Identifying Running Deviations in Long Distance Runners Utilizing Gait Profile Analysis: A Case Control Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Reduction and Analysis
2.3. Statistical Analysis
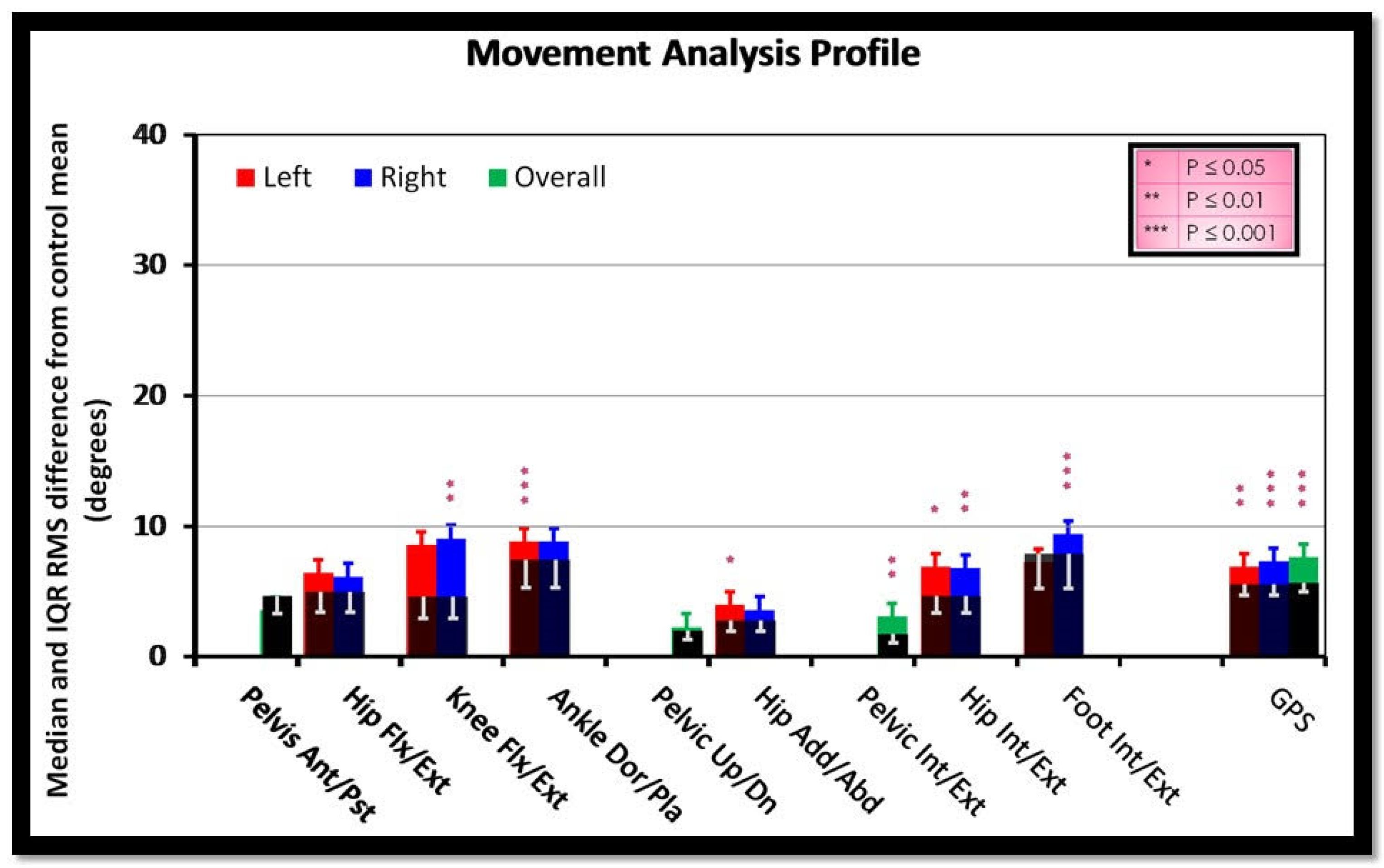
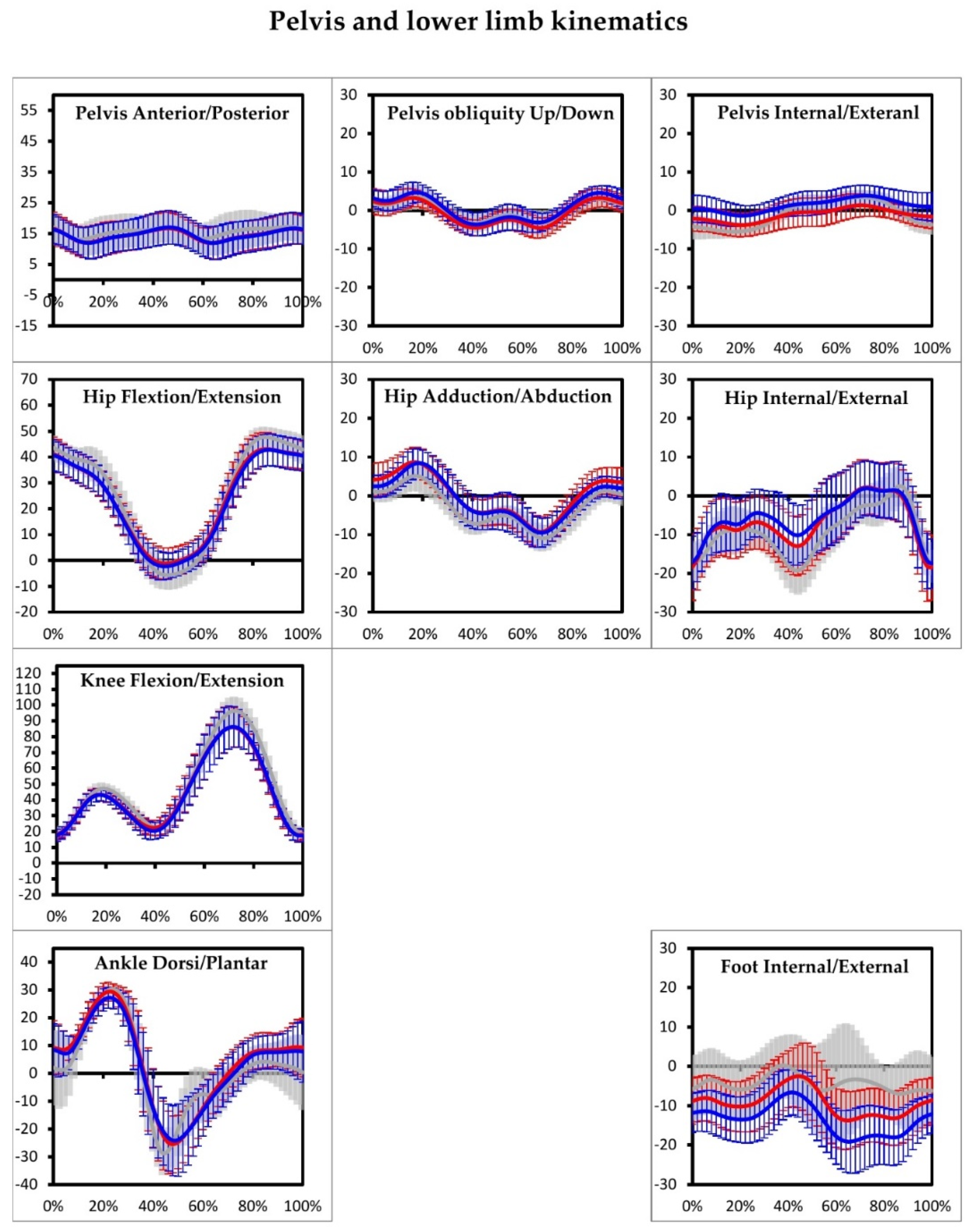
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Fokkema, T.; Burggraaff, R.; Hartgens, F.; Kluitenberg, B.; Verhagen, E.; Backx, F.J.G.; van der Worp, H.; Bierma-Zeinstra, S.M.A.; Koes, B.W.; van Middelkoop, M. Prognosis and prognostic factors of running-related injuries in novice runners: A prospective cohort study. J. Sci. Med. Sport 2019, 22, 259–263. [Google Scholar] [CrossRef] [PubMed]
- van der Worp, M.P.; ten Haaf, D.S.; van Cingel, R.; de Wijer, A.; van der Sanden, M.W.N.; Staal, J.B. Injuries in runners; a systematic review on risk factors and sex differences. PLoS ONE 2015, 10, e0114937. [Google Scholar]
- Lopes, A.D.; Hespanhol Júnior, L.C.; Yeung, S.S.; Costa, L.O. What are the Main Running-Related Musculoskeletal Injuries? Sports Med. 2012, 42, 891–905. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, N.C.; Castello, N.; Cotman, C.W. Exercise and time-dependent benefits to learning and memory. Neuroscience 2010, 167, 588–597. [Google Scholar] [CrossRef] [Green Version]
- van Gent, R.N.; Siem, D.; van Middelkoop, M.; van Os, A.G.; Bierma-Zeinstra, S.M.; Koes, B.W. Incidence and determinants of lower extremity running injuries in long distance runners: A systematic review. Br. J. Sports Med. 2007, 41, 469–480. [Google Scholar] [CrossRef] [Green Version]
- Fields, K.B.; Sykes, J.C.; Walker, K.M.; Jackson, J.C. Prevention of running injuries. Curr. Sports Med. Rep. 2010, 9, 176–182. [Google Scholar] [CrossRef]
- Taunton, J.E.; Ryan, M.B.; Clement, D.B.; McKenzie, D.C.; Lloyd-Smith, D.R.; Zumbo, B.D. A retrospective case-control analysis of 2002 running injuries. Br. J. Sports Med. 2002, 36, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Baker, R.; McGinley, J.L.; Schwartz, M.H.; Beynon, S.; Rozumalski, A.; Graham, H.K.; Tirosh, O. The gait profile score and movement analysis profile. Gait Posture 2009, 30, 265–269. [Google Scholar] [CrossRef]
- Schwartz, M.H.; Rozumalski, A. The Gait Deviation Index: A new comprehensive index of gait pathology. Gait Posture 2008, 28, 351–357. [Google Scholar] [CrossRef]
- Holmes, S.J.; Mudge, A.J.; Wojciechowski, E.A.; Axt, M.W.; Burns, J. Impact of multilevel joint contractures of the hips, knees and ankles on the Gait Profile score in children with cerebral palsy. Clin. Biomech. 2018, 59, 8–14. [Google Scholar] [CrossRef]
- Pau, M.; Caggiari, S.; Mura, A.; Corona, F.; Leban, B.; Coghe, G.; Lorefice, L.; Marrosu, M.G.; Cocco, E. Clinical assessment of gait in individuals with multiple sclerosis using wearable inertial sensors: Comparison with patient-based measure. Mult. Scler. Relat. Disorders. 2016, 10, 187–191. [Google Scholar] [CrossRef]
- Speciali, D.S.; Corrêa, J.C.; Luna, N.M.; Brant, R.; Greve, J.M.; de Godoy, W.; Baker, R.; Lucareli, P.R. Validation of GDI, GPS and GVS for use in Parkinson’s disease through evaluation of effects of subthalamic deep brain stimulation and levodopa. Gait Posture 2014, 39, 1142–1145. [Google Scholar] [CrossRef]
- Crossley, K.M.; Callaghan, M.J.; van Linschoten, R. Patellofemoral pain. Br. J. Sports Med. 2016, 50, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Strauss, E.J.; Kim, S.; Calcei, J.G.; Park, D. Iliotibial band syndrome: Evaluation and management. J. Am. Acad. Orthop. Surg. 2011, 19, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Aderem, J.; Louw, Q.A. Biomechanical risk factors associated with iliotibial band syndrome in runners: A systematic review. BMC Musculoskelet. Disord. 2015, 16, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfayez, S.M.; Ahmed, M.L.; Alomar, A.Z. A review article of medial tibial stress syndrome. J. Musculoskelet. Surg. Res. 2017, 1, 2–5. [Google Scholar]
- Barton, C.J.; Levinger, P.; Menz, H.B.; Webster, K.E. Kinematic gait characteristics associated with patellofemoral pain syndrome: A systematic review. Gait Posture 2009, 30, 405–416. [Google Scholar] [CrossRef]
- Bolgla, L.A.; Malone, T.R.; Umberger, B.R.; Uhl, T.L. Hip strength and hip and knee kinematics during stair descent in females with and without patellofemoral pain syndrome. J. Orthop. Sports Phys. Ther. 2008, 38, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Souza, R.B.; Powers, C.M. Differences in hip kinematics, muscle strength, and muscle activation between subjects with and without patellofemoral pain. J. Orthop. Sports Phys. Ther. 2009, 39, 12–19. [Google Scholar] [CrossRef]
- Neal, B.S.; Barton, C.J.; Gallie, R.; O’Halloran, P.; Morrissey, D. Runners with patellofemoral pain have altered biomechanics which targeted interventions can modify: A systematic review and meta-analysis. Gait Posture 2016, 45, 69–82. [Google Scholar] [CrossRef]
- Miller, R.H.; Lowry, J.L.; Meardon, S.A.; Gillette, J.C. Lower extremity mechanics of iliotibial band syndrome during an exhaustive run. Gait Posture 2007, 26, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.H.; Meardon, S.A.; Derrick, T.R.; Gillette, J.C. Continuous relative phase variability during an exhaustive run in runners with a history of iliotibial band syndrome. J. Appl. Biomech. 2008, 24, 262–270. [Google Scholar] [CrossRef] [Green Version]
- Loudon, J.K.; Reiman, M.P. Lower extremity kinematics in running athletes with and without a history of medial shin pain. Int. J. Sports Phys. Ther. 2012, 7, 356–364. [Google Scholar] [PubMed]
- Newman, P.; Witchalls, J.; Waddington, G.; Adams, R. Risk factors associated with medial tibial stress syndrome in runners: A systematic review and meta-analysis. Open Access J. Sports Med. 2013, 4, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Davis, I.S.; Tenforde, A.S.; Neal, B.S.; Roper, J.L.; Willy, R.W. Gait retraining as an intervention for patellofemoral pain. Curr. Rev. Musculoskelet. Med. 2020, 13, 103–114. [Google Scholar] [CrossRef]
- Letafatkar, A.; Rabiei, P.; Afshari, M. Effect of neuromuscular training augmented with knee valgus control instructions on lower limb biomechanics of male runners. Phys. Ther. Sport 2020, 43, 89–99. [Google Scholar] [CrossRef]
- Napier, C.; Cochrane, C.K.; Taunton, J.E.; Hunt, M.A. Gait modifications to change lower extremity gait biomechanics in runners: A systematic review. Br. J. Sports Med. 2015, 49, 1382–1388. [Google Scholar] [CrossRef]
- Naqvi, U.; Sherman, A.L. Muscle Strength Grading. 2020 Sep 3. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Charalambous, C.P. Measurement of lower extremity kinematics during level walking. In Classic Papers in Orthopaedics; Banaszkiewicz, P., Kader, D., Eds.; Springer: London, UK, 2014; pp. 397–398. [Google Scholar]
- Baker, R.; McGinley, J.L.; Schwartz, M.; Thomason, P.; Rodda, J.; Graham, H.K. The minimal clinically important difference for the Gait Profile Score. Gait Posture 2012, 35, 612–615. [Google Scholar] [CrossRef]
- Liang, B.W.; Wu, W.H.; Meijer, O.G.; Lin, J.H.; Lv, G.R.; Lin, X.C.; Prins, M.R.; Hu, H.; van Dieën, J.H.; Bruijn, S.M. Pelvic step: The contribution of horizontal pelvis rotation to step length in young healthy adults walking on a treadmill. Gait Posture 2014, 39, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Louw, M.; Deary, C. The biomechanical variables involved in the aetiology of iliotibial band syndrome in distance runners—A systematic review of the literature. Phys. Ther. Sport 2014, 15, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Bazett-Jones, D.M.; Cobb, S.C.; Huddleston, W.E.; O’Connor, K.M.; Armstrong, B.S.R.; Earl-Boehm, J.E. Effect of patellofemoral pain on strength and mechanics after an exhaustive run. Med. Sci. Sports Exerc. 2013, 45, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Mei, Q.; Gu, Y.; Xiang, L.; Baker, J.S.; Fernandez, J. Foot pronation contributes to altered lower extremity loading after long distance running. Front. Physiol. 2019, 10, 573. [Google Scholar] [CrossRef] [PubMed]
- Okunuki, T.; Koshino, Y.; Yamanaka, M.; Tsutsumi, K.; Igarashi, M.; Samukawa, M.; Saitoh, H.; Tohyama, H. Forefoot and hindfoot kinematics in subjects with medial tibial stress syndrome during walking and running. J. Orthop. Res. 2019, 37, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.; Younis, A.; MacRae, S. Is there a correlation in frontal plane knee kinematics between running and performing a single leg squat in runners with patellofemoral pain syndrome and asymptomatic runners? Clin. Biomech. 2019, 61, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.M.; Zifchock, R.A.; Hillstrom, H.J. The effects of limb dominance and fatigue on running biomechanics. Gait Posture 2014, 39, 915–919. [Google Scholar] [CrossRef]
- Ceyssens, L.; Vanelderen, R.; Barton, C.; Malliaras, P.; Dingenen, B. Biomechanical risk factors associated with running-related injuries: A systematic review. Sports Med. 2019, 49, 1095–1115. [Google Scholar] [CrossRef] [Green Version]
| N = 30 | Bilateral | Left | Right | Sex (F/M)_ | |
|---|---|---|---|---|---|
| PFPS | 12 | 4 | 4 | 4 | F = 4; M = 8 |
| ITBS | 10 | - | 5 | 5 | F = 5; M = 5 |
| MTSS | 8 | 8 | - | - | F = 3; M = 5 |
| GDI | ||||
|---|---|---|---|---|
| Non-Injured [SD] (Degrees) | Injured [SD] (Degrees) | p-Value | ||
| Hip flexion/extension | Left | 5.3 [1.9] | 6.8 [2.6] | 0.141 |
| Right | 5.24 [1.2] | 6.5 [2.3] | 0.130 | |
| Knee flexion/extension | Left | 7.8 [2.4] | 9.5 [2.9] | 0.255 |
| Right | 6.3 [2.9] | 9.7 [3.4] | 0.008 ** | |
| Ankle dorsi/plantar flexion | Left | 5.0 [3.5] | 9.3 [2.9] | 0.000 ** |
| Right | 7.9 [1.9] | 9.4 [3.4] | 0.181 | |
| Pelvis up/down | Left | 2.2 [0.9] | 2.4 [1.0] | 0.500 |
| Right | 2.2 [0.9] | 2.5 [1.1] | 0.433 | |
| Hip abduction/adduction | Left | 2.9 [1.0] | 4.4 [2.0] | 0.044 * |
| Right | 3.1 [1.3] | 4 [2.0] | 0.229 | |
| Pelvis anterior/posterior | Overall | 4.9 [1.6] | 4.3 [2.8] | 0.175 |
| Pelvic internal/external | Left | 1.9 [0.8] | 3.3 [1.4] | 0.003 |
| Right | 2.7 [1.0] | 3.9 [1.7] | 0.021 | |
| Hip internal/external | Left | 4.9 [1.5] | 7.7 [3.7] | 0.019 * |
| Right | 5.0 [2.6] | 7.7 [4.2] | 0.008 ** | |
| Foot internal/external | Left | 8.5 [3.1] | 8.1 [3.9] | 0.634 |
| Right | 5. [1.8] | 10.6 [4.9] | 0.000 *** | |
| GPS | ||||
| Left | 5.5 [0.8] | 7.1 [1.6] | 0.003 ** | |
| Right | 5.3 [0.8] | 7.5 [1.6] | 0.000 *** | |
| Overall | 5.7 [0.8] | 7.8 [1.6] | 0.000 *** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khamis, S.; Gurel, R.; Arad, M.; Danino, B. Identifying Running Deviations in Long Distance Runners Utilizing Gait Profile Analysis: A Case Control Study. Appl. Sci. 2021, 11, 10898. https://doi.org/10.3390/app112210898
Khamis S, Gurel R, Arad M, Danino B. Identifying Running Deviations in Long Distance Runners Utilizing Gait Profile Analysis: A Case Control Study. Applied Sciences. 2021; 11(22):10898. https://doi.org/10.3390/app112210898
Chicago/Turabian StyleKhamis, Sam, Ron Gurel, Moran Arad, and Barry Danino. 2021. "Identifying Running Deviations in Long Distance Runners Utilizing Gait Profile Analysis: A Case Control Study" Applied Sciences 11, no. 22: 10898. https://doi.org/10.3390/app112210898
APA StyleKhamis, S., Gurel, R., Arad, M., & Danino, B. (2021). Identifying Running Deviations in Long Distance Runners Utilizing Gait Profile Analysis: A Case Control Study. Applied Sciences, 11(22), 10898. https://doi.org/10.3390/app112210898






