Chemical Composition of Hexane-Extracted Plectranthus amboinicus Leaf Essential Oil: Maximizing Contents on Harvested Plant Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Sensory Evaluation (Direct Olfactory Detection) of Plectranthus amboinicus Leaf Volatiles
2.3. Oil Red O Histochemical Staining of Plectranthus amboinicus Leaf Using Freehand Fresh Stain Method
2.4. Oil Quantification of Stained Leaf Sections
2.5. Essential Oil Extraction from Fresh Plectranthus amboinicus Leaves Using a Non-Polar Solvent
2.6. Total Phenolics Content Assay (TPC) of Plectranthus amboinicus Essential Oil Extract
2.7. Total Flavonoids Content (TFC) of Plectranthus amboinicus Essential Oil Extract
2.8. GC-MS Analysis of Plectranthus amboinicus Essential Oil Extract
3. Results and Discussion
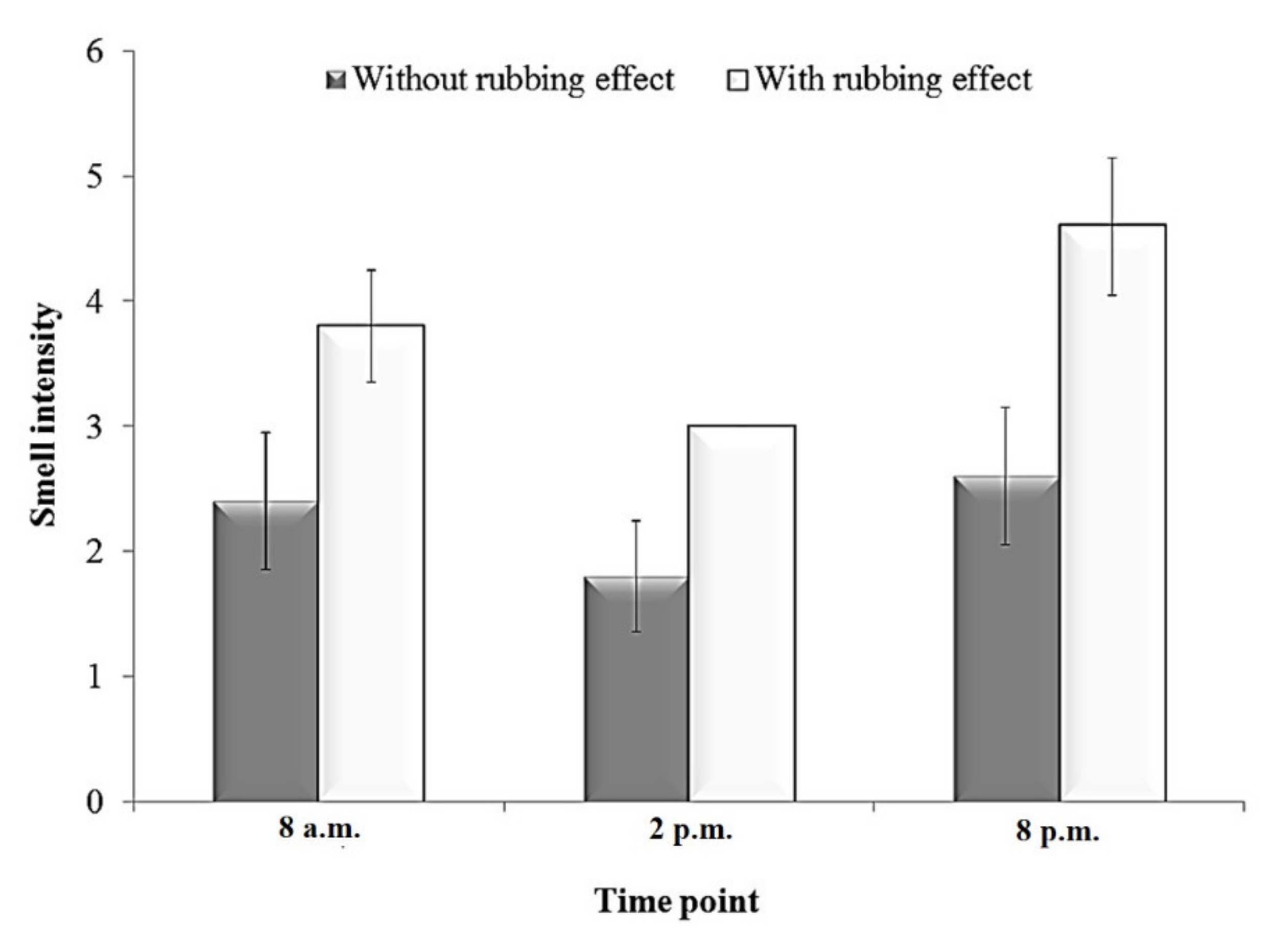
3.1. Sensory Evaluation (Direct Olfactory Detection) of Plectranthus amboinicus Leaf Volatiles
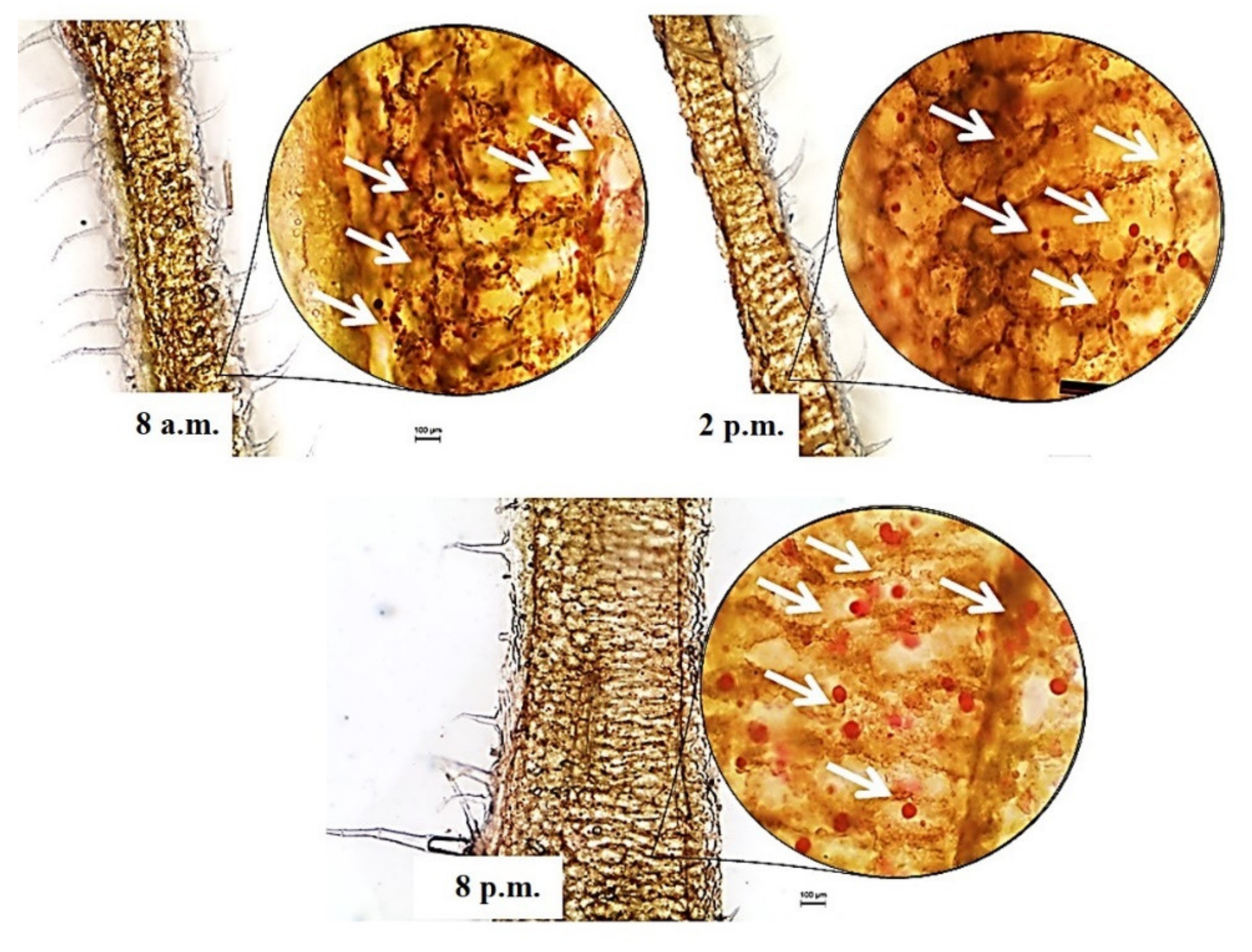
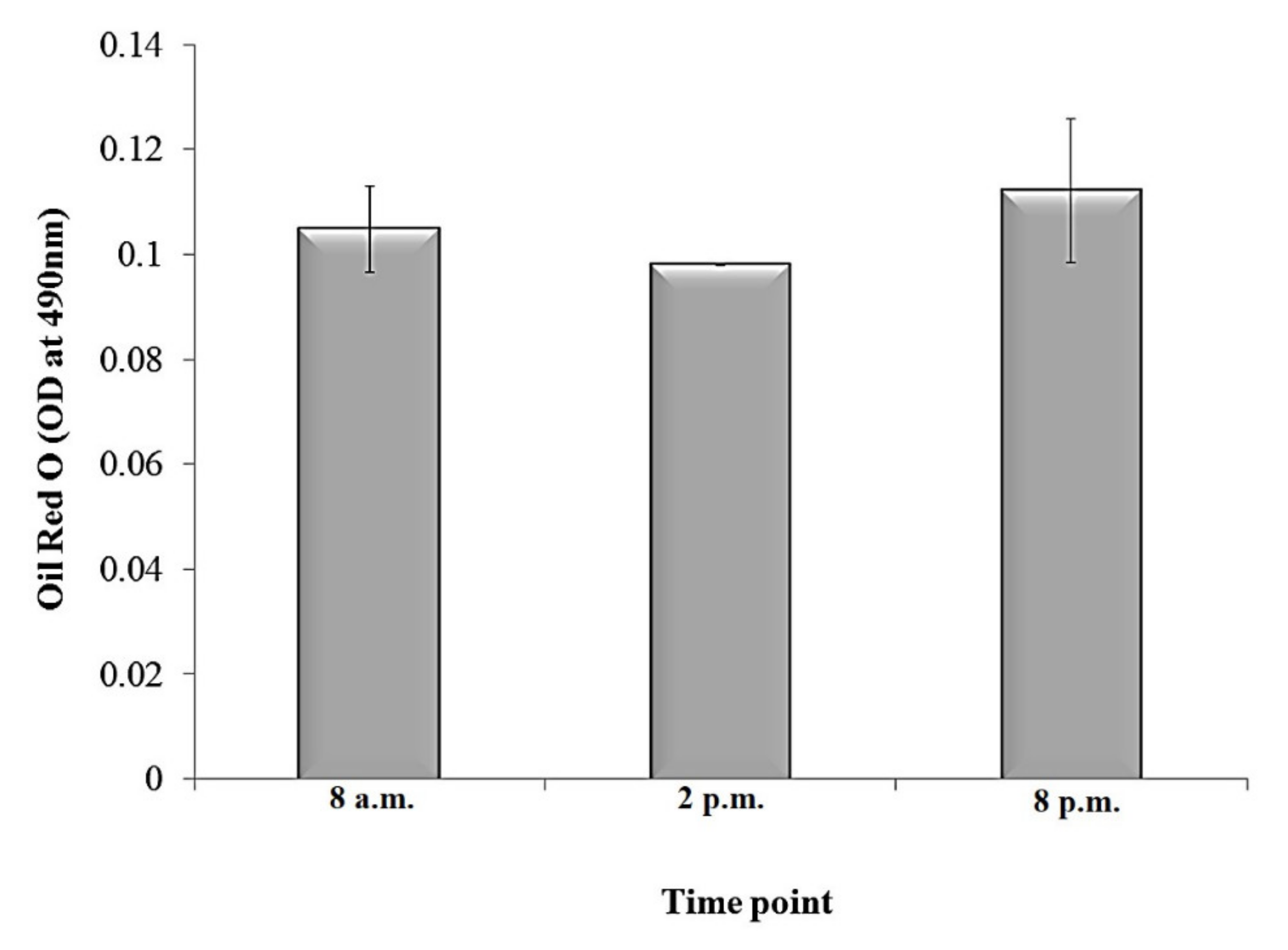
3.2. Oil Red O Histochemical Staining of Plectranthus amboinicus Leaf Using Freehand Fresh Stain Method
3.3. Total Phenolics (TFC) and Total Flavonoids (TFC) Contents of Plectranthus amboinicus Essential Oil Extract
3.4. GC-MS Analysis of Plectranthus amboinicus Essential Oil Extract
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arumugam, G.; Swamy, M.K.; Sinniah, U.R. Plectranthus amboinicus (Lour.) Spreng: Botanical, Phytochemical, Pharmacological and Nutritional Significance. Molecules 2016, 21, 369. [Google Scholar] [CrossRef] [PubMed]
- El Sohafy, S.M.; Metwally, A.M.; Omar, A.A.; Harraz, F.M. Phytochemical investigation and antimicrobial activity of Psidium guajava L. leaves. Pharmacogn. Mag. 2010, 6, 212–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khare, R.S.; Banerjee, S.; Kundu, K. Coleus aromaticus benth—A nutritive medicinal plant of potential therapeutic value. Int. J. Pharma. Bio Sci. 2011, 2, 488–500. [Google Scholar]
- Damanik, R.; Wahlqvist, M.L.; Wattanapenpaiboon, N. Lactagogue effects of Torbangun, a Bataknese traditional cuisine. Asia Pac. J. Clin. Nutr. 2006, 15, 267–274. [Google Scholar] [PubMed]
- De Oliveira, F.F.M.; Torres, A.F.; Gonçalves, T.B.; Santiago, G.M.P.; De Carvalho, C.B.M.; Aguiar, M.B.; Camara, L.M.C.; Rabenhorst, S.H.B.; Martins, A.M.C.; Junior, J.T.V.; et al. Efficacy of Plectranthus amboinicus (Lour.) Spreng in a Murine Model of Methicillin-Resistant Staphylococcus aureus Skin Abscesses. Evid. Based Complement. Altern. Med. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hoehler, T.M. An Energy Balance Concept for Habitability. Astrobiology 2007, 7, 824–838. [Google Scholar] [CrossRef]
- Gurgel, A.P.A.D.; da Silva, J.G.; Grangeiro, A.R.S.; Oliveira, D.C.; Lima, C.M.; da Silva, A.C.; Oliveira, R.A.; Souza, I.A. In vivo study of the anti-inflammatory and antitumor activities of leaves from Plectranthus amboinicus (Lour.) Spreng (Lamiaceae). J. Ethnopharmacol. 2009, 125, 361–363. [Google Scholar] [CrossRef]
- Senthilkumar, A.; Venkatesalu, V. Chemical composition and larvicidal activity of the essential oil of Plectranthus amboinicus (Lour.) Spreng against Anopheles stephensi: A malarial vector mosquito. Parasitol. Res. 2010, 107, 1275–1278. [Google Scholar] [CrossRef]
- Bhatt, P.; Joseph, G.S.; Negi, P.S.; Varadaraj, M.C. Chemical Composition and Nutraceutical Potential of Indian Borage (Plectranthus amboinicus) Stem Extract. J. Chem. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Oyedemi, S.O.; Okoh, A.I.; Mabinya, L.V.; Pirochenva, G.; Afolayan, A.J. The proposed mechanism of bactericidal action of eugenol, α-terpineol and γ-terpinene against Listeria monocytogenes, Streptococcus pyogenes, Proteus vulgaris and Escherichia coli. Afr. J. Biotechnol. 2009, 8, 1280–1286. [Google Scholar] [CrossRef]
- Dai, W.; Sun, C.; Huang, S.; Zhou, Q. Carvacrol suppresses proliferation and invasion in human oral squamous cell carcinoma. Onco Targets Ther. 2016, 9, 2297–2304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashaari, N.S.; Rahim, M.H.A.; Sabri, S.; Lai, K.S.; Song, A.A.-L.; Rahim, R.A.; Abdullah, W.M.A.N.W.; Abdullah, J.O. Functional characterization of a new terpene synthase from Plectranthus amboinicus. PLoS ONE 2020, 15, e0235416. [Google Scholar] [CrossRef] [PubMed]
- Mohd-Hairul, A.R. Chemical Composition of Floral Volatiles and Expression of Scent-Related Genes in Vanda Mimi Palmer. Ph.D. Thesis, Universiti Putra Malaysia, Seri Kembangan, Malaysia, 2010. [Google Scholar]
- Mohamad, N.E.; Yeap, S.K.; Lim, K.L.; Yusof, H.M.; Beh, B.K.; Tan, S.W.; Ho, W.Y.; Sharifuddin, S.A.; Jamaluddin, A.; Long, K.; et al. Antioxidant effects of pineapple vinegar in reversing of paracetamol-induced liver damage in mice. Chin. Med. 2015, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguiar, J.; Gonçalves, J.L.; Alves, V.L.; Câmara, J.S. Chemical Fingerprint of Free Polyphenols and Antioxidant Activity in Dietary Fruits and Vegetables Using a Non-Targeted Approach Based on QuEChERS Ultrasound-Assisted Extraction Combined with UHPLC-PDA. Antioxidants 2020, 9, 305. [Google Scholar] [CrossRef] [Green Version]
- Winter, K.; Holtum, J.A.M. Facultative crassulacean acid metabolism (CAM) plants: Powerful tools for unravelling the functional elements of CAM photosynthesis. J. Exp. Bot. 2014, 65, 3425–3441. [Google Scholar] [CrossRef] [Green Version]
- Muthukumarana, R.; Dharmadasa, R. Pharmacognostical investigation of Plectranthus hadiensis (Forssk.) Schweinf. ex Sprenger. and Plectranthus amboinicus (Lour.) Spreng. World J. Agric. Res. 2014, 2, 240–246. [Google Scholar] [CrossRef]
- Sharma, S.; Sangwan, N.S.; Sangwan, R.S. Developmental process of essential oil glandular trichome collapsing in menthol mint. Curr. Sci. 2003, 84, 544–550. [Google Scholar]
- Lukhoba, C.W.; Simmonds, M.S.; Paton, A.J. Plectranthus: A review of ethnobotanical uses. J. Ethnopharmacol. 2006, 103, 1–24. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.; Pedro, L.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Fanoudi, S.; Alavi, M.S.; Hosseini, M.; Sadeghnia, H.R. Nigella sativa and thymoquinone attenuate oxidative stress and cognitive impairment following cerebral hypoperfusion in rats. Metab. Brain Dis. 2019, 34, 1001–1010. [Google Scholar] [CrossRef]
- Ashraf, S.S.; Rao, M.V.; Kaneez, F.S.; Qadri, S.; Al-Marzouqi, A.H.; Chandranath, I.S.; Adem, A. Nigella sativa Extract as a Potent Antioxidant for Petrochemical-Induced Oxidative Stress. J. Chromatogr. Sci. 2011, 49, 321–326. [Google Scholar] [CrossRef] [Green Version]
- Yildiz, S.; Turan, S.; Kiralan, M.; Ramadan, M.F. Antioxidant properties of thymol, carvacrol, and thymoquinone and its efficiencies on the stabilization of refined and stripped corn oils. J. Food Meas. Charact. 2021, 15, 621–632. [Google Scholar] [CrossRef]
- Khan, M.; Khan, S.T.; Khan, M.; Mousa, A.A.; Mahmood, A.; Alkhathlan, H.Z. Chemical diversity in leaf and stem essential oils of Origanum vulgare L. and their effects on microbicidal activities. AMB Express 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Pinheiro, P.F.; Costa, A.V.; Alves, T.D.A.; Galter, I.N.; Pinheiro, C.A.; Pereira, A.F.; Oliveira, C.M.R.; Fontes, M.M.P. Phytotoxicity and Cytotoxicity of Essential Oil from Leaves of Plectranthus amboinicus, Carvacrol, and Thymol in Plant Bioassays. J. Agric. Food Chem. 2015, 63, 8981–8990. [Google Scholar] [CrossRef]
- Meyers, M. Oregano and Marjoram: An Herb Society of America Guide to the Genus Origanum. Herb Soc. Am. 2005, 1–66. Available online: https://www.herbsociety.org (accessed on 25 May 2021).
- Lopes, P.Q.; Carneiro, F.B.; De Sousa, A.L.B.; Santos, S.G.; Oliveira, E.E.; Soares, L.A.L. Technological Evaluation of Emulsions Containing The Volatile Oil from Leaves of Plectranthus Amboinicus Lour. Pharmacogn. Mag. 2017, 13, 159–167. [Google Scholar]
- Asiimwe, S.; Karlsson, A.B.; Azeem, M.; Mugisha, K.M.; Namutebi, A.; Gakunga, N.J. Chemical composition and toxicological evaluation of the aqueous leaf extracts of Plectranthus amboinicus Lour. Spreng. Int. J. Pharm. Sci. Invent. 2014, 3, 19–27. [Google Scholar]
- Erny Sabrina, M.N.; Razali, M.; Mirfat, A.H.S.; Mohd Shukri, M.A. Antimicrobial activity and bioactive evaluation of Plectranthus amboinicus essential oil. Am. J. Res. Commun. 2014, 2, 121–127. [Google Scholar]
- Swamy, M.K.; Arumugam, G.; Kaur, R.; Ghasemzadeh, A.; Yusoff, M.M.; Sinniah, U.R. GC-MS Based Metabolite Profiling, Antioxidant and Antimicrobial Properties of Different Solvent Extracts of Malaysian Plectranthus amboinicus Leaves. Evid. Based Complement. Altern. Med. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafiee-Hajiabad, M.; Novak, J.; Honermeier, B. Content and composition of essential oil of four Origanum vulgare L. accessions under reduced and normal light intensity conditions. J. Appl. Bot. Food Qual. 2016, 89, 126–134. [Google Scholar] [CrossRef]
- Ibrahim, L.; Karaky, M.; Ayoub, P.; El Ajouz, N.; Ibrahim, S. Chemical composition and antimicrobial activities of essential oil and its components from Lebanese Origanum syriacum L. J. Essent. Oil Res. 2012, 24, 339–345. [Google Scholar] [CrossRef]
- Zein, S. Variation of thymol, carvacrol and thymoquinone production from wild and cultivated Origanum syriacum of South Lebanon. J. Med. Plants Res. 2012, 6, 1692–1696. [Google Scholar] [CrossRef]
- Lukas, B.; Schmiderer, C.; Franz, C.; Novak, J. Composition of Essential Oil Compounds from Different Syrian Populations of Origanum syriacum L. (Lamiaceae). J. Agric. Food Chem. 2009, 57, 1362–1365. [Google Scholar] [CrossRef]
- Grosso, C.; Figueiredo, A.C.; Burillo, J.; Mainar, A.M.; Urieta, J.S.; Barroso, J.G.; Coelho, J.A.; Palavra, A.M.F.; Grosso, A.C.; Fernández, A.M.M. Enrichment of the thymoquinone content in volatile oil from Satureja montana using supercritical fluid extraction. J. Sep. Sci. 2009, 32, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Grosso, C.; Figueiredo, A.C.; Burillo, J.; Mainar, A.M.; Urieta, J.S.; Barroso, J.; Coelho, J.A.; Palavra, A.M.F. Composition and antioxidant activity of Thymus vulgaris volatiles: Comparison between supercritical fluid extraction and hydrodistillation. J. Sep. Sci. 2010, 33, 2211–2218. [Google Scholar] [CrossRef]
- Manter, D.K.; Kelsey, R.G.; Karchesy, J.J. Antimicrobial Activity of Extractable Conifer Heartwood Compounds Toward Phytophthora ramorum. J. Chem. Ecol. 2007, 33, 2133–2147. [Google Scholar] [CrossRef]
- Das, S.; Dey, K.K.; Dey, G.; Pal, I.; Majumder, A.; MaitiChoudhury, S.; Kundu, S.C.; Mandal, M. Antineoplastic and Apoptotic Potential of Traditional Medicines Thymoquinone and Diosgenin in Squamous Cell Carcinoma. PLoS ONE 2012, 7, e46641. [Google Scholar] [CrossRef] [Green Version]
- Yi, T.; Cho, S.-G.; Yi, Z.; Pang, X.; Rodriguez, M.; Wang, Y.; Sethi, G.; Aggarwal, B.B.; Liu, M. Thymoquinone inhibits tumor angiogenesis and tumor growth through suppressing AKT and extracellular signal-regulated kinase signaling pathways. Mol. Cancer Ther. 2008, 7, 1789–1796. [Google Scholar] [CrossRef] [Green Version]
- Gurung, R.L.; Ni Lim, S.; Khaw, A.K.; Soon, J.F.F.; Shenoy, K.; Ali, S.M.; Jayapal, M.; Sethu, S.; Baskar, R.; Hande, M.P. Thymoquinone Induces Telomere Shortening, DNA Damage and Apoptosis in Human Glioblastoma Cells. PLoS ONE 2010, 5, e12124. [Google Scholar] [CrossRef]
- Schneider-Stock, R.; Fakhoury, I.H.; Zaki, A.M.; El-Baba, C.; Gali-Muhtasib, H.U. Thymoquinone: Fifty years of success in the battle against cancer models. Drug Discov. Today 2014, 19, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Yu, H.-M.; Shie, J.-J.; Cheng, T.-J.R.; Wu, C.-Y.; Fang, J.-M.; Wong, C.-H. Chemical constituents of Plectranthus amboinicus and the synthetic analogs possessing anti-inflammatory activity. Bioorg. Med. Chem. 2014, 22, 1766–1772. [Google Scholar] [CrossRef] [PubMed]
- Botnick, I.; Xue, W.; Bar, E.; Ibdah, M.; Schwartz, A.; Joel, D.M.; Lev, E.; Fait, A.; Lewinsohn, E. Distribution of Primary and Specialized Metabolites in Nigella sativa Seeds, a Spice with Vast Traditional and Historical Uses. Molecules 2012, 17, 10159–10177. [Google Scholar] [CrossRef] [PubMed]
- Crocoll, C.; Asbach, J.; Novak, J.; Gershenzon, J.; Degenhardt, J. Terpene synthases of oregano (Origanum vulgare L.) and their roles in the pathway and regulation of terpene biosynthesis. Plant Mol. Biol. 2010, 73, 587–603. [Google Scholar] [CrossRef]
- Zein, S.; Awada, S.; Rachidi, S.; Hajj, A.; Krivoruschko, E.; Kanaan, H. Chemical analysis of essential oil from Lebanese wild and cultivated Origanum syriacum L. (Lamiaceae) before and after flowering. J. Med. Plants Res. 2011, 5, 379–387. [Google Scholar]
- Novák, J.; Lukas, B.; Franz, C. Temperature Influences Thymol and Carvacrol Differentially in Origanum spp. (Lamiaceae). J. Essent. Oil Res. 2010, 22, 412–415. [Google Scholar] [CrossRef]
- Dudai, N.; Putievsky, E.; Ravid, U.; Palevitch, D.; Halevy, A.H. Monoterpene content in Origanum syriacum as affected by environmental conditions and flowering. Physiol. Plant. 1992, 84, 453–459. [Google Scholar] [CrossRef]
- Tibaldi, G.; Fontana, E.; Nicola, S. Growing conditions and postharvest management can affect the essential oil of Origanum vulgare L. ssp. hirtum (Link) Ietswaart. Ind. Crop. Prod. 2011, 34, 1516–1522. [Google Scholar] [CrossRef]
- Dehsheikh, A.B.; Sourestani, M.M.; Dehsheikh, P.B.; Vitalini, S.; Iriti, M.; Mottaghipisheh, J. A Comparative Study of Essential Oil Constituents and Phenolic Compounds of Arabian Lilac (Vitex Trifolia var. Purpurea): An Evidence of Season Effects. Foods 2019, 8, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokkini, S.; Karousou, R.; Dardioti, A.; Krigas, N.; Lanaras, T. Autumn essential oils of Greek oregano. Phytochemistry 1997, 44, 883–886. [Google Scholar] [CrossRef]

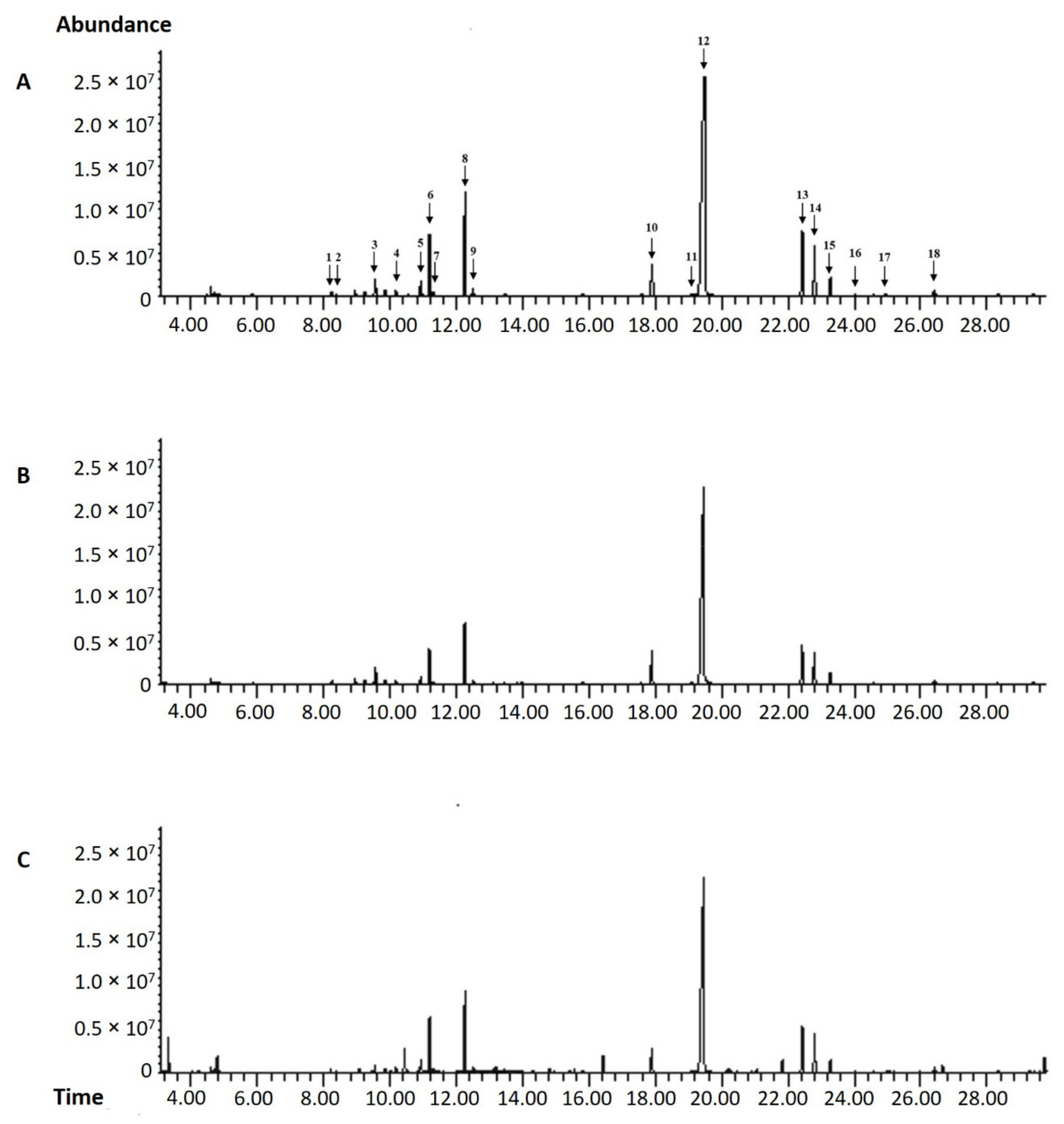
| Peak | Compounds | RT a (min) | RI b | Harvesting Time/Relative Content c (%) | ||
|---|---|---|---|---|---|---|
| 8 a.m. | 2 p.m. | 8 p.m. | ||||
| Monoterpene hydrocarbons | ||||||
| 1 | β-Thujene | 8.21 | 926 | 0.25 | 0.21 | 0.39 |
| 2 | α-pinene | 8.39 | 932 | 0.10 | 0.10 | 0.12 |
| 3 | β-Myrcene | 10.17 | 991 | 0.47 | 0.41 | 0.63 |
| 4 | α-Phellandrene | 10.55 | 1004 | 0.17 | 0.14 | 0.17 |
| 5 | α-Terpinene | 10.93 | 1016 | 1.25 | 1.02 | 1.40 |
| 6 | p-Cymene | 11.19 | 1025 | 5.42 | 5.03 | 6.44 |
| 7 | Limonene | 11.30 | 1028 | 0.26 | 0.23 | 0.38 |
| 8 | γ-Terpinene | 12.26 | 1060 | 10.11 | 8.89 | 10.33 |
| Oxygenated monoterpenes | ||||||
| 9 | β-Terpineol | 12.50 | 1067 | 0.69 | 0.77 | 1.03 |
| 10 | Thymoquinone | 17.90 | 1252 | 3.92 | 5.65 | 3.31 |
| 11 | Thymol | 19.10 | 1296 | 0.51 | 0.49 | 0.27 |
| 12 | Carvacrol | 19.45 | 1309 | 59.25 | 59.79 | 48.13 |
| Sesquiterpene hydrocarbons | ||||||
| 13 | Caryophyllene | 22.43 | 1424 | 6.66 | 6.29 | 6.17 |
| 14 | Trans-α-Bergamotene | 22.80 | 1439 | 4.94 | 4.79 | 4.84 |
| 15 | Humulene | 23.28 | 1458 | 1.86 | 1.80 | 1.75 |
| 16 | (E)-β-Farnesene | 24.01 | 1487 | 0.12 | 0.11 | 0.14 |
| 17 | β-Bisabolene | 24.58 | 1511 | 0.26 | 0.26 | 0.26 |
| Oxygenated sesquiterpenes | ||||||
| 18 | Caryophyllene oxide | 26.41 | 1590 | 0.96 | 1.08 | 1.11 |
| Alkane and alcohol | ||||||
| 19 | 3-Methylcyclopentanol | 5.86 | 841 | 0.26 | 0.29 | 0.17 |
| 20 | 4-Ethyl-octane | 9.07 | 954 | - | - | 0.37 |
| 21 | 3-Ethyl-octane | 9.44 | 967 | - | - | 0.29 |
| 22 | 3-methyl-nonane | 9.54 | 970 | - | - | 0.89 |
| 23 | 1-Octen-3-ol | 9.86 | 980 | 0.54 | 0.63 | 0.51 |
| 24 | 3,8-Dimethy decane | 15.56 | 1171 | - | - | 0.55 |
| 25 | 3-Methylpentadecane | 25.98 | 1571 | - | - | 0.35 |
| Chemical class | ||||||
| Monoterpene hydrocarbons | 18.03 | 16.03 | 19.86 | |||
| Sesquiterpene hydrocarbons | 64.37 | 66.70 | 52.74 | |||
| Total monoterpenes | 82.40 | 82.73 | 72.60 | |||
| Sesquiterpene hydrocarbons | 13.84 | 13.25 | 13.16 | |||
| Oxygenated sesquiterpenes | 0.96 | 1.08 | 1.11 | |||
| Total sesquiterpenes | 14.80 | 14.33 | 14.27 | |||
| Others | 0.80 | 0.92 | 3.13 | |||
| Total | 98.00 | 97.98 | 90.00 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashaari, N.S.; Mohamad, N.E.; Afzinizam, A.H.; Ab. Rahim, M.-H.; Lai, K.S.; Ong Abdullah, J. Chemical Composition of Hexane-Extracted Plectranthus amboinicus Leaf Essential Oil: Maximizing Contents on Harvested Plant Materials. Appl. Sci. 2021, 11, 10838. https://doi.org/10.3390/app112210838
Ashaari NS, Mohamad NE, Afzinizam AH, Ab. Rahim M-H, Lai KS, Ong Abdullah J. Chemical Composition of Hexane-Extracted Plectranthus amboinicus Leaf Essential Oil: Maximizing Contents on Harvested Plant Materials. Applied Sciences. 2021; 11(22):10838. https://doi.org/10.3390/app112210838
Chicago/Turabian StyleAshaari, Nur Suhanawati, Nurul Elyani Mohamad, Amirul Hafizin Afzinizam, Mohd-Hairul Ab. Rahim, Kok Song Lai, and Janna Ong Abdullah. 2021. "Chemical Composition of Hexane-Extracted Plectranthus amboinicus Leaf Essential Oil: Maximizing Contents on Harvested Plant Materials" Applied Sciences 11, no. 22: 10838. https://doi.org/10.3390/app112210838






