Microbial Fuel Cell United with Other Existing Technologies for Enhanced Power Generation and Efficient Wastewater Treatment
Abstract
:1. Introduction
Stand-Alone Bioelectrochemical Systems and Their Limitations
2. Types of Integrated Microbial Fuel Cell System
2.1. Integrated MFC-Capacitive Deionization (MFC-CDI) System
2.2. Membrane-Based MFCs and Membrane Bioreactor-Microbial Fuel Cells
2.3. FO-MFC Integration
| Type of Integration | Substrate | TEA | Application | Power Density and Efficiency in Wastewater Treatment | References | |
|---|---|---|---|---|---|---|
| Aerobic Reactor | Molasses | - | Acid orange removal | 90% removal | 52 mW/m2 | [28] |
| AHPB | AHPB sewage | O2 | H2 generation | 2.72 mol H2/mol glucose | 4200 mW/m3 | [49] |
| Capacitive deionization | Synthetic wastewater + sodium acetate | O2 | Deionization | 0.63 V | 60% NaCl removal | [27] |
| Capacitive deionization | Synthetic wastewater | O2 | COD removal | 97% removal | 9.3 mW/m2 | [27] |
| Catalytic oxidation reactor | Glucose | PBS + Congo red | Congo red removal | 90% Congo red degradation | 808.3 mW/m3 | [50] |
| Dark fermentation + aerobic digester | Waste mix | O2 | H2 + methane production from waste | H2 = 37.7 L/kg waste | Methane = 214 L/kg waste | [37] |
| Electric membrane bioreactor | Synthetic wastewater + glucose | O2 | COD removal | 97% removal | 111 mW/m3 | [51] |
| Electric membrane bioreactor | Wastewater | O2 | COD removal | 95.3% removal | 0.15 W/m3 | [44] |
| AD-SCMFC (1 m3) | Pretreated pharmaceutical wastewater | O2 | COD removal | 35% removal | 1.25 A/m2 | [52] |
| CW-DCMFC (30 L) | Dewatered alum sludge | O2 | COD removal | 92% removal | 0.448 W/m3 | [53] |
| Hybrid AA/O- SCMFC (1 m3) | Domestic wastewater | O2 | COD removal | 95% removal | 0.0036 W/m3 | [54] |
| Septic tank-SCMFC (18 units, 700 L) disinfection | Domestic wastewater | O2 | COD removal | 87% removal | 0.00043 W/m3 | [55] |
2.4. Integration of MFCs with Dark Fermentation
2.5. Sediment Microbial Fuel Cells
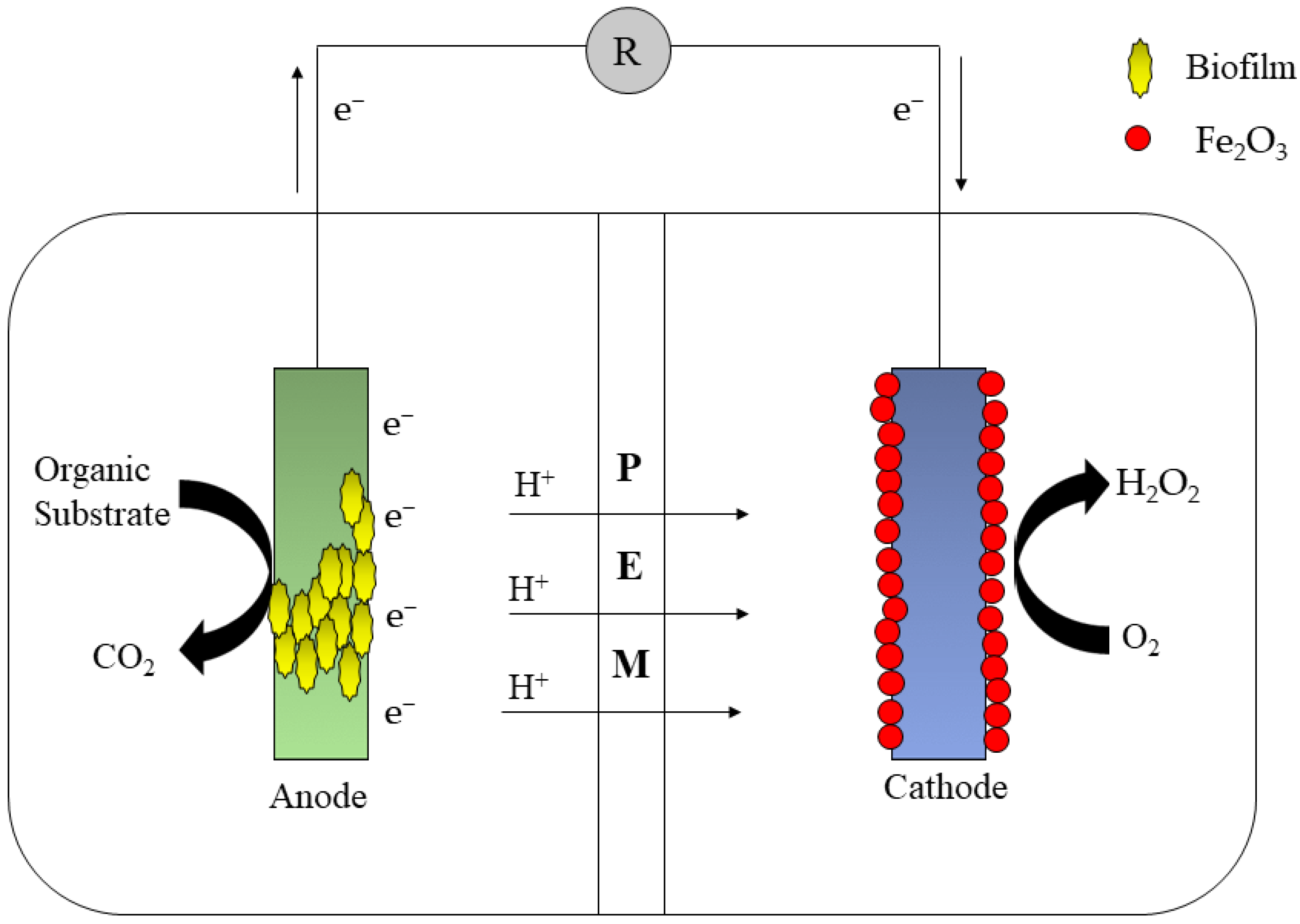
2.6. Integration of MFC with MDC
2.7. Integrated Constructed Wetland MFC
| Type of Integrated MFCs-CW | Power Density | COD Removal Rate | References |
|---|---|---|---|
| Vertical flow MFCs-CW | 531.04 mW/m3 | 72.17% | [88] |
| Up-flow_ downflow CW | 50.268 mW/m3 | 81% | [89] |
| Horizontal subsurface flow, continuous mode-CW | 36 mW/m2 | 71% | [83] |
| Horizontal flow bed-CW | - | 60–70% | [90] |
| Vertical CW | 53,714.08 mW/m2 | 82.32% | [91] |
2.8. Integration of Microbial Fuel Cells with Microalgae
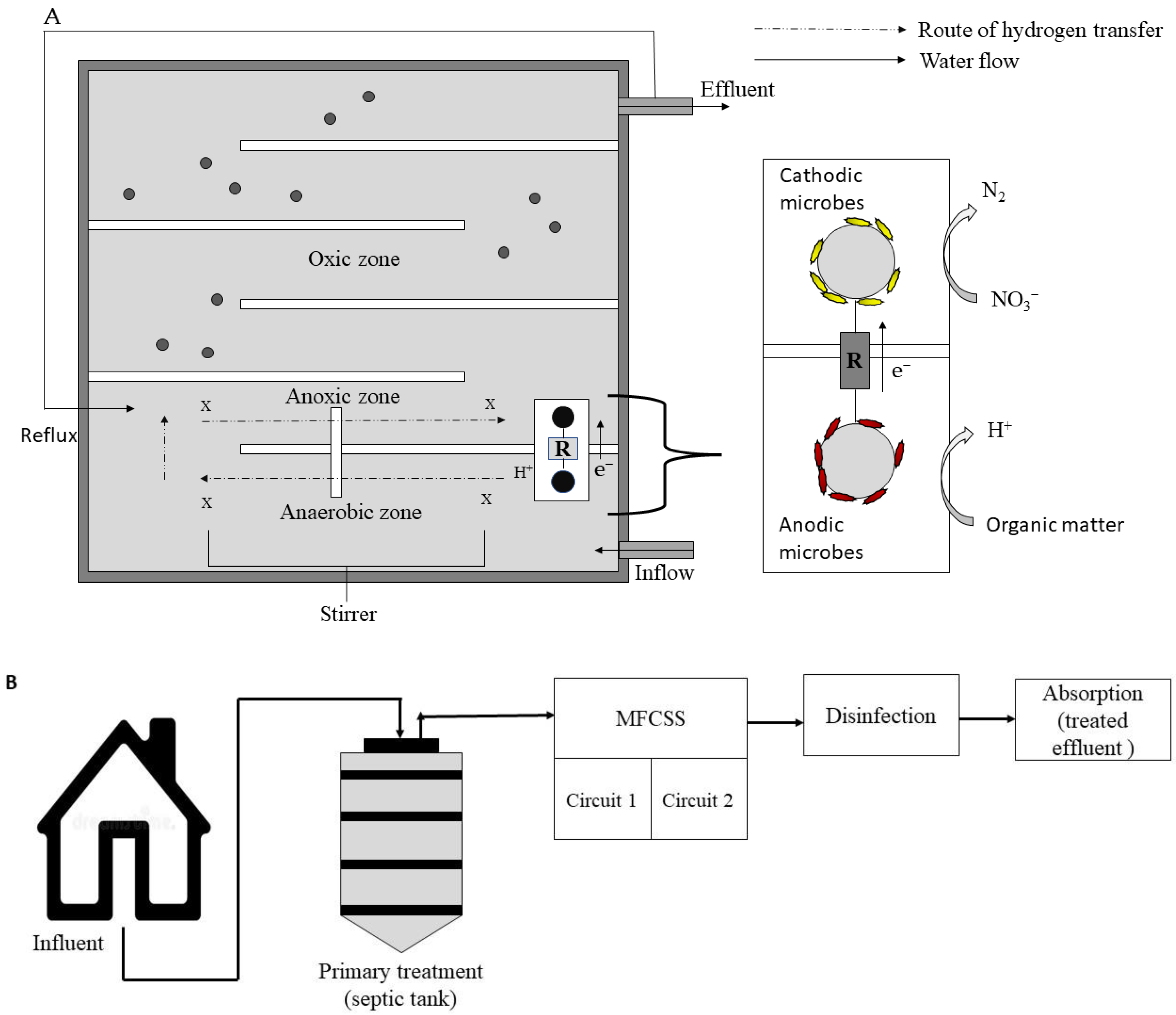
2.9. Anaerobic-Anoxic-Oxic (AO/O) Integrated with MFC
2.10. Other Integrated MFCs
3. Life-Cycle Assessment (LCA) of Integrated Microbial Electrochemical Systems
3.1. MFC-FO
3.2. MDC-FO
4. Challenges Associated with Integrated MFC
4.1. High Operational Cost
4.2. Reduced Power Density
4.3. Maintenance and Optimization of Hybrid Systems
4.4. Evaluating Sustainability
4.5. Commercialization and Practicality
5. Future Prospects and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yaqoob, A.A.; Khatoon, A.; Mohd Setapar, S.H.; Umar, K.; Parveen, T.; Mohamad Ibrahim, M.N.; Ahmad, A.; Rafatullah, M. Outlook on the Role of Microbial Fuel Cells in Remediation of Environmental Pollutants with Electricity Generation. Catalysts 2020, 10, 819. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Mohamad Nasir, M.I.; Umar, K.; Parveen, T.; Ahmad, A.; Lokhat, D.; Siti Hamidah, M.S. A Glimpse into the Microbial Fuel Cells for Wastewater Treatment with Energy Generation. Desalination Water Treat. 2021, 214, 379–389. [Google Scholar] [CrossRef]
- Lee, D.-J.; Chang, J.-S.; Lai, J.-Y. Microalgae–Microbial Fuel Cell: A Mini Review. Bioresour. Technol. 2015, 198, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Savla, N.; Khilari, S.; Pandit, S.; Jung, S.P. Effective Cathode Catalysts for Oxygen Reduction Reactions in Microbial Fuel Cell. In Bioelectrochemical Systems: Vol.1 Principles and Processes; Kumar, P., Kuppam, C., Eds.; Springer: Singapore, 2020; pp. 189–210. ISBN 9789811568725. [Google Scholar]
- Savla, N.; Shinde, A.; Sonawane, K.; Mekuto, L.; Chowdhary, P.; Pandit, S. 17-Microbial hydrogen production: Fundamentals to application. In Microorganisms for Sustainable Environment and Health; Chowdhary, P., Raj, A., Verma, D., Akhter, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 343–365. ISBN 978-0-12-819001-2. [Google Scholar]
- Dange, P.; Savla, N.; Pandit, S.; Bobba, R.; Jung, S.P.; Kumar Gupta, P.; Sahni, M.; Prasad, R. A Comprehensive Review on Oxygen Reduction Reaction in Microbial Fuel Cells. J. Renew. Mater. 2022, 10, 665–697. [Google Scholar] [CrossRef]
- Pandit, S.; Savla, N.; Jung, S.P. 16-Recent advancements in scaling up microbial fuel cells. In Integrated Microbial Fuel Cells for Wastewater Treatment; Abbassi, R., Yadav, A.K., Khan, F., Garaniya, V., Eds.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 349–368. ISBN 978-0-12-817493-7. [Google Scholar]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Guerrero-Barajas, C. Modern Trend of Anodes in Microbial Fuel Cells (MFCs): An Overview. Environ. Technol. Innov. 2021, 23, 101579. [Google Scholar] [CrossRef]
- He, L.; Du, P.; Chen, Y.; Lu, H.; Cheng, X.; Chang, B.; Wang, Z. Advances in Microbial Fuel Cells for Wastewater Treatment. Renew. Sustain. Energy Rev. 2017, 71, 388–403. [Google Scholar] [CrossRef]
- Pandit, S.; Savla, N.; Sonawane, J.M.; Sani, A.M.; Gupta, P.K.; Mathuriya, A.S.; Rai, A.K.; Jadhav, D.A.; Jung, S.P.; Prasad, R. Agricultural Waste and Wastewater as Feedstock for Bioelectricity Generation Using Microbial Fuel Cells: Recent Advances. Fermentation 2021, 7, 169. [Google Scholar] [CrossRef]
- Sani, A.; Savla, N.; Pandit, S.; Singh Mathuriya, A.; Gupta, P.K.; Khanna, N.; Pramod Babu, R.; Kumar, S. Recent Advances in Bioelectricity Generation through the Simultaneous Valorization of Lignocellulosic Biomass and Wastewater Treatment in Microbial Fuel Cell. Sustain. Energy Technol. Assess. 2021, 48, 101572. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, Y.; Doherty, L.; Hu, Y.; Hao, X. The Integrated Processes for Wastewater Treatment Based on the Principle of Microbial Fuel Cells: A Review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 60–91. [Google Scholar] [CrossRef]
- Ahn, Y.; Logan, B.E. Domestic Wastewater Treatment Using Multi-Electrode Continuous Flow MFCs with a Separator Electrode Assembly Design. Appl. Microbiol. Biotechnol. 2013, 97, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Ghadge, A.N.; Ghangrekar, M.M. Performance of Low Cost Scalable Air–Cathode Microbial Fuel Cell Made from Clayware Separator Using Multiple Electrodes. Bioresour. Technol. 2015, 182, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; He, W.; Liu, J.; Wang, X.; Qu, Y.; Ren, N. A Horizontal Plug Flow and Stackable Pilot Microbial Fuel Cell for Municipal Wastewater Treatment. Bioresour. Technol. 2014, 156, 132–138. [Google Scholar] [CrossRef]
- Liu, H.; Logan, B.E. Electricity Generation Using an Air-Cathode Single Chamber Microbial Fuel Cell in the Presence and Absence of a Proton Exchange Membrane. Environ. Sci. Technol. 2004, 38, 4040–4046. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Rabaey, K.; Rozendal, R.A.; Yuan, Z.; Keller, J. Decolorization of Azo Dyes in Bioelectrochemical Systems. Environ. Sci. Technol. 2009, 43, 5137–5143. [Google Scholar] [CrossRef]
- Zhang, B.; He, Z. Integrated Salinity Reduction and Water Recovery in an Osmotic Microbial Desalination Cell. RSC Adv. 2012, 2, 3265–3269. [Google Scholar] [CrossRef]
- Sheng, C.; Lu, Y.; Gao, X.; Yao, H. Fine Ash Formation during Pulverized Coal CombustionA Comparison of O2/CO2 Combustion versus Air Combustion. Energy Fuels 2007, 21, 435–440. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, S.; Feng, Y.; Merrill, M.D.; Saito, T.; Logan, B.E. Use of Carbon Mesh Anodes and the Effect of Different Pretreatment Methods on Power Production in Microbial Fuel Cells. Environ. Sci. Technol. 2009, 43, 6870–6874. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Luo, H.; Jenkins, P.E.; Ren, Z. Concurrent Desalination and Hydrogen Generation Using Microbial Electrolysis and Desalination Cells. Environ. Sci. Technol. 2011, 45, 340–344. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Wang, Z.; Lu, Y.; Li, X.; Ren, Y. Integrating Microbial Fuel Cells with Anaerobic Acidification and Forward Osmosis Membrane for Enhancing Bio-Electricity and Water Recovery from Low-Strength Wastewater. Water Res. 2017, 110, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Kadier, A.; Simayi, Y.; Abdeshahian, P.; Azman, N.F.; Chandrasekhar, K.; Kalil, M.S. A Comprehensive Review of Microbial Electrolysis Cells (MEC) Reactor Designs and Configurations for Sustainable Hydrogen Gas Production. Alex. Eng. J. 2016, 55, 427–443. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Bian, X.; Wang, H.; Wang, X.; Ren, Y.; Li, X. Simultaneously Recovering Electricity and Water from Wastewater by Osmotic Microbial Fuel Cells: Performance and Membrane Fouling. Front. Environ. Sci. Eng. 2018, 12, 5. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, H.; Zhou, S.; Shi, C.; Wang, C.; Ni, J. A Novel UASB-MFC-BAF Integrated System for High Strength Molasses Wastewater Treatment and Bioelectricity Generation. Bioresour. Technol. 2009, 100, 5687–5693. [Google Scholar] [CrossRef]
- Yuan, L.; Yang, X.; Liang, P.; Wang, L.; Huang, Z.-H.; Wei, J.; Huang, X. Capacitive Deionization Coupled with Microbial Fuel Cells to Desalinate Low-Concentration Salt Water. Bioresour. Technol. 2012, 110, 735–738. [Google Scholar] [CrossRef]
- Ren, L.; Ahn, Y.; Logan, B.E. A Two-Stage Microbial Fuel Cell and Anaerobic Fluidized Bed Membrane Bioreactor (MFC-AFMBR) System for Effective Domestic Wastewater Treatment. Environ. Sci. Technol. 2014, 48, 4199–4206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, J.; Choi, S.; Yu, H.; Kim, H.; Kim, C. Directly Applicable Microbial Fuel Cells in Aeration Tank for Wastewater Treatment. Bioelectrochemistry 2009, 78, 72–79. [Google Scholar] [CrossRef]
- Liu, X.-W.; Wang, Y.-P.; Huang, Y.-X.; Sun, X.-F.; Sheng, G.-P.; Zeng, R.J.; Li, F.; Dong, F.; Wang, S.-G.; Tong, Z.-H.; et al. Integration of a Microbial Fuel Cell with Activated Sludge Process for Energy-Saving Wastewater Treatment: Taking a Sequencing Batch Reactor as an Example. Biotechnol. Bioeng. 2011, 108, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Sun, D.; Cao, G.; Wang, H.; Ren, N.; Wu, W.-M.; Logan, B.E. Integrated Hydrogen Production Process from Cellulose by Combining Dark Fermentation, Microbial Fuel Cells, and a Microbial Electrolysis Cell. Bioresour. Technol. 2011, 102, 4137–4143. [Google Scholar] [CrossRef] [PubMed]
- Fernando, E.; Keshavarz, T.; Kyazze, G. Complete Degradation of the Azo Dye Acid Orange-7 and Bioelectricity Generation in an Integrated Microbial Fuel Cell, Aerobic Two-Stage Bioreactor System in Continuous Flow Mode at Ambient Temperature. Bioresour. Technol. 2014, 156, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Liu, L.; Yang, F. Development of a Novel Proton Exchange Membrane-Free Integrated MFC System with Electric Membrane Bioreactor and Air Contact Oxidation Bed for Efficient and Energy-Saving Wastewater Treatment. Bioresour. Technol. 2017, 238, 472–483. [Google Scholar] [CrossRef]
- Hegab, H.M.; ElMekawy, A.; Zou, L.; Mulcahy, D.; Saint, C.P.; Ginic-Markovic, M. The Controversial Antibacterial Activity of Graphene-Based Materials. Carbon 2016, 105, 362–376. [Google Scholar] [CrossRef]
- Asif, M.B.; Maqbool, T.; Zhang, Z. Electrochemical Membrane Bioreactors: State-of-the-Art and Future Prospects. Sci. Total Environ. 2020, 741, 140233. [Google Scholar] [CrossRef]
- Ajayi, F.F.; Kim, K.-Y.; Chae, K.-J.; Choi, M.-J.; Chang, I.S.; Kim, I.S. Optimization Studies of Bio-Hydrogen Production in a Coupled Microbial Electrolysis-Dye Sensitized Solar Cell System. Photochem. Photobiol. Sci. 2010, 9, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Manzini, E.; Scaglia, B.; Schievano, A.; Adani, F. Dark Fermentation Effectiveness as a Key Step for Waste Biomass Refineries: Influence of Organic Matter Macromolecular Composition and Bioavailability. Int. J. Energy Res. 2015, 39, 1519–1527. [Google Scholar] [CrossRef]
- Su, X.; Tian, Y.; Sun, Z.; Lu, Y.; Li, Z. Performance of a Combined System of Microbial Fuel Cell and Membrane Bioreactor: Wastewater Treatment, Sludge Reduction, Energy Recovery and Membrane Fouling. Biosens. Bioelectron. 2013, 49, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cheng, S. Microbial Fuel Cells for Energy Production from Wastewaters: The Way toward Practical Application. J. Zhejiang Univ. Sci. A 2014, 15, 841–861. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Yun, H.; Khan, A.; Salama, E.-S.; Redina, M.M.; Liu, P.; Li, X. Two-Stage Microbial Fuel Cell (MFC) and Membrane Bioreactor (MBR) System for Enhancing Wastewater Treatment and Resource Recovery Based on MFC as a Biosensor. Environ. Res. 2022, 204, 112089. [Google Scholar] [CrossRef]
- Bhowmick, G.D.; Ghangrekar, M.M.; Banerjee, R. Improved Wastewater Treatment by Using Integrated Microbial Fuel Cell-Membrane Bioreactor System Along with Ruthenium/activated Carbon Cathode Catalyst to Enhance Bio-energy Recovery. In Climate Impacts on Water Resources in India: Environment and Health; Pandey, A., Mishra, S.K., Kansal, M.L., Singh, R.D., Singh, V.P., Eds.; Water Science and Technology Library; Springer: New York, NY, USA, 2021; pp. 183–192. ISBN 978-3-030-51427-3. [Google Scholar]
- Savla, N.; Pandit, S.; Khanna, N.; Mathuriya, A.S.; Jung, S.P. Microbially Powered Electrochemical Systems Coupled with Membrane-Based Technology for Sustainable Desalination and Efficient Wastewater Treatment. J. Korean Soc. Environ. Eng. 2020, 42, 360–380. [Google Scholar] [CrossRef]
- Rozendal, R.A.; Hamelers, H.V.M.; Buisman, C.J.N. Effects of Membrane Cation Transport on PH and Microbial Fuel Cell Performance. Environ. Sci. Technol. 2006, 40, 5206–5211. [Google Scholar] [CrossRef]
- Chen, X.; Xia, X.; Liang, P.; Cao, X.; Sun, H.; Huang, X. Stacked Microbial Desalination Cells to Enhance Water Desalination Efficiency. Environ. Sci. Technol. 2011, 45, 2465–2470. [Google Scholar] [CrossRef]
- Zhang, F.; Saito, T.; Cheng, S.; Hickner, M.A.; Logan, B.E. Microbial Fuel Cell Cathodes With Poly(Dimethylsiloxane) Diffusion Layers Constructed around Stainless Steel Mesh Current Collectors. Environ. Sci. Technol. 2010, 44, 1490–1495. [Google Scholar] [CrossRef]
- Zhang, F.; Brastad, K.S.; He, Z. Integrating Forward Osmosis into Microbial Fuel Cells for Wastewater Treatment, Water Extraction and Bioelectricity Generation. Environ. Sci. Technol. 2011, 45, 6690–6696. [Google Scholar] [CrossRef]
- Gao, T.; Zhang, H.; Xu, X.; Teng, J. Integrating Microbial Electrolysis Cell Based on Electrochemical Carbon Dioxide Reduction into Anaerobic Osmosis Membrane Reactor for Biogas Upgrading. Water Res. 2021, 190, 116679. [Google Scholar] [CrossRef]
- Xue, W.; He, Y.; Yumunthama, S.; Udomkittayachai, N.; Hu, Y.; Tabucanon, A.S.; Zhang, X.; Kurniawan, T.A. Membrane Cleaning and Performance Insight of Osmotic Microbial Fuel Cell. Chemosphere 2021, 285, 131549. [Google Scholar] [CrossRef] [PubMed]
- Biffinger, J.C.; Ray, R.; Little, B.J.; Fitzgerald, L.A.; Ribbens, M.; Finkel, S.E.; Ringeisen, B.R. Simultaneous Analysis of Physiological and Electrical Output Changes in an Operating Microbial Fuel Cell with Shewanella Oneidensis. Biotechnol. Bioeng. 2009, 103, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, S.; Kakade, A.; Kulshreshtha, S.; Liu, P.; Li, X. A Review on Microbial Electrocatalysis Systems Coupled with Membrane Bioreactor to Improve Wastewater Treatment. Microorganisms 2019, 7, 372. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zheng, Y.; Jia, H.; Zhang, H. Bioelectricity Generation in an Integrated System Combining Microbial Fuel Cell and Tubular Membrane Reactor: Effects of Operation Parameters Performing a Microbial Fuel Cell-Based Biosensor for Tubular Membrane Bioreactor. Bioresour. Technol. 2014, 170, 483–490. [Google Scholar] [CrossRef]
- Wang, A.-J.; Wang, H.-C.; Cheng, H.-Y.; Liang, B.; Liu, W.-Z.; Han, J.-L.; Zhang, B.; Wang, S.-S. Electrochemistry-Stimulated Environmental Bioremediation: Development of Applicable Modular Electrode and System Scale-Up. Environ. Sci. Ecotechnol. 2020, 3, 100050. [Google Scholar] [CrossRef]
- Tang, C.; Zhao, Y.; Kang, C.; Yang, Y.; Morgan, D.; Xu, L. Towards Concurrent Pollutants Removal and High Energy Harvesting in a Pilot-Scale CW-MFC: Insight into the Cathode Conditions and Electrodes Connection. Chem. Eng. J. 2019, 373, 150–160. [Google Scholar] [CrossRef]
- Liu, R.; Tursun, H.; Hou, X.; Odey, F.; Li, Y.; Wang, X.; Xie, T. Microbial Community Dynamics in a Pilot-Scale MFC-AA/O System Treating Domestic Sewage. Bioresour. Technol. 2017, 241, 439–447. [Google Scholar] [CrossRef]
- Valladares Linares, R.; Domínguez-Maldonado, J.; Rodríguez-Leal, E.; Patrón, G.; Castillo-Hernández, A.; Miranda, A.; Diaz Romero, D.; Moreno-Cervera, R.; Camara-chale, G.; Borroto, C.G.; et al. Scale up of Microbial Fuel Cell Stack System for Residential Wastewater Treatment in Continuous Mode Operation. Water 2019, 11, 217. [Google Scholar] [CrossRef] [Green Version]
- Keskin, T.; Abo-Hashesh, M.; Hallenbeck, P.C. Photofermentative Hydrogen Production from Wastes. Bioresour. Technol. 2011, 102, 8557–8568. [Google Scholar] [CrossRef] [PubMed]
- Chookaew, T.; Prasertsan, P.; Ren, Z.J. Two-Stage Conversion of Crude Glycerol to Energy Using Dark Fermentation Linked with Microbial Fuel Cell or Microbial Electrolysis Cell. New Biotechnol. 2014, 31, 179–184. [Google Scholar] [CrossRef]
- Estrada-Arriaga, E.B.; Hernández-Romano, J.; Mijaylova-Nacheva, P.; Gutiérrez-Macías, T.; Morales-Morales, C. Assessment of a Novel Single-Stage Integrated Dark Fermentation-Microbial Fuel Cell System Coupled to Proton-Exchange Membrane Fuel Cell to Generate Bio-Hydrogen and Recover Electricity from Wastewater. Biomass Bioenergy 2021, 147, 106016. [Google Scholar] [CrossRef]
- Gebreslassie, T.R.; Nguyen, P.K.T.; Yoon, H.H.; Kim, J. Co-Production of Hydrogen and Electricity from Macroalgae by Simultaneous Dark Fermentation and Microbial Fuel Cell. Bioresour. Technol. 2021, 336, 125269. [Google Scholar] [CrossRef] [PubMed]
- Reimers, C.E.; Tender, L.M.; Fertig, S.; Wang, W. Harvesting Energy from the Marine Sediment−Water Interface. Environ. Sci. Technol. 2001, 35, 192–195. [Google Scholar] [CrossRef]
- He, Z.; Shao, H.; Angenent, L.T. Increased Power Production from a Sediment Microbial Fuel Cell with a Rotating Cathode. Biosens. Bioelectron. 2007, 22, 3252–3255. [Google Scholar] [CrossRef]
- Bond, D.R.; Holmes, D.E.; Tender, L.M.; Lovley, D.R. Electrode-Reducing Microorganisms That Harvest Energy from Marine Sediments. Science 2002, 295, 483–485. [Google Scholar] [CrossRef] [Green Version]
- Fadzli, F.S.; Rashid, M.; Yaqoob, A.A.; Mohamad Ibrahim, M.N. Electricity Generation and Heavy Metal Remediation by Utilizing Yam (Dioscorea Alata) Waste in Benthic Microbial Fuel Cells (BMFCs). Biochem. Eng. J. 2021, 172, 108067. [Google Scholar] [CrossRef]
- Daud, N.N.M.; Ahmad, A.; Yaqoob, A.A.; Ibrahim, M.N.M. Application of Rotten Rice as a Substrate for Bacterial Species to Generate Energy and the Removal of Toxic Metals from Wastewater through Microbial Fuel Cells. Environ. Sci. Pollut. Res. Int. 2021, 1–12. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Mohamad Ibrahim, M.N.; Umar, K.; Bhawani, S.A.; Khan, A.; Asiri, A.M.; Khan, M.R.; Azam, M.; AlAmmari, A.M. Cellulose Derived Graphene/Polyaniline Nanocomposite Anode for Energy Generation and Bioremediation of Toxic Metals via Benthic Microbial Fuel Cells. Polymers 2021, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- Venkata Mohan, S.; Mohanakrishna, G.; Chiranjeevi, P. Sustainable Power Generation from Floating Macrophytes Based Ecological Microenvironment through Embedded Fuel Cells along with Simultaneous Wastewater Treatment. Bioresour. Technol. 2011, 102, 7036–7042. [Google Scholar] [CrossRef]
- Huang, D.-Y.; Zhou, S.-G.; Chen, Q.; Zhao, B.; Yuan, Y.; Zhuang, L. Enhanced Anaerobic Degradation of Organic Pollutants in a Soil Microbial Fuel Cell. Chem. Eng. J. 2011, 172, 647–653. [Google Scholar] [CrossRef]
- Quaglio, M.; Ahmed, D.; Massaglia, G.; Sacco, A.; Margaria, V.; Pirri, C.F. Enhanced Power Extraction with Sediment Microbial Fuel Cells by Anode Alternation. Fuels 2021, 2, 168–178. [Google Scholar] [CrossRef]
- Taşkan, B.; Bakır, M.; Taşkan, E. Enhanced Power Generation from Algal Biomass Using Multi-Anode Membrane-Less Sediment Microbial Fuel Cell. Int. J. Energy Res. 2021, 45, 2011–2022. [Google Scholar] [CrossRef]
- Li, H.; He, W.; Qu, Y.; Li, C.; Tian, Y.; Feng, Y. Pilot-Scale Benthic Microbial Electrochemical System (BMES) for the Bioremediation of Polluted River Sediment. J. Power Sources 2017, 356, 430–437. [Google Scholar] [CrossRef]
- Li, H.; Tian, Y.; Qu, Y.; Qiu, Y.; Liu, J.; Feng, Y. A Pilot-Scale Benthic Microbial Electrochemical System (BMES) for Enhanced Organic Removal in Sediment Restoration. Sci. Rep. 2017, 7, 39802. [Google Scholar] [CrossRef] [Green Version]
- Walter, X.A.; Merino-Jiménez, I.; Greenman, J.; Ieropoulos, I. PEE POWER® Urinal II – Urinal Scale-up with Microbial Fuel Cell Scale-down for Improved Lighting. J. Power Sources 2018, 392, 150–158. [Google Scholar] [CrossRef]
- Prasad, J.; Tripathi, R.K. Scale-up and Control the Voltage of Sediment Microbial Fuel Cell for Charging a Cell Phone. Biosens. Bioelectron. 2021, 172, 112767. [Google Scholar] [CrossRef]
- Prasad, J.; Tripathi, R.K. Effect of Sediment Microbial Fuel Cell Stacks on 9 V/12 V DC Power Supply. Int. J. Hydrogen Energy 2021, 46, 14628–14638. [Google Scholar] [CrossRef]
- Zahid, M.; Savla, N.; Pandit, S.; Thakur, V.K.; Jung, S.P.; Gupta, P.K.; Prasad, R.; Marsili, E. Microbial Desalination Cell: Desalination through Conserving Energy. Desalination 2022, 521, 115381. [Google Scholar] [CrossRef]
- Cao, X.; Huang, X.; Liang, P.; Xiao, K.; Zhou, Y.; Zhang, X.; Logan, B.E. A New Method for Water Desalination Using Microbial Desalination Cells. Environ. Sci. Technol. 2009, 43, 7148–7152. [Google Scholar] [CrossRef] [PubMed]
- Mohanakrishna, G.; Venkata Mohan, S.; Sarma, P.N. Bio-Electrochemical Treatment of Distillery Wastewater in Microbial Fuel Cell Facilitating Decolorization and Desalination along with Power Generation. J. Hazard. Mater. 2010, 177, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; He, Z. Improving Water Desalination by Hydraulically Coupling an Osmotic Microbial Fuel Cell with a Microbial Desalination Cell. J. Membr. Sci. 2013, 441, 18–24. [Google Scholar] [CrossRef]
- Zhang, F.; He, Z. Scaling up Microbial Desalination Cell System with a Post-Aerobic Process for Simultaneous Wastewater Treatment and Seawater Desalination. Desalination 2015, 360, 28–34. [Google Scholar] [CrossRef]
- Yadav, A.K.; Abbassi, R.; Kumar, N.; Satya, S.; Sreekrishnan, T.R.; Mishra, B.K. The Removal of Heavy Metals in Wetland Microcosms: Effects of Bed Depth, Plant Species, and Metal Mobility. Chem. Eng. J. 2012, 211–212, 501–507. [Google Scholar] [CrossRef]
- Villaseñor, J.; Capilla, P.; Rodrigo, M.A.; Cañizares, P.; Fernández, F.J. Operation of a Horizontal Subsurface Flow Constructed Wetland – Microbial Fuel Cell Treating Wastewater under Different Organic Loading Rates. Water Res. 2013, 47, 6731–6738. [Google Scholar] [CrossRef]
- Zhao, Y.; Collum, S.; Phelan, M.; Goodbody, T.; Doherty, L.; Hu, Y. Preliminary Investigation of Constructed Wetland Incorporating Microbial Fuel Cell: Batch and Continuous Flow Trials. Chem. Eng. J. 2013, 229, 364–370. [Google Scholar] [CrossRef] [Green Version]
- Corbella, C.; Guivernau, M.; Viñas, M.; Puigagut, J. Operational, Design and Microbial Aspects Related to Power Production with Microbial Fuel Cells Implemented in Constructed Wetlands. Water Res. 2015, 84, 232–242. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Vargas, C.A.; Arias, C.A.; Carvalho, P.; Zhang, L.; Esteve-Núñez, A.; Brix, H. Electroactive Biofilm-Based Constructed Wetland (EABB-CW): A Mesocosm-Scale Test of an Innovative Setup for Wastewater Treatment. Sci. Total Environ. 2019, 659, 796–806. [Google Scholar] [CrossRef]
- Wen, H.; Zhu, H.; Yan, B.; Xu, Y.; Shutes, B. Treatment of Typical Antibiotics in Constructed Wetlands Integrated with Microbial Fuel Cells: Roles of Plant and Circuit Operation Mode. Chemosphere 2020, 250, 126252. [Google Scholar] [CrossRef] [PubMed]
- Mittal, Y.; Dash, S.; Srivastava, P.; Mishra, P.M.; Aminabhavi, T.M.; Yadav, A.K. Azo Dye Containing Wastewater Treatment in Earthen Membrane Based Unplanted Two Chambered Constructed Wetlands-Microbial Fuel Cells: A New Design for Enhanced Performance. Chem. Eng. J. 2022, 427, 131856. [Google Scholar] [CrossRef]
- Mu, C.; Wang, L.; Wang, L. Removal of Cr(VI) and Electricity Production by Constructed Wetland Combined with Microbial Fuel Cell (CW-MFC): Influence of Filler Media. J. Clean. Prod. 2021, 320, 128860. [Google Scholar] [CrossRef]
- Srivastava, P.; Dwivedi, S.; Kumar, N.; Abbassi, R.; Garaniya, V.; Yadav, A.K. Performance Assessment of Aeration and Radial Oxygen Loss Assisted Cathode Based Integrated Constructed Wetland-Microbial Fuel Cell Systems. Bioresour. Technol. 2017, 244, 1178–1182. [Google Scholar] [CrossRef] [PubMed]
- Doherty, L.; Zhao, Y.; Zhao, X.; Wang, W. Nutrient and Organics Removal from Swine Slurry with Simultaneous Electricity Generation in an Alum Sludge-Based Constructed Wetland Incorporating Microbial Fuel Cell Technology. Chem. Eng. J. 2015, 266, 74–81. [Google Scholar] [CrossRef]
- Hartl, M.; Bedoya-Ríos, D.F.; Fernández-Gatell, M.; Rousseau, D.P.L.; Du Laing, G.; Garfí, M.; Puigagut, J. Contaminants Removal and Bacterial Activity Enhancement along the Flow Path of Constructed Wetland Microbial Fuel Cells. Sci. Total Environ. 2019, 652, 1195–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Cao, F.; Kong, Q.; Zhou, L.; Yuan, Q.; Zhu, Y.; Wang, Q.; Du, Y.; Wang, Z. Electricity Production and Evolution of Microbial Community in the Constructed Wetland-Microbial Fuel Cell. Chem. Eng. J. 2018, 339, 479–486. [Google Scholar] [CrossRef]
- Bombelli, P.; Bradley, R.W.; Scott, A.M.; Philips, A.J.; McCormick, A.J.; Cruz, S.M.; Anderson, A.; Yunus, K.; Bendall, D.S.; Cameron, P.J.; et al. Quantitative Analysis of the Factors Limiting Solar Power Transduction by Synechocystis Sp. PCC 6803 in Biological Photovoltaic Devices. Energy Environ. Sci. 2011, 4, 4690–4698. [Google Scholar] [CrossRef]
- Kakarla, R.; Min, B. Photoautotrophic Microalgae Scenedesmus Obliquus Attached on a Cathode as Oxygen Producers for Microbial Fuel Cell (MFC) Operation. Int. J. Hydrogen Energy 2014, 39, 10275–10283. [Google Scholar] [CrossRef]
- Kondaveeti, S.; Choi, K.S.; Kakarla, R.; Min, B. Microalgae Scenedesmus Obliquus as Renewable Biomass Feedstock for Electricity Generation in Microbial Fuel Cells (MFCs). Front. Environ. Sci. Eng. 2014, 8, 784–791. [Google Scholar] [CrossRef]
- Rashid, N.; Cui, Y.-F.; Saif Ur Rehman, M.; Han, J.-I. Enhanced Electricity Generation by Using Algae Biomass and Activated Sludge in Microbial Fuel Cell. Sci. Total Environ. 2013, 456–457, 91–94. [Google Scholar] [CrossRef]
- Cao, X.; Huang, X.; Liang, P.; Boon, N.; Fan, M.; Zhang, L.; Zhang, X. A Completely Anoxic Microbial Fuel Cell Using a Photo-Biocathode for Cathodic Carbon Dioxide Reduction. Energy Environ. Sci. 2009, 2, 498–501. [Google Scholar] [CrossRef]
- Wu, X.; Song, T.; Zhu, X.; Wei, P.; Zhou, C.C. Construction and Operation of Microbial Fuel Cell with Chlorella Vulgaris Biocathode for Electricity Generation. Appl. Biochem. Biotechnol. 2013, 171, 2082–2092. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, H.; Zhang, Y.; Antonopoulou, G.; Ntaikou, I.; Lyberatos, G.; Yan, Q. In Situ Biogas Upgrading via Cathodic Biohydrogen Using Mitigated Ammonia Nitrogen during the Anaerobic Digestion of Taihu Blue Algae in an Integrated Bioelectrochemical System (BES). Bioresour. Technol. 2021, 341, 125902. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-Z.; Zhu, C.-X.; Tang, Y.-L.; Li, H.-N. Research Progresses in Microbial Fuel Cells for Antibiotic Wastewater Treatment. Chin. J. Agrometeorol. 2020, 41, 275. [Google Scholar] [CrossRef]
- Castro, C. The Green Latrine: Development of a Large Scale Microbial Fuel Cell for the Treatment of Human Waste in Developing Areas. Environ. Water Resour. Eng. Masters Proj. 2014. [Google Scholar] [CrossRef]
- Yazdi, H.; Alzate-Gaviria, L.; Ren, Z.J. Pluggable Microbial Fuel Cell Stacks for Septic Wastewater Treatment and Electricity Production. Bioresour. Technol. 2015, 180, 258–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzate-Gaviria, L.; García-Rodríguez, O.; Flota-Bañuelos, M.; Del Rio Jorge-Rivera, F.; Cámara-Chalé, G.; Domínguez-Maldonado, J. Stacked-MFC into a Typical Septic Tank Used in Public Housing. Biofuels 2016, 7, 79–86. [Google Scholar] [CrossRef]
- Savla, N.; Pandit, S.; Verma, J.P.; Awasthi, A.K.; Sana, S.S.; Prasad, R. Techno-Economical Evaluation and Life Cycle Assessment of Microbial Electrochemical Systems: A Review. Curr. Res. Green Sustain. Chem. 2021, 4, 100111. [Google Scholar] [CrossRef]
- Eberle, A.; Hanes, R.; Key, A.; Ghosh, T. An Overview of the Circular Economy Lifecycle Assessment and VIsualization (CELAVI) Framework; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2020. Available online: https://www.nrel.gov/docs/fy20osti/77599.pdf (accessed on 26 October 2021).
- Ardakani, M.N.; Badalians Gholikandi, G. Microbial Fuel Cells (MFCs) in Integration with Anaerobic Treatment Processes (AnTPs) and Membrane Bioreactors (MBRs) for Simultaneous Efficient Wastewater/Sludge Treatment and Energy Recovery -A State-of-the-Art Review. Biomass Bioenergy 2020, 141, 105726. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Mohamad Ibrahim, M.N.; Rafatullah, M.; Chua, Y.S.; Ahmad, A.; Umar, K. Recent Advances in Anodes for Microbial Fuel Cells: An Overview. Material 2020, 13, 2078. [Google Scholar] [CrossRef] [PubMed]
- Savla, N.; Anand, R.; Pandit, S.; Prasad, R. Utilization of Nanomaterials as Anode Modifiers for Improving Microbial Fuel Cells Performance. J. Renew. Mater. 2020, 8, 1581–1605. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Rodríguez-Couto, S. Development and Modification of Materials to Build Cost-Effective Anodes for Microbial Fuel Cells (MFCs): An Overview. Biochem. Eng. J. 2020, 164, 107779. [Google Scholar] [CrossRef]
- Cheng, S.; Xing, D.; Call, D.F.; Logan, B.E. Direct Biological Conversion of Electrical Current into Methane by Electromethanogenesis. Environ. Sci. Technol. 2009, 43, 3953–3958. [Google Scholar] [CrossRef] [PubMed]
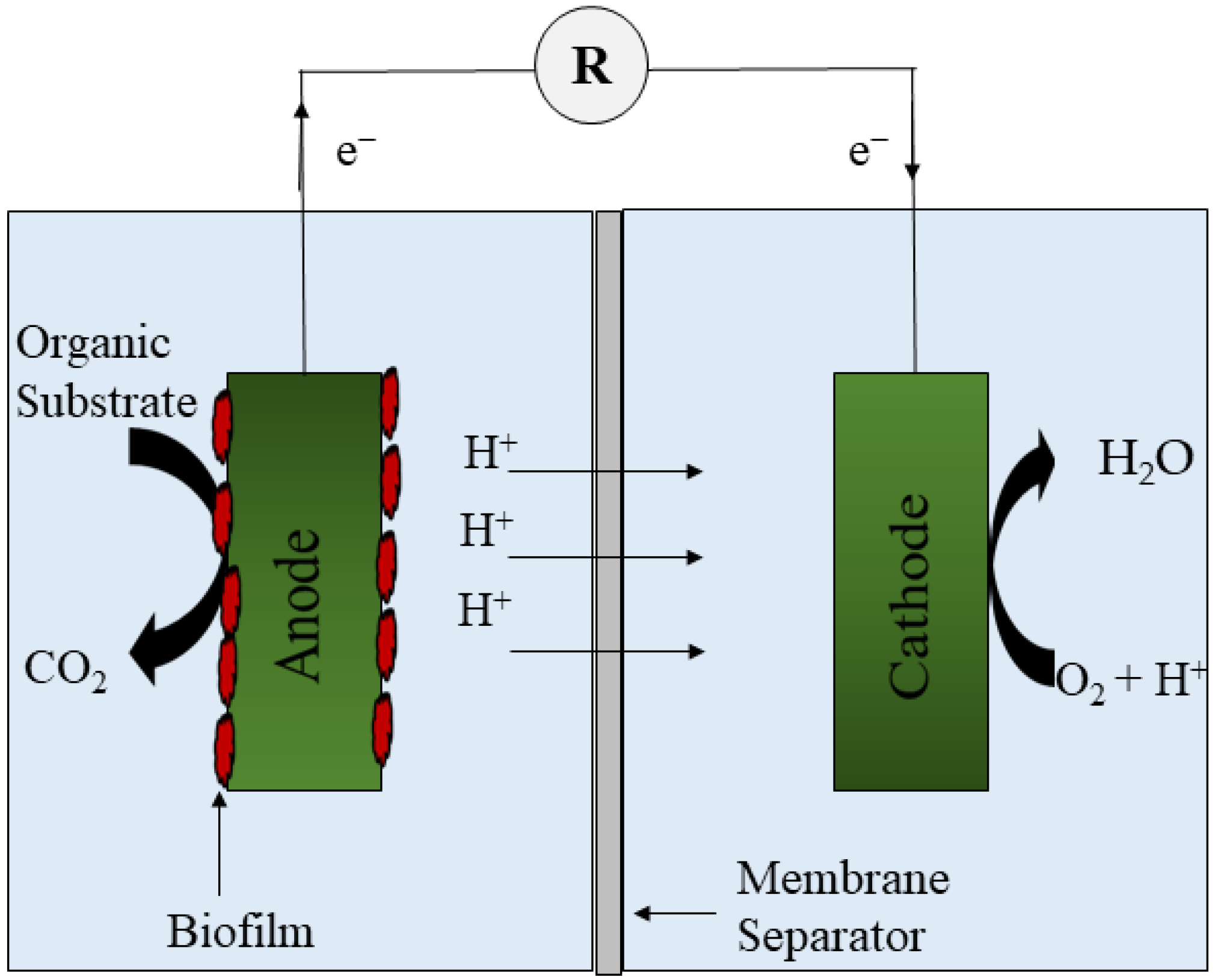
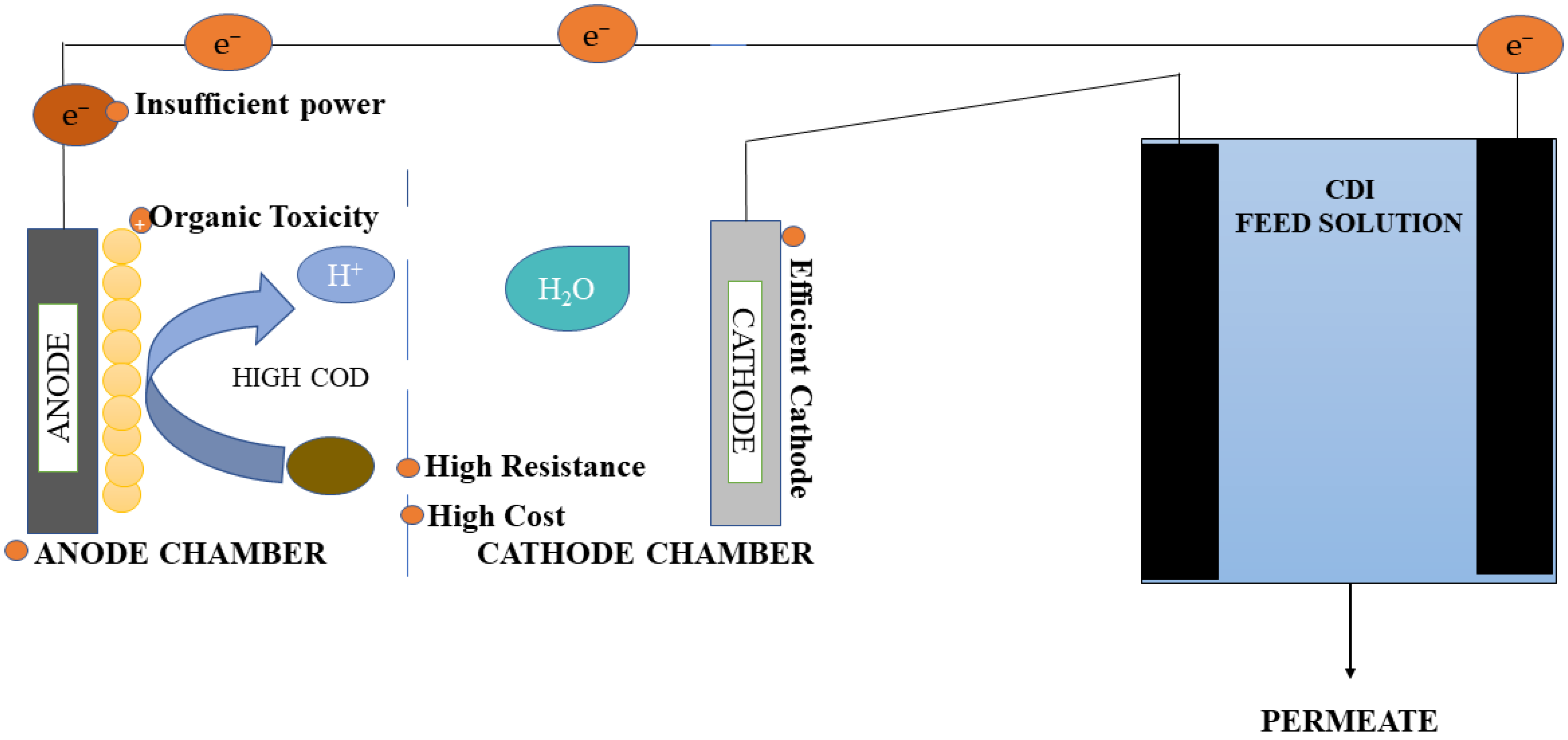
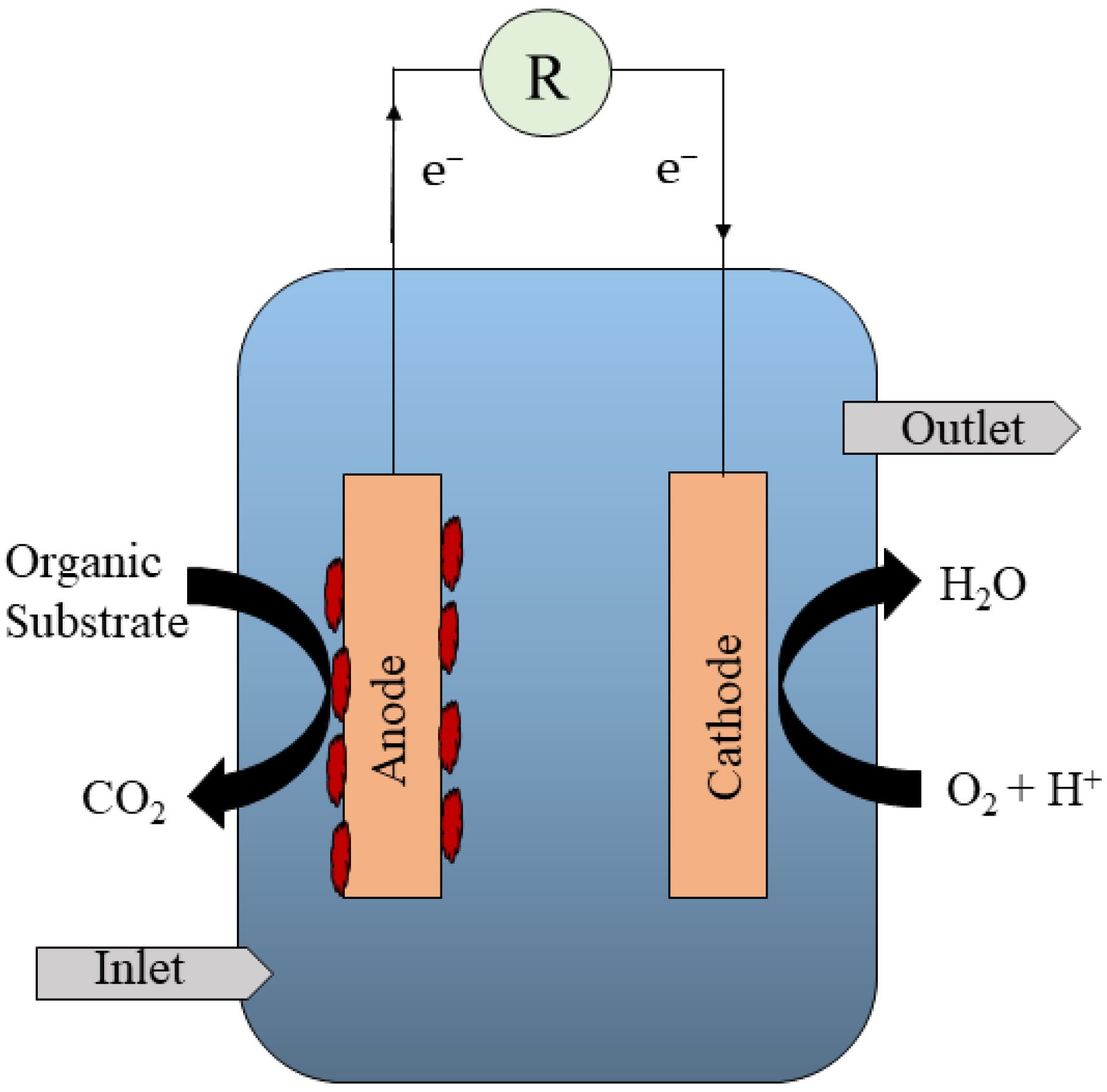

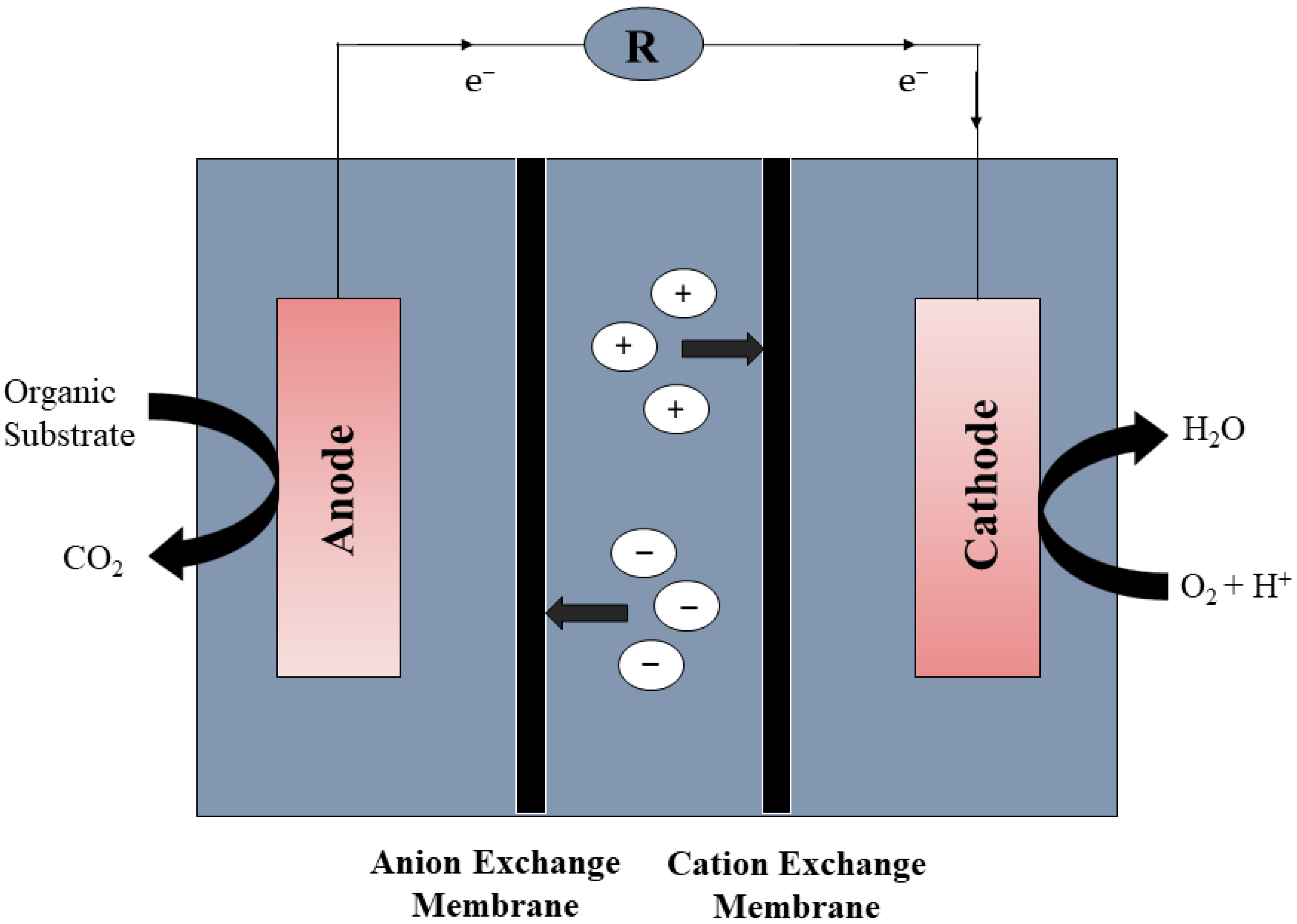


| Sr. No | Reactor Type | Substrate | COD, Conductivity, pH | Cathode | Anode | Power Density (W/m3) | Reference |
|---|---|---|---|---|---|---|---|
| 1. | Sediment MFC, 350 L | Synthetic wastewater | - | Activated carbon | C mesh | 0.0064 | [70] |
| 2. | Sediment MFC, 195 L | River sediment | 13.5 mS/m pH 6.8–7.4 | Activated carbon/stainless steel | C mesh | 0.0415 | [71] |
| 3. | Self-stratifying SCMFC (38 units), 19.2–57.6 L | Urine | 5.6–6.8 g/L pH 8.5–9.2 | Microporous carbon | C veil fibers | 7.3–9.9 | [72] |
| 4. | Sediment MFC, 72 units, 72 L | River sediment water | 890 g/L 27.206 mS/m pH 8 | Zinc | Copper | 0.0019 | [73] |
| Sediment MFC, 35 units, 35 L | 0.0069 | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patwardhan, S.B.; Savla, N.; Pandit, S.; Gupta, P.K.; Mathuriya, A.S.; Lahiri, D.; Jadhav, D.A.; Rai, A.K.; KanuPriya; Ray, R.R.; et al. Microbial Fuel Cell United with Other Existing Technologies for Enhanced Power Generation and Efficient Wastewater Treatment. Appl. Sci. 2021, 11, 10777. https://doi.org/10.3390/app112210777
Patwardhan SB, Savla N, Pandit S, Gupta PK, Mathuriya AS, Lahiri D, Jadhav DA, Rai AK, KanuPriya, Ray RR, et al. Microbial Fuel Cell United with Other Existing Technologies for Enhanced Power Generation and Efficient Wastewater Treatment. Applied Sciences. 2021; 11(22):10777. https://doi.org/10.3390/app112210777
Chicago/Turabian StylePatwardhan, Sanchita Bipin, Nishit Savla, Soumya Pandit, Piyush Kumar Gupta, Abhilasha Singh Mathuriya, Dibyajit Lahiri, Dipak A. Jadhav, Ashutosh Kumar Rai, KanuPriya, Rina Rani Ray, and et al. 2021. "Microbial Fuel Cell United with Other Existing Technologies for Enhanced Power Generation and Efficient Wastewater Treatment" Applied Sciences 11, no. 22: 10777. https://doi.org/10.3390/app112210777
APA StylePatwardhan, S. B., Savla, N., Pandit, S., Gupta, P. K., Mathuriya, A. S., Lahiri, D., Jadhav, D. A., Rai, A. K., KanuPriya, Ray, R. R., Singh, V., Kumar, V., & Prasad, R. (2021). Microbial Fuel Cell United with Other Existing Technologies for Enhanced Power Generation and Efficient Wastewater Treatment. Applied Sciences, 11(22), 10777. https://doi.org/10.3390/app112210777











