Existing and Emerging Breast Cancer Detection Technologies and Its Challenges: A Review
Abstract
1. Introduction
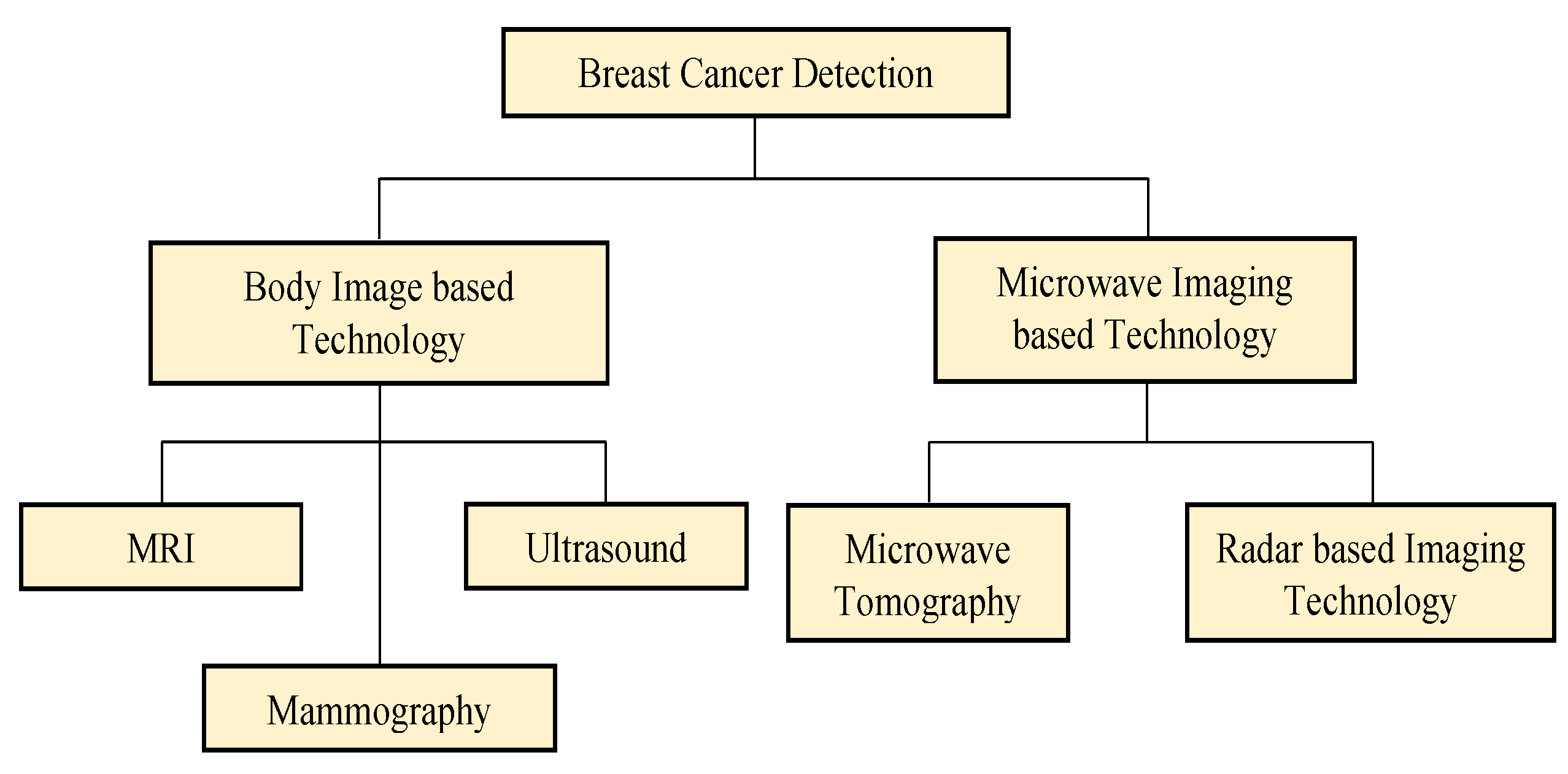
2. Existing Breast Cancer Detection Techniques & Technologies
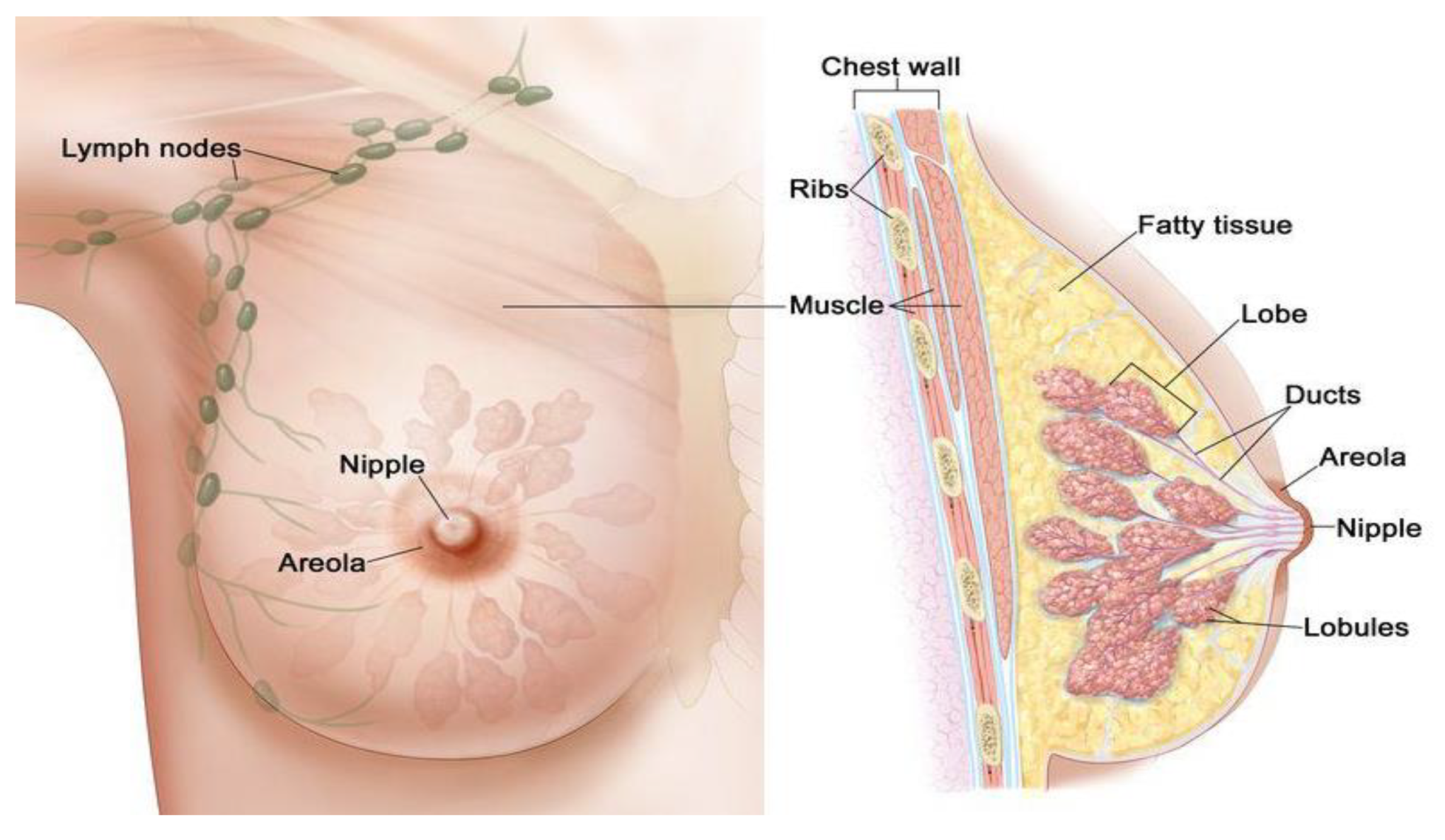
2.1. Body Image-Based Technology
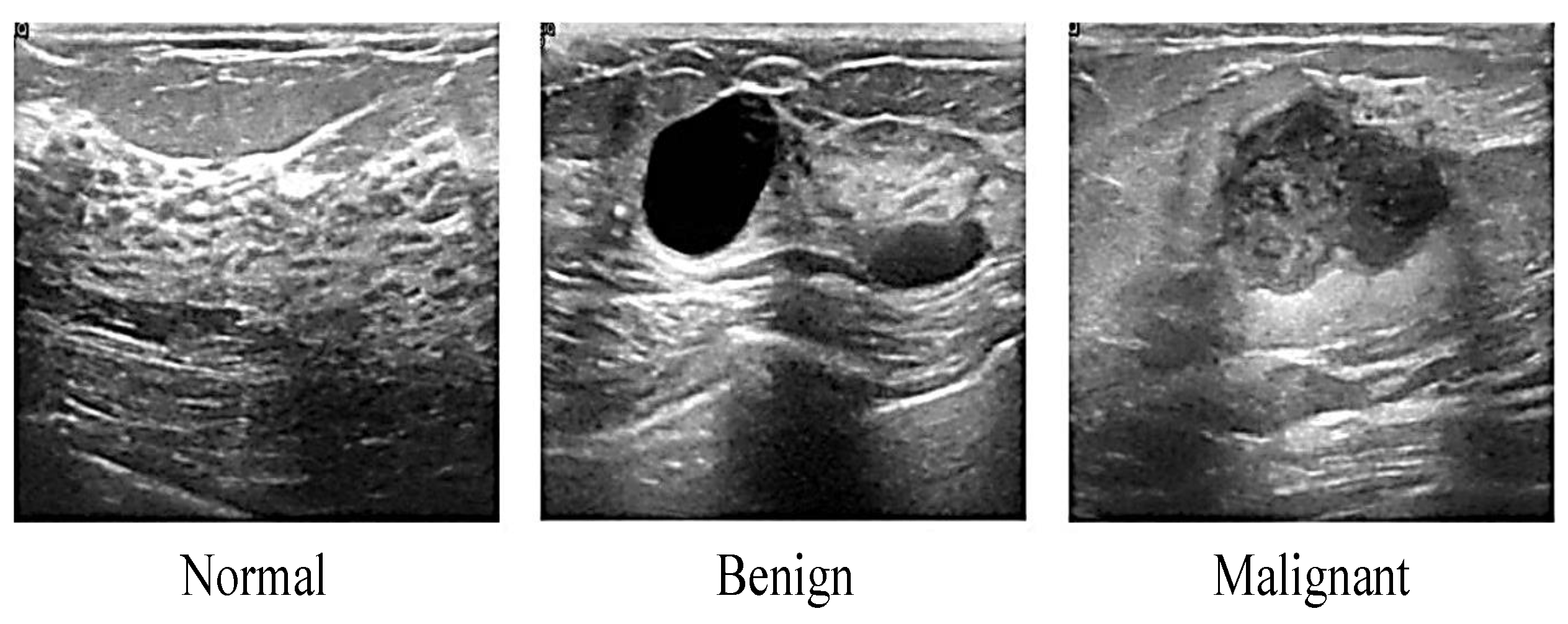
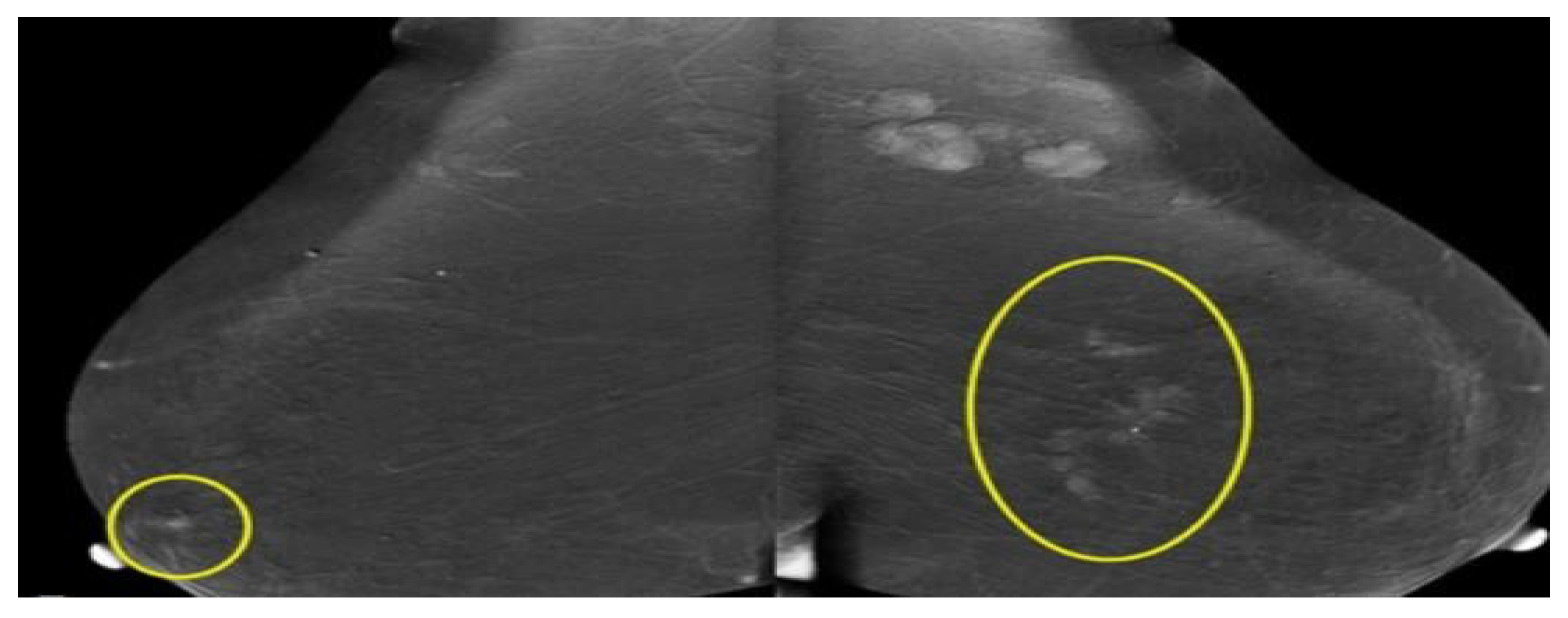
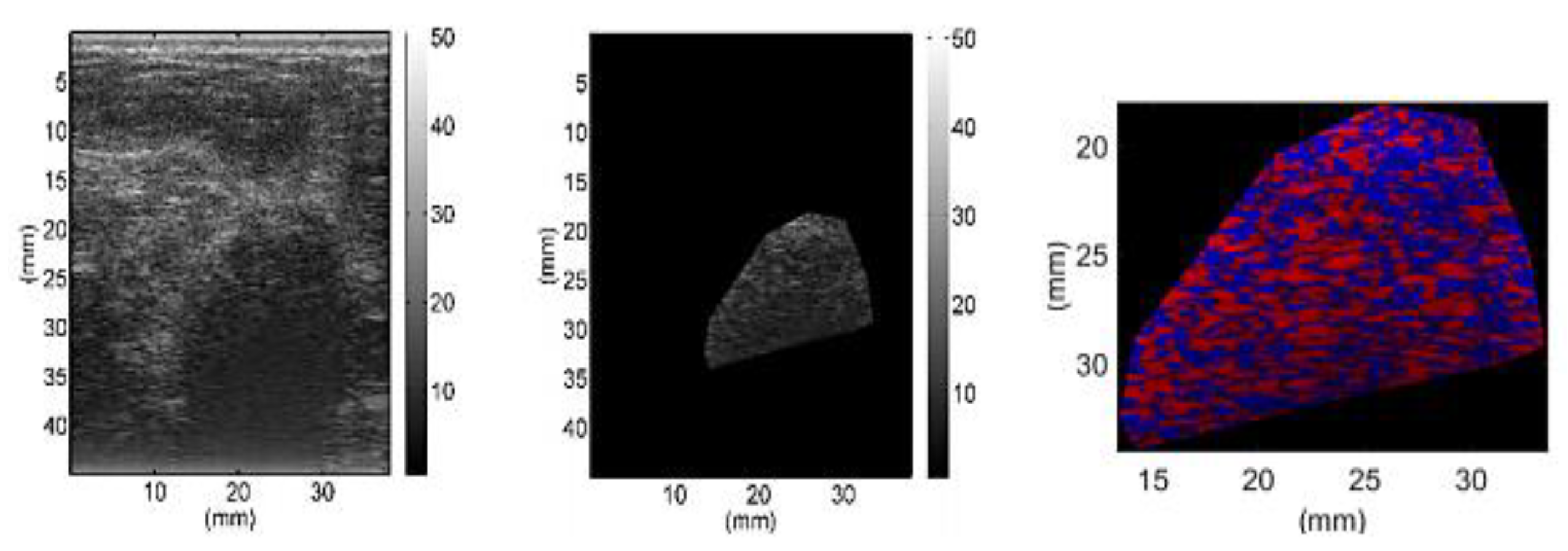
2.1.1. Ultrasound
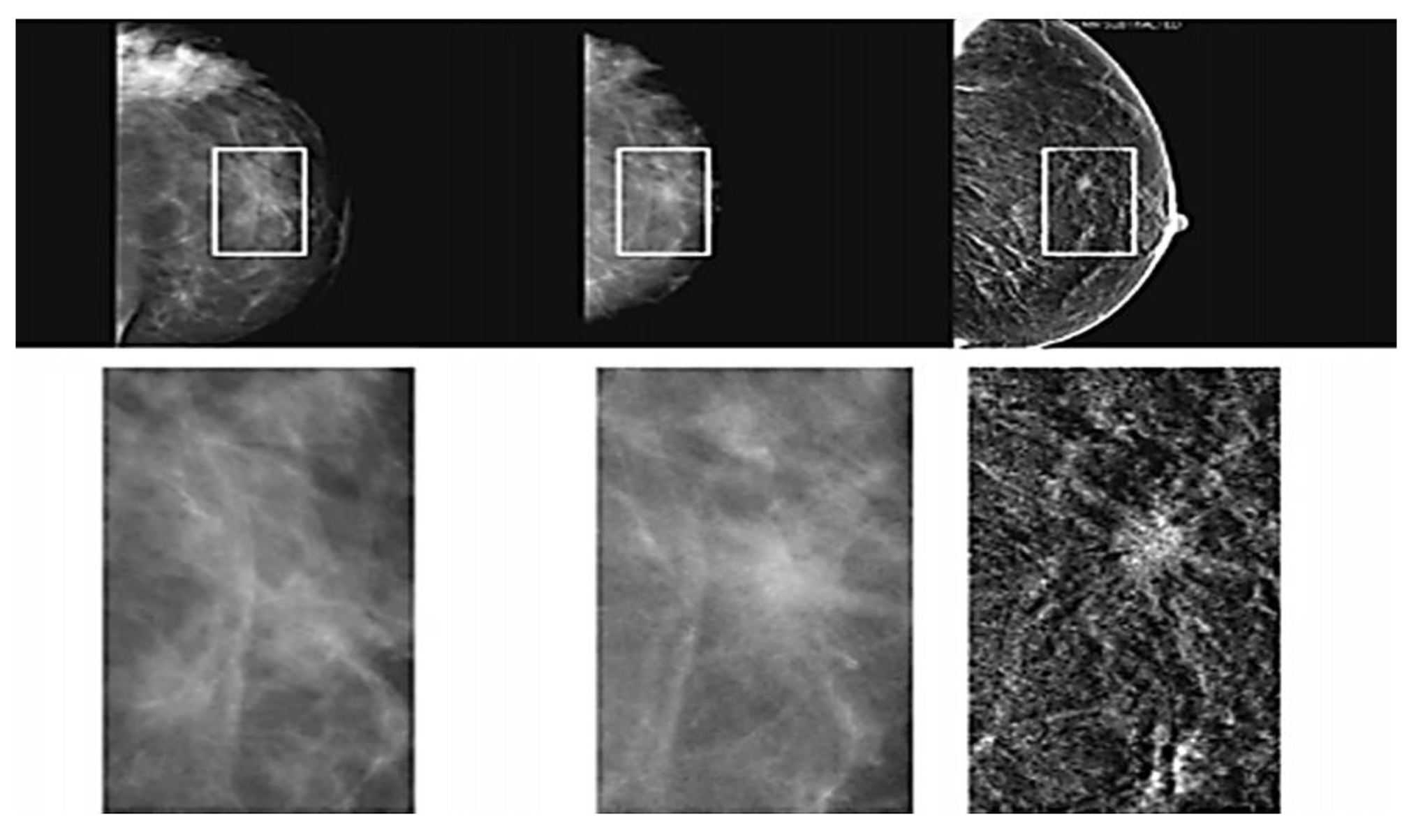
2.1.2. Mammography
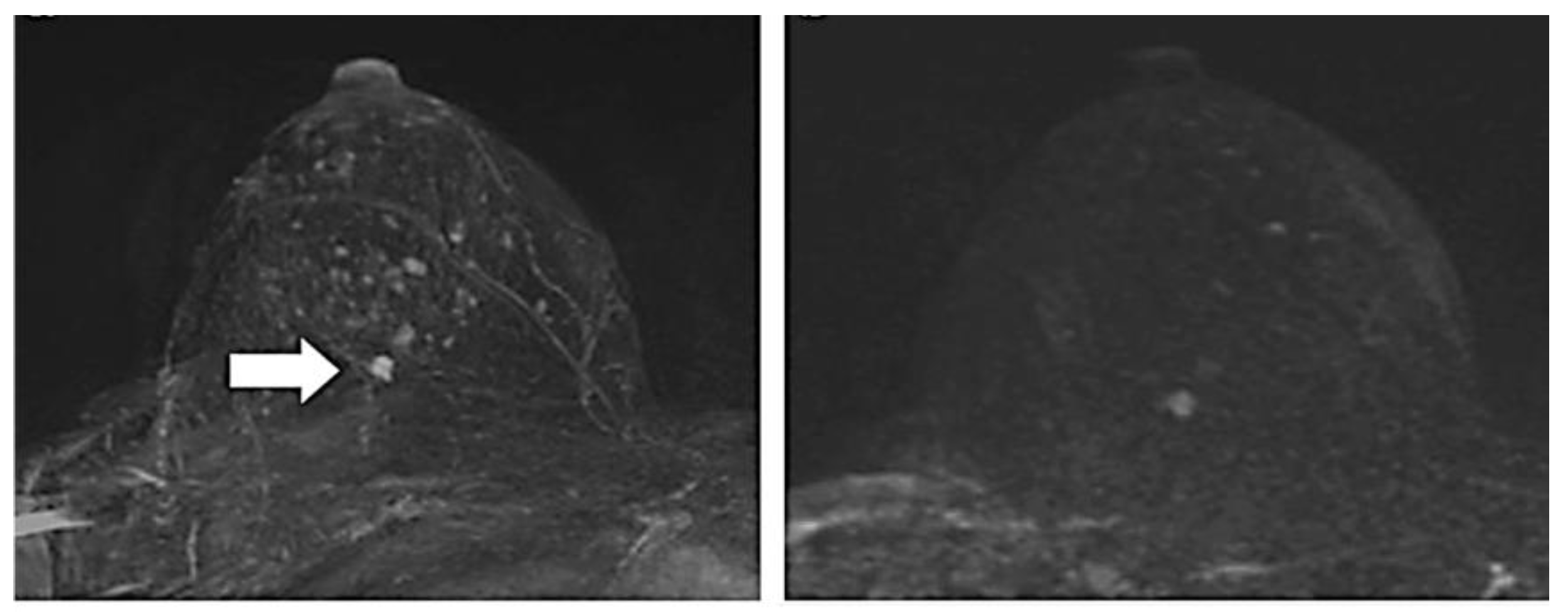
2.1.3. Magnetic Resonance Imaging (MRI)
2.2. Microwave Imaging-Based Technology
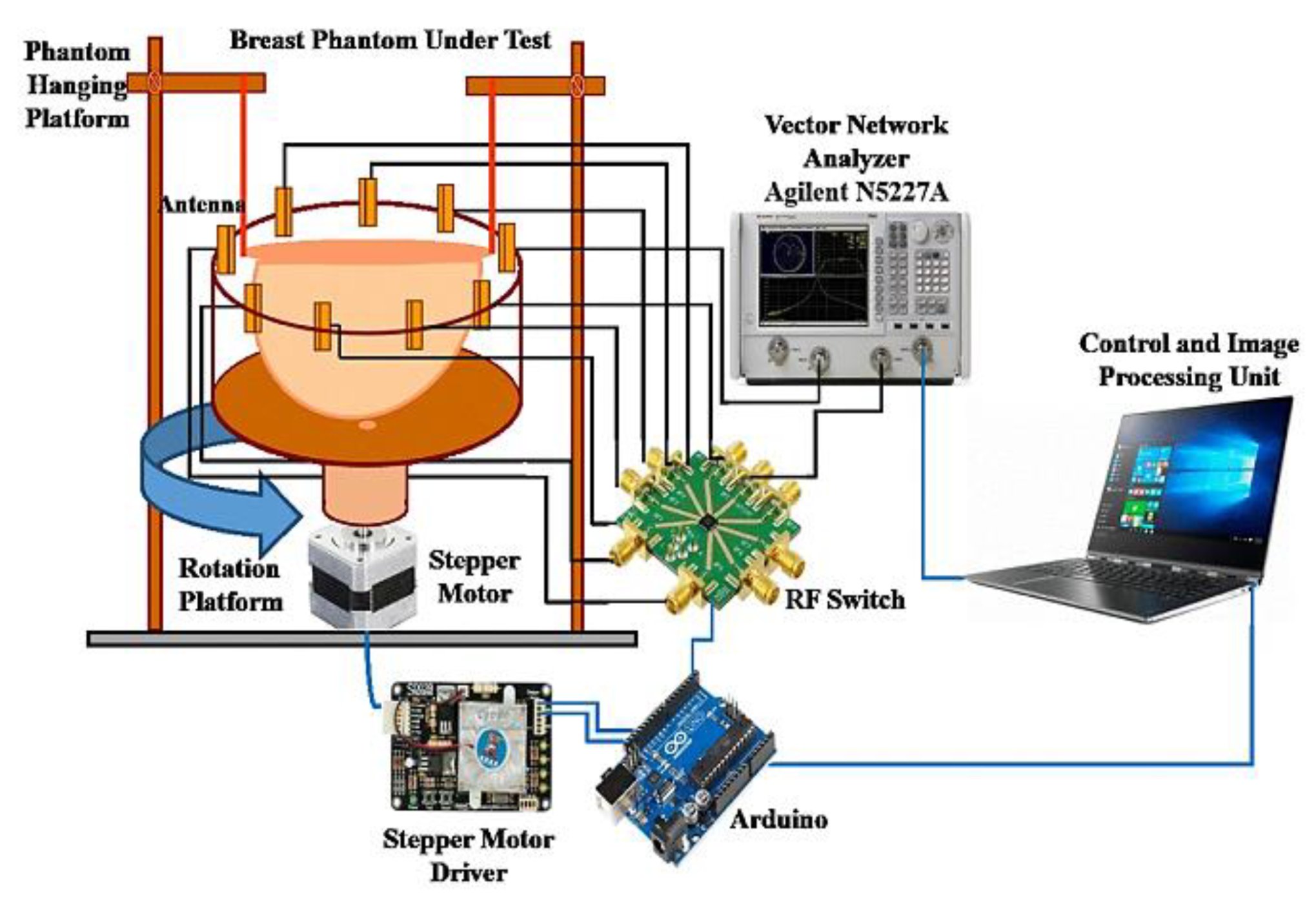
2.2.1. Microwave Tomography Technique
2.2.2. Radar-Based Technique
3. Emerging Breast Cancer Detection Technique
3.1. Artificial Intelligence in Breast Cancer Detection—The Progression
3.2. Bias and Challenges of Artificial Intelligence in Breast Cancer Detection
4. Summary of Previous Research on Breast Cancer Detection
4.1. Image Processing
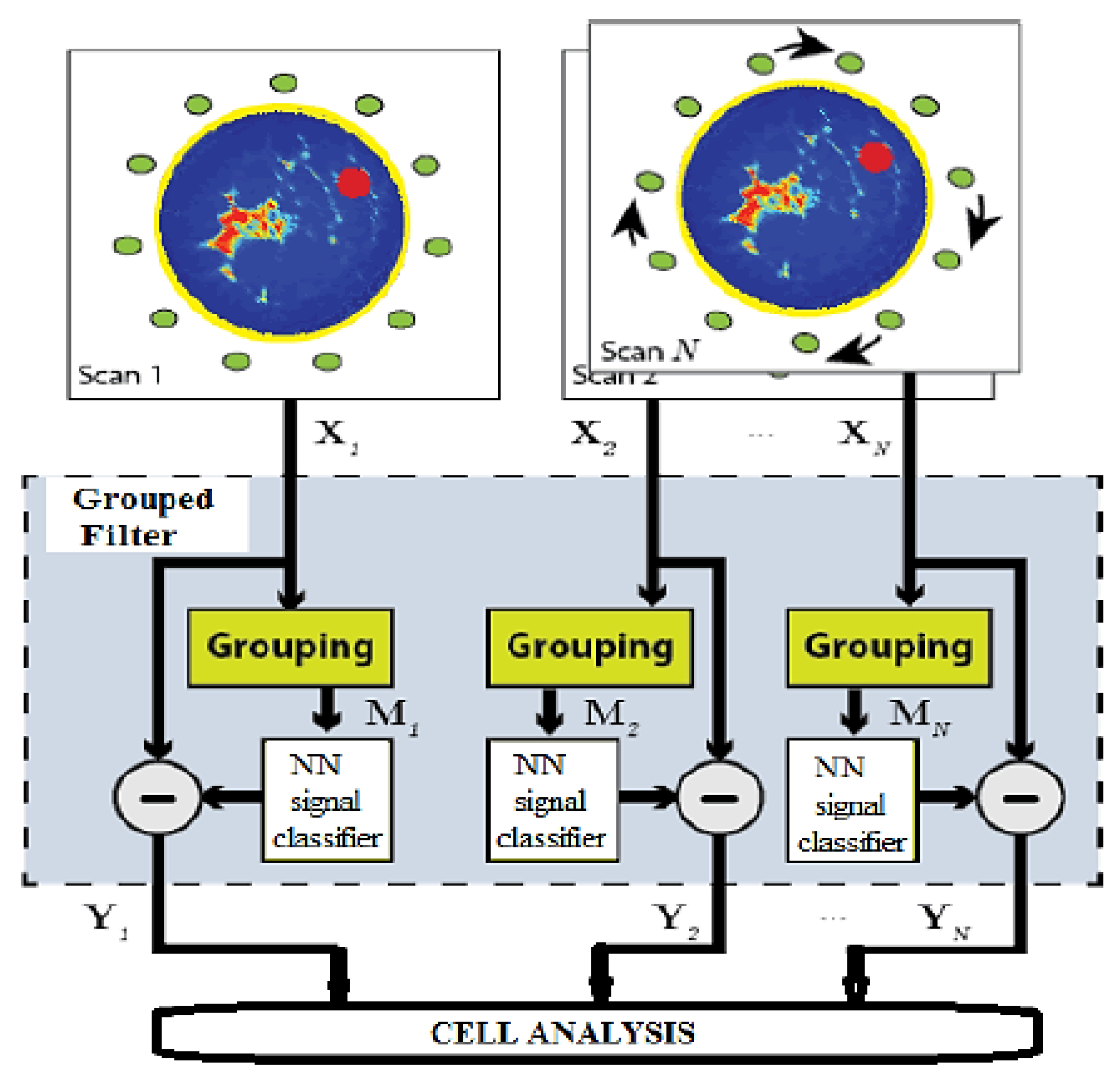
4.2. Signal Processing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Cancer in Malaysia. Available online: https://gco.iarc.fr/today/data/factsheets/populations/458-malaysia-fact-sheets.pdf (accessed on 10 February 2021).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Hjelm, T.E.; Matovu, A.; Mugisha, N.; Löfgren, J. Breast cancer care in Uganda: A multicenter study on the frequency of breast cancer surgery in relation to t.he incidence of breast cancer. PLoS ONE 2019, 14, e0219601. [Google Scholar] [CrossRef]
- Bera, A.; Subramanian, M.; Karaian, J.; Eklund, M. Functional role of vitronectin in breast cancer. PLoS ONE 2020, 15, e0242141. [Google Scholar] [CrossRef]
- Amir, P.N.; Ali, N.; Raman, R.K.; Raman, S.; Bahtiar, B. Malaysian Study on Cancer survival, 1st ed.; National Cancer Institute Ministry of Health: Putrajaya, Malaysia, 2018; pp. 1–57. [Google Scholar]
- Wang, L. Early diagnosis of breast cancer. Sensors 2017, 17, 1572. [Google Scholar] [CrossRef]
- Al-hadidi, M.D.R.; Alarabeyyat, A.; Alhanahnah, M. Breast Cancer Detection using K-nearest Neighbor Machine Learning Algorithm. In Proceedings of the 9th International Conference on Development in eSystems Engineering (DeSE), Liverpool, UK, 31 August–2 September 2016; pp. 35–39. [Google Scholar] [CrossRef]
- 1 in 30 Malaysian Women Will Have Breast Cancer, So Get Checked Now. Available online: https://www.thestar.com.my/lifestyle/family/2019/10/16/breast-cancer-2/ (accessed on 10 February 2021).
- Yip, C.H.; Pathy, N.B.; Teo, S.H. A review of breast cancer research in Malaysia. Med. J. Malays. 2014, 69, 8–22. [Google Scholar]
- Azizah, M.; Nor Saleha, I.T.; Noor Hashimah, A.; Asmah, Z.A.; Mastulu, W. Malaysian National Cancer Registry Report 2007–2011, 1st ed.; National Cancer Institute, Ministry of Health: Putrajaya, Malaysia, 2016; pp. 1–203. [Google Scholar]
- Mehmood, M.; Ayub, E.; Ahmad, F.; Alruwaili, M.; Alrowaili, Z.A.; Alanazi, S. Machine learning enabled early detection of breast cancer by structural analysis of mammograms. Comput. Mater. Continua 2021, 67, 641–657. [Google Scholar] [CrossRef]
- Wanandi, S.I.; Limanto, A.; Yunita, E.; Syahrani, R.A.; Louisa, M.; Wibowo, A.E. In silico and in vitro studies on the anti-cancer activity of andrographolide targeting survivin in human breast cancer stem cells. PLoS ONE 2020, 15, e0240020. [Google Scholar] [CrossRef]
- Tripodi, D.; Amabile, M.I.; Varanese, M.; D’Andrea, V.; Sorrenti, S.; Cannistrà, C. Large cell anaplastic lymphoma associated with breast implant: A rare case report presentation and discussion of possible management. Gland Surg. 2021, 10, 2076–2080. [Google Scholar] [CrossRef]
- Bonsu, A.B.; Ncama, B.P. Integration of breast cancer prevention and early detection into cancer palliative care model. PLoS ONE 2019, 14, e0212806. [Google Scholar] [CrossRef]
- Seidler, S.J.; Huber, D. Overview of diagnosis and treatment of breast cancer in young women. EC Gynaecol. 2020, 2, 18–25. [Google Scholar]
- Tan, K.F.; Adam, F.; Hami, R.; Shariff, N.M.; Mujar, N.M.M. Review of breast cancer in young women. Med. J. Malays. 2020, 16, 8–22. [Google Scholar]
- Cheng, W.; Shen, X.; Xing, M. Correction: Decreased breast cancer-specific mortality risk in patients with a history of thyroid cancer. PLoS ONE 2019, 14, e0221093. [Google Scholar] [CrossRef]
- Cartmell, K.B.; Sterba, K.R.; Pickett, K.; Zapka, J.; Alberg, A.J.; Sood, A.J. Availability of patient-centered cancer support services: A statewide survey of cancer centers. PLoS ONE 2018, 13, e0194649. [Google Scholar] [CrossRef]
- Ekici, S.; Jawzal, H. Breast cancer diagnosis using thermography and convolutional neural networks. Med. Hypotheses 2020, 137, 109542. [Google Scholar] [CrossRef]
- McKinney, S.M.; Sieniek, M.; Godbole, V.; Godwin, J.; Antropova, N.; Ashrafian, H. International evaluation of an AI system for breast cancer screening. Nature 2020, 577, 89–94. [Google Scholar] [CrossRef]
- Wu, J.; Hicks, C. Breast cancer type classification using machine learning. J. Personal. Med. 2021, 11, 61. [Google Scholar] [CrossRef]
- Ann, H.J.; Su-Shi, L.S.; Zakaria, Z. Non-invasive breast cancer assessment using magnetic induction spectroscopy technique. Int. J. Integr. Eng. 2017, 9, 54–60. [Google Scholar]
- Kwon, S.; Lee, S. Recent advances in microwave imaging for breast cancer detection. Int. J. Biomed. Imaging 2016, 2016, 5054912. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Li, Y.; Coates, M.; Men, A. Microwave breast cancer detection using empirical mode decomposition features. arXiv 2017, arXiv:1702.07608. [Google Scholar]
- Cheng, Y.; Fu, M. Dielectric properties for non-invasive detection of normal, benign, and malignant breast tissues using microwave theories. Thoracic Cancer 2018, 9, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Martellosio, A.; Pasian, M.; Bozzi, M.; Perregrini, L.; Mazzanti, A.; Svelto, F. Dielectric properties characterization from 0.5 to 50 GHz of breast cancer tissues. IEEE Trans. Microw. Theory Tech. 2017, 65, 998–1011. [Google Scholar] [CrossRef]
- Lazebnik, M.; McCartney, L.; Bozzi, M.; Popovic, D.; Watkins, C.B.; Lindstrom, M.J.; Harter, J.; Sewall, S.; Magliocco, A.; Booske, J.H.; et al. A large-scale study of the ultrawideband microwave dielectric properties of normal breast tissue obtained from reduction surgeries. Phys. Med. Biol. 2007, 52, 2637–2656. [Google Scholar] [CrossRef] [PubMed]
- Lazebnik, M.; Popovic, D.; McCartney, L.; Watkins, C.B.; Lindstrom, M.J.; Harter, J.; Sewall, S.; Ogilvie, T.; Magliocco, A.; Breslin, T.M.; et al. A large-scale study of the ultrawideband microwave dielectric properties of normal, benign and malignant breast tissues obtained from cancer surgeries. Phys. Med. Biol. 2007, 52, 6093–6115. [Google Scholar] [CrossRef]
- Di Meo, S.; Espin-Lopez, P.F.; Martellosio, A.; Pasian, M.; Bozzi, M.; Perregrini, L.; Mazzanti, A.; Svelto, F.; Summers, P.E.; Renne, G.; et al. Dielectric properties of breast tissues: Experimental results up to 50 GHz. In Proceedings of the 12th European Conference on Antennas and Propagation (EuCAP 2018), London, UK, 9–13 April 2018; pp. 1–5. [Google Scholar] [CrossRef]
- Sadoughi, F.; Kazemy, Z.; Hamedan, F.; Owji, L.; Rahmanikatigari, M.; Azadboni, T.T. Artificial intelligence methods for the diagnosis of breast cancer by image processing: A review. Breast Cancer Targets Therapy 2018, 10, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Celik, A.R.; Helhel, S. An overview of electromagnetic methods for breast cancer detection and a novel antenna design for microwave imaging. In Proceedings of the International Conference on Engineering Technologies (ICENTE’17), Konya, Turkey, 7–9 December 2017; pp. 377–383. [Google Scholar]
- Lewis, M.; Prashar, A.; Toms, A.P. The diagnosis of thoracic outlet syndrome. J. Vasc. Diagn. 2014, 2, 113–120. [Google Scholar] [CrossRef][Green Version]
- Lui, G.K.; Saidi, A.; Bhatt, A.B.; Burchill, L.J.; Deen, J.F.; Earing, M.G. Diagnosis and management of noncardiac complications in adults with congenital heart disease: A scientific statement from the american heart association. Circulation 2017, 136, e348–e392. [Google Scholar] [CrossRef]
- Carovac, A.; Smajlovic, F. Junuzovic, D. Application of Ultrasound in Medicine. Acta Inform. Med. 2011, 19, 168–171. [Google Scholar] [CrossRef]
- Al-dhabyani, W.; Gomaa, M.; Khaled, H.; Fahmy, A. Dataset of breast ultrasound images. Data Brief 2019, 28, 104863. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, S.; Dolcetti, V.; Fresilli, D. The Role of CEUS in the Evaluation of Thyroid Cancer: From Diagnosis to Local Staging. MDPI Clin. Med. 2021, 10, 4559. [Google Scholar] [CrossRef]
- Emine, D.; Suzana, M.K.; Halit Ymeri, A.K. Comparative accuracy of mammography and ultrasound in women with breast symptoms according to age and breast density. Bosn. J. Basic Med. Sci. 2009, 57, 205–216. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, Z.; Shen, G.; Zhang, Y.; Li, L.; Wu, Z. MRI evaluation of pulmonary lesions and lung tissue changes induced by tuberculosis. Int. J. Infect. Dis. 2019, 82, 138–146. [Google Scholar] [CrossRef]
- Joy, J.E.; Penhoet, E.E.; Petitti, D.B. Saving Women’s Lives: Strategies for Improving Breast Cancer Detection and Diagnosis, 1st ed.; The National Academies: Washington, DC, USA, 2005; pp. 1–361. [Google Scholar]
- Dlama Zira, J.; Chukwuemeka Nzotta, C. Radiation doses for mammography and its relationship with anthropo-technical parameters. Int. J. Radiol. Radiat. Therapy 2018, 5, 14–18. [Google Scholar] [CrossRef][Green Version]
- Gresik, C. How Dangerous Is Radiation from a Mammogram? Available online: https://www.eehealth.org/blog/2018/04/radiation-from-a-mammogram/#:~:text=On%20average%2C%20the%20total%20radiation,-just%20from%20their%20natural%20surroundings (accessed on 10 April 2021).
- Kamal, R.; Mansour, S.; Farouk, A. Contrast-enhanced mammography in comparison with dynamic contrast-enhanced MRI: Which modality is appropriate for whom? Egypt. J. Radiol. Nucl. Med. 2021, 52, 216. [Google Scholar] [CrossRef]
- Hogg, P.; Kelly, J.; Mercer, C. Digital Mammography, 1st ed.; Springer: London, UK, 2015; pp. 1–307. [Google Scholar] [CrossRef]
- Arleo, E.K.; Hendrick, R.E.; Helvie, M.A.; Sickles, E.A. Comparison of recommendations for screening mammography using CISNET models. Cancer 2017, 123, 3673–3680. [Google Scholar] [CrossRef]
- Iranmakani, S.; Mortezazadeh, T.; Sajadian, F.; Ghaziani, M.F.; Ghafari, A.; Khezerloo, D. A review of various modalities in breast imaging: Technical aspects and clinical outcomes. Egypt. J. Radiol. Nucl. Med. 2020, 51, 57. [Google Scholar] [CrossRef]
- Mahmud, M.Z.; Islam, M.T.; Misran, N.; Almutairi, A.F.; Cho, M. Ultra-wideband (UWB) antenna sensor based microwave breast imaging: A review. Sensors 2018, 18, 2951. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, Z. Comparison of CT and MRI images for the prediction of soft-tissue sarcoma grading and lung metastasis via a convolutional neural networks model. Clin. Radiol. 2020, 75, 64–69. [Google Scholar] [CrossRef]
- Arteaga-Marrero, N.; Villa, E.; González-Fernández, J.; Martín, Y.; Ruiz-Alzola, J. Polyvinyl alcohol cryogel phantoms of biological tissues for wideband operation at microwave frequencies. PLoS ONE 2019, 14, e0219997. [Google Scholar] [CrossRef]
- Khan, S.U.; Ullah, N.; Ahmed, I.; Ahmad, I.; Mahsud, M.I. MRI imaging, comparison of MRI with other modalities, noise in MRI images and machine learning techniques for noise removal: A review. Curr. Med. Imaging 2018, 15, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.M.; Kuhl, C.K.; Moy, L. Contrast-enhanced MRI for breast cancer screening. J. Mag. Resonance Imaging 2019, 50, 377–390. [Google Scholar] [CrossRef]
- Gunduru, M.; Grigorian, C. Breast magnetic resonance imaging MRI. Radiol. Technol. 2020, 91, 1–6. [Google Scholar]
- Mann, R.M.; Cho, N.; Moy, L. Breast MRI: State of the Art. Radiology 2019, 292, 520–536. [Google Scholar] [CrossRef]
- Broadhouse, K.M. The Physics of MRI and How We Use It to Reveal the Mysteries of the Mind. Available online: https://kids.frontiersin.org/articles/10.3389/frym.2019.00023 (accessed on 5 March 2021).
- Rahman, A.; Islam, M.T.; Singh, M.J.; Kibria, S. Electromagnetic performances analysis of an ultra-wideband and flexible material antenna in microwave breast imaging: To implement a wearable medical bra. Nature 2016, 18, 38906. [Google Scholar] [CrossRef]
- Khuda, I.E. Feasibility of the Detection of Breast Cancer Using Ultra-Wide Band (UWB) Technology in Comparison with Other Screening Techniques; IntechOpen: London, UK, 2018; pp. 1–14. [Google Scholar] [CrossRef]
- Natalia, K. Microwave Imaging for Breast Cancer. IEEE Microw. Mag. 2011, 78–94. [Google Scholar] [CrossRef]
- Aldhaeebi, M.A.; Alzoubi, K.; Almoneef, T.S.; Bamatra, S.M.; Attia, H.; Ramahi, O.M. Review of microwaves techniques for breast cancer detection. Sensors 2020, 20, 2390. [Google Scholar] [CrossRef]
- Zhang, H.; Li, M.; Yang, F.; Xu, S.; Yin, Y.; Zhou, H. A feasibility study of 2-d microwave thorax imaging based on the supervised descent method. Electronics 2021, 10, 352. [Google Scholar] [CrossRef]
- Mobashsher, A.T.; Bialkowski, K.S.; Abbosh, A.M.; Crozier, S. Design and experimental evaluation of a non-invasive microwave head imaging system for intracranial haemorrhage detection. PLoS ONE 2016, 11, e0152351. [Google Scholar] [CrossRef]
- Wang, Z.; Lim, E.G.; Tang, Y.; Leach, M. Medical applications of microwave imaging. Sci. World J. 2014, 2014, 147016. [Google Scholar] [CrossRef]
- Zamani, A.; Darvazehban, A.; Rezaeieh, S.A.; Abbosh, A. Three-Dimensional Electromagnetic Torso Imaging using Reconfigurable Antennas. In Proceedings of the 13th European Conference on Antennas and Propagation (EuCAP 2019), Krakow, Poland, 31 March–5 April 2019; pp. 1–3. [Google Scholar]
- Lavoie, B.R.; Okoniewski, M.; Fear, E.C. Estimating the effective permittivity for reconstructing accurate microwave-radar images. PLoS ONE 2016, 11, e0160849. [Google Scholar] [CrossRef]
- Rezaeieh, S.A.; Darvazehban, A.; Janani, A.S.; Abbosh, A.M. Electromagnetic torso scanning: A review of devices, algorithms, and systems. Biosensors 2021, 11, 135. [Google Scholar] [CrossRef]
- Oziel, M.; Korenstein, R.; Rubinsky, B. Radar based technology for non-contact monitoring of accumulation of blood in the head: A numerical study. PLoS ONE 2017, 12, e0186381. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Espin-Lopez, P.F.; Martellosio, A.; Pasian, M.; Bozzi, M.; Perregrini, L.; Mazzanti, A.; Svelto, F.; Summers, P.E.; Renne, G.; et al. On the Feasibility of Breast Cancer Imaging Systems at Millimeter-Waves Frequencies. IEEE Trans. Microw. Theory Technol. 2017, 65, 1795–1806. [Google Scholar] [CrossRef]
- Di Meo, S.; Espin-Lopez, P.F.; Martellosio, A.; Pasian, M. Experimental Validation on Tissue-Mimicking Phantoms of Millimeter-Wave Imaging for Breast Cancer Detection. Appl. Sci. 2021, 11, 432. [Google Scholar] [CrossRef]
- Iliopoulus, I.; Di Meo, S.; Pasian, M. Enhancement of Penetration of Millimeter Waves by Field Focusing Applied to Breast Cancer Detection. IEEE Trans. Biomed. Eng. 2021, 68, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Microwave Imaging Shows Great Potential. Available online: https://www.diagnosticimaging.com/view/microwave-imaging-shows-great-potential (accessed on 3 June 2021).
- Sani, L.; Vispa, A.; Loretoni, R.; Duranti, M.; Ghavami, N.; Sánchez-Bayuela, D.A. Breast lesion detection through MammoWave device: Empirical detection capability assessment of microwave images’ parameters. PLoS ONE 2021, 16, e0250005. [Google Scholar] [CrossRef]
- Fedeli, A.; Maffongelli, M.; Monleone, R.; Pagnamenta, C.; Pastorino, M.; Poretti, S. A tomograph prototype for quantitative microwave imaging: Preliminary experimental results. J. Imaging 2018, 4, 139. [Google Scholar] [CrossRef]
- Friedrich, C.; Bourguignon, S.; Idier, J.; Goussard, Y. Three-dimensional microwave imaging: Fast and accurate computations with block resolution algorithms. Sensors 2020, 20, 6282. [Google Scholar] [CrossRef]
- Chandra, R.; Balasingham, I.; Zhou, H.; Narayanan, R.M. Medical image analysis and informatics: Computer-aided diagnosis and therapy. Med. Image Anal. Inform. Comput.-Aided Diag. Therapy 2017, 1–518. [Google Scholar] [CrossRef]
- Benny, R.; Anjit, T.A.; Mythili, P. An overview of microwave imaging for breast tumor detection. Prog. Electromagn. Res. 2020, 87, 61–91. [Google Scholar] [CrossRef]
- dos Santos, V.R.N.; Almeida, E.R.; Porsani, J.L.; Teixeira, F.L.; Soldovieri, F. A controlled-site comparison of microwave tomography and time-reversal imaging techniques for GPR surveys. Remote Sens. 2018, 10, 214. [Google Scholar] [CrossRef]
- Rahiman, M.H.F.; Thomas, T.W.K.; Soh, P.J.; Rahim, R.A.; Jamaludin, J.; Ramli, M.F. Microwave tomography sensing for potential agarwood trees imaging. Comput. Electron. Agric. 2019, 164, 104901. [Google Scholar] [CrossRef]
- Bevacqua, M.T.; Di Meo, S.; Crocco, L. Millimeter-waves breast cancer imaging via inverse scattering techniques. IEEE J. Electromag. RF Microw. Med. Biol. 2021, 5, 246–253. [Google Scholar] [CrossRef]
- Tomasz, M.; Paul, M.; Jack, S.P.; Peter, M. Fast 3-D Tomographic Microwave Imaging for Breast Cancer Detection. IEEE Trans. Med. Imaging 2012, 31, 133–138. [Google Scholar] [CrossRef]
- Burfeindt, M.J.; Shea, J.D.; Veen, B.D.V. Beamforming-Enhanced Inverse Scattering for Microwave Breast Imaging. IEEE Trans. Antennas Propog. 2014, 62, 5126–15132. [Google Scholar] [CrossRef]
- Rahiman, M.H.F.; Kiata, T.T.W.; Jack, S.P.; Rahim, R.A. Microwave tomography application and approaches—A review. J. Teknol. 2015, 73, 133–138. [Google Scholar] [CrossRef][Green Version]
- Wahab, Y.A.; Rahim, R.A.; Rahiman, M.H.F.; Aw, S.R.; Yunus, F.R.M.; Goh, C.L. Non-invasive process tomography in chemical mixtures—A review. Sens. Actuators 2015, 210, 602–617. [Google Scholar] [CrossRef]
- Islam, M.T.; Mahmud, M.Z.; Islam, M.T.; Kibria, S.; Samsuzzaman, M. A low cost and portable microwave imaging system for breast tumor detection using UWB directional antenna array. Nat. Res. 2019, 1–13. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, D.; Oliveira, B.L.; Glavin, M.; Jones, E.; O’Halloran, M. Comparing radar-based breast imaging algorithm performance with realistic patient-specific permittivity estimation. J. Imaging 2019, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Byrne, D.; Sarafianou, M.; Craddock, I.J. Compound Radar Approach for Breast Imaging. IEEE Trans. Biomed. Eng. 2017, 64, 40–51. [Google Scholar] [CrossRef]
- Shao, W.; Edalati, A.; McCollough, T.R.; McCollough, W.J. A Time-Domain Measurement System for UWB Microwave Imaging. IEEE Trans. Microw. Theory Technol. 2018, 66, 2265–2275. [Google Scholar] [CrossRef]
- Klemm, M.; Craddock, I.J.; Leendertz, J.A.; Preece, A.; Benjamin, R. Radar-based breast cancer detection using a hemispherical antenna array—Experimental results. IEEE Trans. Antennas Propag. 2009, 57, 1692–1704. [Google Scholar] [CrossRef]
- Kuwahara, Y. Microwave Imaging for Early Breast Cancer Detection; IntechOpen: London, UK, 2017; pp. 45–71. [Google Scholar] [CrossRef]
- Shere, M.; Lyburn, I.; Sidebottom, R.; Massey, H.; Gillett, C.; Jones, L. MARIA® M5: A multicentre clinical study to evaluate the ability of the Micrima radio-wave radar breast imaging system (MARIA®) to detect lesions in the symptomatic breast. Eur. J. Radiol. 2019, 116, 61–67. [Google Scholar] [CrossRef]
- Shimizu, H.; Nakayama, K.I. Artificial intelligence in oncology. Cancer Sci. 2020, 111, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Kaul, V.; Enslin, S.; Gross, S.A. History of artificial intelligence in medicine. Gastrointest. Endoscopy 2020, 92, 807–812. [Google Scholar] [CrossRef]
- Alanazi, S.A.; Kamruzzaman, M.M.; Islam Sarker, M.N.; Alruwaili, M.; Alhwaiti, Y.; Alshammari, N. Boosting breast cancer detection using convolutional neural network. J. Healthc. Eng. 2021, 2021, 5528622. [Google Scholar] [CrossRef]
- Jha, S.; Topol, E.J. Adapting to artificial intelligence: Radiologists and pathologists as information specialists. J. Am. Med. Assoc. 2016, 316, 2353–2354. [Google Scholar] [CrossRef] [PubMed]
- Pesapane, F.; Codari, M.; Sardanelli, F. Artificial intelligence in medical imaging: Threat or opportunity? Radiologists again at the forefront of innovation in medicine. Eur. Radiol. Exper. 2018, 2, 35. [Google Scholar] [CrossRef]
- Ahuja, A.S. The impact of artificial intelligence in medicine on the future role of the physician. PeerJ 2019, 7, e7702. [Google Scholar] [CrossRef]
- Dembrower, K.; Wahlin, E.; Liu, Y.; Salim, M.; Smith, K.; Lindholm, P. Effect of artificial intelligence-based triaging of breast cancer screening mammograms on cancer detection and radiologist workload: A retrospective simulation study. Lancet Digital Health 2020, 2, e468–e474. [Google Scholar] [CrossRef]
- AI Helps Radiologists Improve Accuracy in Breast Cancer Detection with Lesser Recalls. Available online: https://www.healthcareitnews.com/news/asia-pacific/ai-helps-radiologists-improve-accuracy-breast-cancer-detection-lesser-recalls (accessed on 13 March 2021).
- Houssein, E.H.; Emam, M.M.; Ali, A.A.; Suganthan, P.N. Deep and machine learning techniques for medical imaging-based breast cancer: A comprehensive review. Expert Syst. Appl. 2020, 167, 114161. [Google Scholar] [CrossRef]
- Tai, M. The impact of artificial intelligence on human society and bioethics. Tzu Chi Med. J. 2020, 32, 339–343. [Google Scholar] [CrossRef]
- Houssami, N.; Lee, C.; Buist, D.S.; Tao, D. Artificial intelligence for breast cancer screening: Opportunity or hype? Breast 2017, 36, 31–33. [Google Scholar] [CrossRef]
- Cheung, H.; Rubin, D. Challenges and opportunities for artificial intelligence in oncological imaging. Clin. Radiol. 2021, 76, 728–736. [Google Scholar] [CrossRef]
- Gonzalez-Hernandez, J.L.; Recinella, A.N.; Kandlikar, S.G.; Dabydeen, D.; Medeiros, L.; Phatak, P. Technology, application and potential of dynamic breast thermography for the detection of breast cancer. Int. J. Heat Mass Transf. 2019, 131, 558–573. [Google Scholar] [CrossRef]
- Diaz, O.; Randriguez, R.; Gubern, A. Are artificial intelligence systems useful in breast cancer screening programmes? Radiología 2021, 236–244. [Google Scholar] [CrossRef]
- Houssami, N.; Kirkpatrick, J.N.; Noguchi, N. Artificial Intelligence (AI) for the early detection of breast cancer: A scoping review to assess AI’s potential in breast screening practice. Expert Rev. Med. Devices 2019, 16, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Dustler, Evaluating AI in breast cancer screening: A complex task. Lancet Digital Health 2020, 2, e106–e107. [CrossRef]
- Vaka, A.R.; Soni, B.; Sudheer, R.K. Breast cancer detection by leveraging Machine Learning. ICT Express 2020, 6, 320–324. [Google Scholar] [CrossRef]
- Ouyang, Y.; Tsui, P.; Wu, S.; Wu, W.; Zhou, Z. Classification of benign and malignant breast tumors using H-Scan ultrasound imaging. Diagnostics 2019, 9, 182. [Google Scholar] [CrossRef]
- Shirazi, A.Z.; Javad, S.; Mahdavi, S.; Mohammadi, Z. A novel and reliable computational intelligence system for breast cancer detection. Int. Feder. Med. Biol. Eng. 2019, 1–12. [Google Scholar] [CrossRef]
- Yuvarani, S.; Venkateswaran, J. Early detection of breast cancer using signal calibration neural network (SCNN) technique. Comput. Life Sci. Smarter Technol. Adv. 2016, S153, 153–157. [Google Scholar]
- Rana, S.P.; Dey, M.; Tiberi, G.; Sani, L.; Vispa, A.; Raspa, G. Machine learning approaches for automated lesion detection in microwave breast imaging clinical data. Nat. Sci. Rep. 2019, 9, 10510. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, S.A.; Khatun, S. UWB imaging for breast cancer detection using neural network. Prog. Electromagn. Res. 2009, 7, 79–93. [Google Scholar] [CrossRef]
- Alshehri, S.A.; Khatun, S.; Jantan, A.B.; Abdullah, R.S.A.R.; Mahmood, R.; Awang, Z. Experimental breast tumor detection using NN-based UWB Imaging. Prog. Electromagn. Res. 2011, 111, 447–465. [Google Scholar] [CrossRef]
- Reza, K.J.; Khatun, S.; Jamlos, M.F.; Abdul, Z.I. Proficient feature extraction strategy for performance enhancement of NN based early breast tumor detection. Int. J. Eng. Technol. 2014, 5, 4689–4696. [Google Scholar]
- Vijayasarveswari, V.; Khatun, S.; Fakir, M.M.; Jusoh., M.; Ali, S. UWB based low-cost and non-invasive practical breast cancer early detection. In Proceedings of the 11th Asian Conference on Chemical Sensors (ACCS 2015), AIP Conference Proceedings, Penang, Malaysia, 16–18 November 2015; pp. 1–5. [Google Scholar] [CrossRef]
- Asri, H.; Mousannif, H.; Al Moatassime, H.; Noel, T. Using machine learning algorithms for breast cancer risk prediction and diagnosis. Procedia Comput. Sci. 2016, 83, 1064–1069. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, N.; Cui, X. Aided diagnosis methods of breast cancer based on machine learning. J. Phys. 2017, 887, 012072. [Google Scholar] [CrossRef]
- Huang, M.; Chen, C.; Lin, W.; Ke, S.; Tsai, C. SVM and SVM ensembles in breast cancer prediction. PLoS ONE 2017, e0161501. [Google Scholar] [CrossRef] [PubMed]
- Vijayasarveswari, V.; Jusoh, M. Scattering performance verification based on UWB imaging and neural network. In Proceedings of the IEEE 13th International Colloquium on Signal Processing and its Applications, Penang, Malaysia, 10–12 March 2017; pp. 238–242. [Google Scholar] [CrossRef]
- Vijayasarveswari, V.; Jusoh, M.; Khatun, S. Experimental UWB based efficient breast cancer early detection. Indian J. Sci. Technol. 2017, 10, 1–6. [Google Scholar] [CrossRef]
- Chaurasia, V.; Pal, S.; Tiwari, B.B. Prediction of benign and malignant breast cancer using data mining techniques. J. Algorithm Comput. Technol. 2018, 12, 119–126. [Google Scholar] [CrossRef]
- Agarap, A.F.M. On Breast Cancer Detection: An Application of Machine Learning Algorithms on the Winconsin Diagnostic Dataset. In Proceedings of the International Conference on Machine Learning and Soft Computing (ICMLSC), Haiphong City, Vietnam, 2–4 February 2018; pp. 5–9. [Google Scholar] [CrossRef]
- Shravya, C.; Pravalika, K.; Subhani, S. Prediction of beast cancer using supervised machine learning techniques. J. Algorithm Comput. Technol. 2019, 8, 1106–1110. [Google Scholar] [CrossRef]
- Ragab, D.A.; Sharkas, M.; Marshall, S.; Ren, J. Breast cancer detection using deep convolutional neural networks and support vector machines. PeerJ 2019, 7, e6201. [Google Scholar] [CrossRef] [PubMed]
- Chtihrakkannan, R.; Kavitha, P.; Mangayarkarasi, T.; Karthikeyan, R. Breast cancer detection using machine learning. Int. J. Innov. Technol. Explore Eng. 2019, 8, 3123–3126. [Google Scholar] [CrossRef]
- Dhahri, H.; Al Maghayreh, E.; Mahmood, A.; Elkilani, W.; Faisal, N.M. Breast cancer detection using machine learning. Autom. Breast Cancer Diag. Based Mach. Learn. Algorithms 2019, 1–11. [Google Scholar] [CrossRef]
- Shen, L.; Margolies, L.R.; Rothstein, J.H.; Fluder, E.; McBride, R.; Sieh, W. Deep Learning to Improve Breast Cancer Detection on Screening Mammography. Sci. Rep. Nat. Res. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Kesavan, S.; Latha, G.C.P. Breast cancer detection and classification using machine learning. TEST Eng. Manag. 2020, 82, 6667–6670. [Google Scholar]
- Srivenkatesh, M. Prediction of breast cancer disease using machine learning algorithms. Int. J. Innov. Technol. Explor. Eng. 2020, 9, 2868–2878. [Google Scholar] [CrossRef]
- Vijayasarveswari, V.; Andrew, A.M.; Jusoh, M.; Sabapathy, T.; Raof, R.A.A.; Yasin, M.N.M. Multi-stage feature selection (MSFS) algorithm for UWB-based early breast cancer size prediction. PLoS ONE 2020, 15, e0229367. [Google Scholar] [CrossRef]
- Bari, B.S.; Khatun, S.; Ghazali, K.H.; Fakir, M.M.; Samsudin, W.N.A.W.; Jusof, M.F.M. Ultra wide and (UWB) based early breast cancer Detection using artificial intelligence. Lect. Notes Electr. Eng. 2020, 632, 505–515. [Google Scholar] [CrossRef]
- Nrea, S.H.; Gezahegn, Y.G.; Boltena, A.S.; Hagos, G. Breast cancer detection using convolutional Neural Network. In Proceedings of the International Conference on Learning Representations (ICLR), Millennium Hall, Addis Ababa, 26–30 April 2020; pp. 1–8. [Google Scholar] [CrossRef]
- Agrawal, P.; Deb, S.; Raju, S.N. Breast Cancer and Prostate Cancer Detection using Classification Algorithms. Int. J. Eng. Res. Technol. (IJERT) 2020, 9, 40–46. [Google Scholar] [CrossRef]
- Abdollahi, J.; Keshandehghan, A.; Gardaneh, M.; Panahi, Y.; Gardaneh, M. Accurate Detection of Breast Cancer Metastasis Using a Hybrid Model of Artificial Intelligence Algorithm. Archiv. Breast Cancer 2020, 7, 22–28. [Google Scholar] [CrossRef]
- Kousalya, K.; Krishnakumar, B.; Shanthosh, C.I.; Sharmila, R.; Sneha, V. Diagnosis of Breast Cancer using Machine Learning Algorithm. Int. J. Adv. Sci. Technol. 2020, 29, 970–974. [Google Scholar]
- Karthikeyan, B.; Gollamudi, S.; Singamsetty, H.V.; Gade, P.K.; Mekala, S.Y. Breast Cancer Detection Using Machine Learning. Int. J. Adv. Trends Comput. Sci. Eng. 2020, 9, 981–984. [Google Scholar] [CrossRef]
- Islam, M.M.; Haque, M.R.; Iqbal, H.; Hasan, M.M.; Hasan, M.; Kabir, M.N. Breast Cancer Prediction: A Comparative Study Using Machine Learning Techniques. SN Comput. Sci. 2020, 1, 290. [Google Scholar] [CrossRef]
- Jabbar, M.A. Breast cancer data classification using ensemble machine learning. Eng. Appl. Sci. Res. 2021, 48, 65–72. [Google Scholar] [CrossRef]
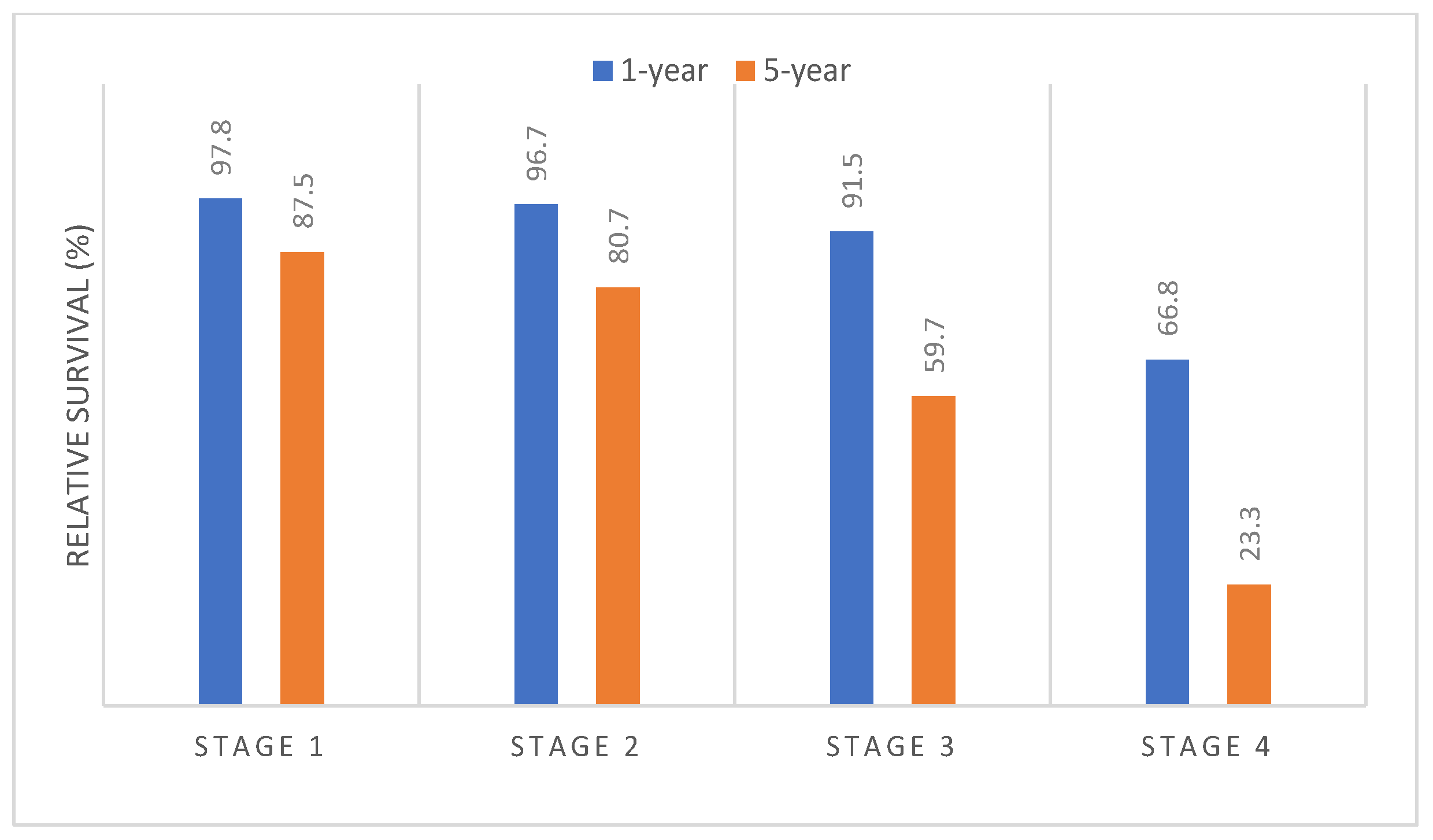



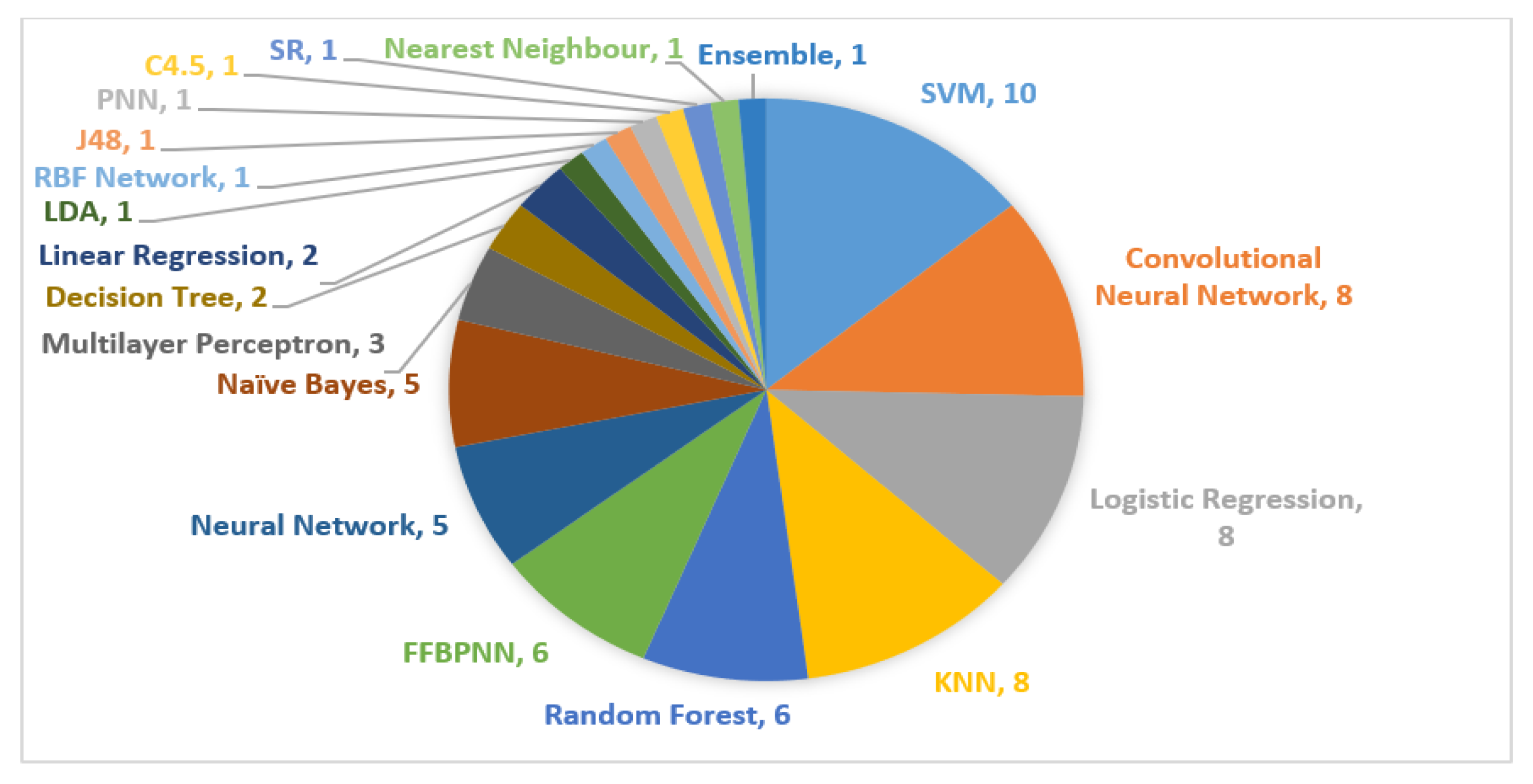

| Cancer Types | Total Numbers of Cases | Cases Recorded | 5-Year Relative Survival (%) by Stages | ||||
|---|---|---|---|---|---|---|---|
| No. | % | 1 | 2 | 3 | 4 | ||
| Female Breast | 17,009 | 11444 | 67.3 | 87.5 | 80.7 | 59.7 | 23.3 |
| Cervix Uteri | 4015 | 2631 | 65.5 | 75.3 | 52.3 | 32.1 | 23.0 |
| Ovary | 3084 | 2164 | 70.2 | 82.8 | 59.7 | 37.1 | 20.7 |
| Corpus Uteri | 2038 | 1374 | 67.4 | 91.3 | 74.9 | 50.2 | 19.5 |
| Variable | No | Relative Survival by Year (%) | |||
|---|---|---|---|---|---|
| 1-Year | 3-Year | 5-Year | |||
| Overall | Female | 17,009 | 89.7 | 74.3 | 66.8 |
| Age group by (years) | 15–44 | 4435 | 89.4 | 72.3 | 63.6 |
| 45–54 | 5936 | 90.0 | 74.6 | 66.7 | |
| 55–64 | 4152 | 89.5 | 73.8 | 65.8 | |
| 65–74 | 1829 | 88.5 | 73.7 | 68.1 | |
| Major ethnic group | Malay | 7568 | 86.1 | 67.0 | 57.9 |
| Chinese | 7014 | 93.4 | 82.5 | 76.5 | |
| Indian | 1578 | 93.5 | 77.1 | 70.5 | |
| Stage at diagnosis | Stage 1 | 2428 | 97.8 | 91.2 | 87.5 |
| Stage 2 | 4291 | 96.7 | 86.8 | 80.7 | |
| Stage 3 | 2654 | 91.5 | 70.2 | 59.7 | |
| Stage 4 | 2071 | 66.8 | 35.6 | 23.3 | |
| Type | Advantages | Disadvantages | Technique |
|---|---|---|---|
| Ultrasound | Inexpensive Suitable dense breast Quick and painless Nonionizing radiation Widely available | Low resolution Low sensitivity Low specificity High operator dependency | This technology uses a high frequency of sound waves to produce an image of organs and structures within the body |
| Mammogram | The old standard Regular check-up Provide high | False negative Not convenient Compressed Ionizing | This imaging uses a low-dose (ionizing radiation) X-ray system to discover inside the breast |
| MRI | High sensitivity Image in any angle Painless Nonionizing radiation | Long scan time Claustrophobia Expensive Less widely available | MRI uses magnetic fields and radio waves to create detailed images of organs and tissues in the body |
| Microwave Imaging | Nonionizing radiation Non-invasive Inexpensive Comfortable | Not available in clinic or hospital | This technology uses a microwave |
| Modality | Sensitivity | Specificity | Accuracy | Advantage | Limitations |
|---|---|---|---|---|---|
| Mammogram | 67.8% (120/177) | 75% (61/81) | 70.2% (181/258) | Low cost | False positive and negative |
| Mammogram and clinical | 77.4% (137/177) | 72% (58/81) | 75.6% (195/258) | Low cost | Lower accuracy |
| Clinical examination | 50.3% (89/177) | 92% (75/81) | 63.6% (164/258) | Easy process | A small tumor cant detect |
| Ultrasound | 83% (147/177) | 34% (28/81) | 67.8% (175/258) | Better than X-ray | Difficult to detect solid tumor |
| Mammogram and ultrasound | 91.5% (162/177) | 23% (19/81) | 70.2% (181/258) | Cost effective | Unwanted compression |
| Mammogram, ultrasound, and clinical | 93.2% (165/177) | 22% (18/81) | 70.9% (183/258) | Good detection | Complex signal processing |
| MRI | 94.4% (167/177) | 26% (21/81) | 72.9% (188/258) | Provide high resolution | Higher cost time consuming |
| Mammogram, clinical exam, and MRI | 99.4% (176/177) | 7% (6/81) | 70.5% (182/258) | Best solution ever found | Complex, expensive, and time |
| Artificial Intelligence: technique enables computers to mimic human behavior |
| Machine Learning (ML): the subset of AI technique; pattern identification and analysis; machines can improve with experience from provided data sets |
| Deep Learning (DL): the subset of ML technique; composed of multi-layer neural networks |
| Year | Author and Year | Dataset | Method | Processing | Parameter | Result (%) |
|---|---|---|---|---|---|---|
| 2009 | S.A. Alshehri, et al. [109] | Breast phantom | FFBPNN | Signal | Presence Location | Presence = 100 Location = 94.4 |
| 2011 | S.A. Alshehri, et al. [110] | Breast phantom Homogenous and Heterogeneous | Neural Network module | Signal | Existence Size Location | Homogenous: Existence = 100 Size = 95.8 Location = 94.3 Heterogeneous: Existence = 100 Size = 93.4 Location = 93.1 |
| 2014 | K. J. Reza, et al. [111] | Breast phantom | FFBPNN | Signal | Size | Size = 99.99 |
| 2015 | V. Vijayasarveswari, et al. [112] | Breast phantom | FFBPNN | Signal | Existence Location Size | Existence = 100 Location = 80.43 Size = 85.86 |
| 2016 | Moh’d Rasoul Al-Hadidi et al. [7] | Mammography | Back Propagation Neural Network (BPNN) model and the Logistic Regression (LR) | Image | 240 Feature | BPNN = 93.00 |
| 2016 | Hiba Asri, et al. [113] | Wisconsin Breast Cancer Dataset | SVM NB KNN C4.5 | Image | - | SVM = 97.13 |
| 2017 | Y.Zhao et al. [114] | First Affiliated Hospital of China Medical University | LDA KNN Logistic Regression | Image | thyroid, Her-2, PR,ER, Ki67, metastasis, and lymph nodes | LDA = 92.60 KNN = 96.30 Logistic Regression = 85.19 |
| 2017 | Min-Wei Huang et al. [115] | UCI machine learning repository | SVM GA | Image | Small scale dataset Large scale dataset | GA+RBF SVM ensembles = 98.28 GA+Poly SVM ensembles = 99.50 |
| 2017 | AZ. Shirazi et al. [106] | Mammographic image analysis | Hybrid between Self-Organizing Map (SOM) and Complex-Valued Neural Network (CVNN) | Image | Breast mass shape, margin, density, age, breast imaging and data system | Health = 94.00 Disease = 95 |
| 2017 | V. Vijayasarveswari, et al. [116] | Breast phantom | Neural network | Signal | Existence Location Size | Forward scattered signal = 84.17 Backward scattered signal = 87.55 |
| 2017 | V. Vijayasarveswari, et al. [117] | Breast phantom 125 data sample | FFBPNN K-fold cross validation (KFFBPNN) | Signal | Existence Location Size | FFBPNN = 85.43 KFFBPNN = 90.23 |
| 2018 | Vikas Chaurasia, et al. [118] | Wisconsin Breast Cancer Dataset | NB RBF Network J48 | Image | Benign and malignant | NB = 97.36 RBF = 96.77 J48 = 93.41 |
| 2018 | A.Agarap et al. [119] | Wisconsin Diagnostic Breast Cancer (WDBC) | GRU-SVM, Linear regression, MLP, NN, SR and SVM | Image | Breast mass; benign and malignant | MLP = 99.04 |
| 2019 | Ch. Shravya, et al. [120] | *UCI Repository ML database | *LR KNN SVM | *Image | *Breast mass; benign and malignant | LR = 92.10 KNN = 92.23 SVM = 92.78 |
| 2019 | S. Rana, et al. [108] | Real patient as a subject | Nearest Neighbour Multilayer Perceptron SVM | Signal | Healthy breast and breast with lesion | SVM = 98 |
| 2019 | D. Ragab et al. [121] | Curated Breast Imaging Subset of DDSM (CBIS-DDSM) | Deep Convolutional Neural Network (DCNN) | Image | Benign and malignant masses | DCNN-SVM = 87.2 |
| 2019 | R. Chtihrakkannan et al. [122] | Mammogram | ANN, SVM, KNN and DNN | Image | Existence | DNN = 92 |
| 2019 | H. Dhahri et al. [123] | Wisconsin Breast Cancer Dataset | Genetic Programming (GP) | Image | Breast mass; benign and malignant | GP = 98.24 |
| 2019 | L. Shen, et al. [124] | Digital Database for Screening Mammography (CBIS-DDSM) | Convolutional network method | Image | Benign and malignant masses | AUC = 0.98 |
| 2020 | S Kesavan, et al. [125] | Mammogram database picture | KNN classifier Convolution Neural Network | Image | - | Proposed CAD framework |
| 2020 | Muktevi Srivenkatesh, et al. [126] | Wisconsin Breast Cancer Dataset | KNN SVM LR Naive Bayes Random Forest | Image | ID, diagnosis, radius mean, texture mean, and perimeter mean | KNN = 64 SVM = 75.32 LR = 47.36 Naive Bayes = 52.63 RF = 98.24 |
| 2020 | Sami Ekici et al. [19] | Thermal image | Using CNN and optimized by Bayes Optimization | Image | - | 98.95 |
| 2020 | V. Vijayasarveswari, et al. [127] | Breast phantom | Data Normalize 4 stages of Multi Stage Feature Selection (MSFS) NB, SVM, and PNN | Signal | Existence Location Size | 8-HybridFeature NB = 91.98 SVM = 90.44 PNN = 80.05 |
| 2020 | BS. Bari et al. [128] | Breast phantom | Feedforward back propagation Neural Network (FFBPNN) with feed forward net | Signal | Existence Location Size | Existence = 100 Size = 92.43 Location = 91.31 |
| 2020 | S. Nrea et al. [129] | Mammogram from different hospital | Convolutional Neural Network with Region Proposed Network and Regin of Interest model proposed R-CNN | Image | Benign or malignant abnormality | R-CNN = 91.86 |
| 2020 | P. Agrawal et al. [130] | Breast Cancer Winconsin Diagnostic Dataset | SVM, Decision Tree, Logistic Regression, Random Forest and Naïve Bayes | Image | Breast mass; benign and malignant | Random forest with stratified K-Fold = 96.05 |
| 2020 | J. Abdollahi et al. [131] | Wisconsin Breast Cancer Dataset | Logistic Regression, K-Nearest Neighbours, Discrete Cosine Transform, Random Forest, SVM, Ensemble and Multilayer Perceptron combined with Genetic Algorithm (MLP-GA) | Image | Breast mass; benign and malignant | MLP-GA = 98 |
| 2020 | K. Kousalya et al. [132] | Wisconsin Breast Cancer Dataset | Linear Regression, Naïve bayes and Random Forest | Image | Breast mass; benign and malignant | Random Forest = 95.7 Naïve Bayes = 94.4 Linear Regression = 95.1 |
| 2020 | B. Kharthikeyan et al. [133] | UCI Machine Learning Repository Dataset | Logistic Regression, Random Forest and Decision Tree | Image | Benign and malignant | Logistic Regression = 99.3 Random Forest = 96.5 Decision Tree = 93.7 |
| 2020 | M. Islam et al. [134] | Wisconsin Breast Cancer Dataset | SVM, K-Nearest Neighbors, Random Forests, Artificial Neural Networks (ANNs) and Logistic Regression (LR) | Image | Benign and malignant | ANNs = 98.57 LR = 95.7 |
| 2021 | S. Alanazi et al. [90] | Kaggle 162 H&E - Invasive Ductal Carcinoma (IDC) Segmentation | Convolutional Neural Network (CNN) | Image | IDC positive and IDC negative | CNN = 87 |
| 2021 | M. Jabbar et al. [135] | Wisconsin Breast Cancer Dataset | Bayesian Network Radial Basis Function | Image | Breast mass; benign and malignant | RBF+BN = 97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul Halim, A.A.; Andrew, A.M.; Mohd Yasin, M.N.; Abd Rahman, M.A.; Jusoh, M.; Veeraperumal, V.; Rahim, H.A.; Illahi, U.; Abdul Karim, M.K.; Scavino, E. Existing and Emerging Breast Cancer Detection Technologies and Its Challenges: A Review. Appl. Sci. 2021, 11, 10753. https://doi.org/10.3390/app112210753
Abdul Halim AA, Andrew AM, Mohd Yasin MN, Abd Rahman MA, Jusoh M, Veeraperumal V, Rahim HA, Illahi U, Abdul Karim MK, Scavino E. Existing and Emerging Breast Cancer Detection Technologies and Its Challenges: A Review. Applied Sciences. 2021; 11(22):10753. https://doi.org/10.3390/app112210753
Chicago/Turabian StyleAbdul Halim, Ahmad Ashraf, Allan Melvin Andrew, Mohd Najib Mohd Yasin, Mohd Amiruddin Abd Rahman, Muzammil Jusoh, Vijayasarveswari Veeraperumal, Hasliza A Rahim, Usman Illahi, Muhammad Khalis Abdul Karim, and Edgar Scavino. 2021. "Existing and Emerging Breast Cancer Detection Technologies and Its Challenges: A Review" Applied Sciences 11, no. 22: 10753. https://doi.org/10.3390/app112210753
APA StyleAbdul Halim, A. A., Andrew, A. M., Mohd Yasin, M. N., Abd Rahman, M. A., Jusoh, M., Veeraperumal, V., Rahim, H. A., Illahi, U., Abdul Karim, M. K., & Scavino, E. (2021). Existing and Emerging Breast Cancer Detection Technologies and Its Challenges: A Review. Applied Sciences, 11(22), 10753. https://doi.org/10.3390/app112210753











