.M.; Brenner, D.J. Germicidal Efficacy and Mammalian Skin Safety of 222-nm UV Light. Radiat. Res. 2017, 187, 493–501. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.W.; Marr, L.C.; Li, Y.; Dancer, S.J. COVID-19 has redefined airborne transmission. BMJ 2021, 373, n913. [Google Scholar] [CrossRef] [PubMed]
- Astrid, F.; Beata, Z.; Miriam, V.D.N.; Julia, E.; Elisabeth, P.; Magda, D.-E. The use of a UV-C disinfection robot in the routine cleaning process: A field study in an Academic hospital. Antimicrob. Resist. Infect. Control 2021, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-H.; Wu, U.-I.; Tai, H.-M.; Sheng, W.-H. Effectiveness of an ultraviolet-C disinfection system for reduction of healthcare-associated pathogens. J. Microbiol. Immunol. Infect. 2019, 52, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Cadnum, J.L.; Jencson, A.L.; Gestrich, S.A.; Livingston, S.H.; Karaman, B.A.; Benner, K.; Wilson, B.M.; Donskey, C.J. A comparison of the efficacy of multiple ultraviolet light room decontamination devices in a radiology procedure room. Infect. Control Hosp. Epidemiol. 2019, 40, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Guettari, M.; Gharbi, I.; Hamza, S. UVC disinfection robot. Environ. Sci. Pollut. Res. 2021, 28, 40394–40399. [Google Scholar] [CrossRef] [PubMed]
| Radiation (μJ/cm2) | ||||
|---|---|---|---|---|
| Exposure Time | 5 s | 10 s | 20 s | 40 s |
| UVC LED (275 nm) | 399 | 798 | 1596 | 3192 |
| Mercury lamp (254 nm) | 4250 | 8500 | 17,000 | 34,000 |
| Excimer lamp (222 nm) | 35 | 70 | 140 | 280 |
| Log-Reduction Value (LRV) | ||||
|---|---|---|---|---|
| Exposure Time | 5 s | 10 s | 20 s | 40 s |
| UVC LED (275 nm) | 4.23 | >6 | >6 | >6 |
| Mercury lamp (254 nm) | 1.17 | 3.34 | >6 | >6 |
| Excimer lamp (222 nm) | 0.33 | 0.6 | 1.83 | 1.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.-J.; Liao, C.-C.; Chang, C.-S.; Lee, C.-Y.; Chen, S.-Y.; Huang, S.-B.; Yeh, Y.-F.; Singh, K.J.; Kuo, H.-C.; Lin, Y.-L.; et al. The Effectiveness of Far-Ultraviolet (UVC) Light Prototype Devices with Different Wavelengths on Disinfecting SARS-CoV-2. Appl. Sci. 2021, 11, 10661. https://doi.org/10.3390/app112210661
Liang J-J, Liao C-C, Chang C-S, Lee C-Y, Chen S-Y, Huang S-B, Yeh Y-F, Singh KJ, Kuo H-C, Lin Y-L, et al. The Effectiveness of Far-Ultraviolet (UVC) Light Prototype Devices with Different Wavelengths on Disinfecting SARS-CoV-2. Applied Sciences. 2021; 11(22):10661. https://doi.org/10.3390/app112210661
Chicago/Turabian StyleLiang, Jian-Jong, Chun-Che Liao, Chih-Shin Chang, Chih-Yin Lee, Si-Yu Chen, Shao-Bo Huang, Yin-Fu Yeh, Konthoujam James Singh, Hao-Chung Kuo, Yi-Ling Lin, and et al. 2021. "The Effectiveness of Far-Ultraviolet (UVC) Light Prototype Devices with Different Wavelengths on Disinfecting SARS-CoV-2" Applied Sciences 11, no. 22: 10661. https://doi.org/10.3390/app112210661








