Bioactivity Potential of Industrial Sunflower Meal Ethanol-Wash Solute Obtained as Waste from Protein Isolation Process
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Sunflower Meal Ethanol Wash Solute (SEWS)
2.2. Chemical Analyzes
2.3. Gas Chromatography-Mass Spectrometry (GC–MS) Analyzes of SEWS
2.4. Determination of Antioxidant Activity
2.5. Determination of Antimicrobial Activity
2.5.1. Test Microorganisms and Culture Media
2.5.2. Antimicrobial Assay
2.6. Statistical Analyzes
3. Results and Discussion
3.1. Preparation and Chemical Characterization of SEWS
3.2. Antioxidant Properties of SEWS
3.3. Antimicrobial Activity of SEWS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adeleke, B.S.; Babalola, O.O. Oilseed crop sunflower (Helianthus annuus) as a source of food: Nutritional and health benefits. Food Sci. Nutr. 2020, 8, 4666–4684. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, W.; Lai, Y.; Zhang, B.; Zhang, D. Edible plant oil: Global status, health issues, and perspectives. Front. Plant Sci. 2020, 11, 1315. [Google Scholar] [CrossRef]
- Yegorov, B.; Turpurova, T.; Sharabaeva, E.; Bondar, Y. Prospects of using by-products of sunflower oil production in compound feed industry. Food Sci. Technol. 2019, 13, 106–113. [Google Scholar] [CrossRef]
- Ancuța, P.; Sonia, A. Oil press-cakes and meals valorization through circular economy approaches: A review. Appl. Sci. 2020, 10, 7432. [Google Scholar] [CrossRef]
- Lević, J.D.; Sredanović, S.A.; Đuragić, O.M. Sunflower meal protein as a feed for broilers. Acta Period. Technol. 2005, 36, 3–10. [Google Scholar] [CrossRef]
- de Oliveira Filho, J.G.; Egea, M.B. Sunflower seed byproduct and its fractions for food application: An attempt to improve the sustainability of the oil process. J. Food Sci. 2021, 86, 1497–1510. [Google Scholar] [CrossRef]
- Baiano, A. Recovery of biomolecules from food wastes—A review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhou, M.; Liu, M. A survey of nutrients and toxic factors in commercial rapeseed meal in China and evaluation of detoxificationby water extraction. Anim. Feed Sci. Technol. 1994, 45, 257–270. [Google Scholar] [CrossRef]
- Laguna, O.; Guyot, S.; Yu, X.; Broudiscou, L.P.; Chapoutot, P.; Solé-Jamault, V.; Anton, M.; Quinsac, A.; Sicaire, A.G.; Fine, F.; et al. The PHENOLEO project or how to separate and add-value to phenolic compounds present in rapeseed and sunflower meals. OCL 2020, 27, 61. [Google Scholar] [CrossRef]
- Kalaydzhiev, H.; Ivanova, P.; Stoyanova, M.; Pavlov, A.; Rustad, T.; Silva, C.L.; Chalova, V.I. Valorization of rapeseed meal: Influence of ethanol antinutrients removal on protein extractability, amino acid composition and fractional profile. Waste Biomass Valor. 2020, 11, 2709–2719. [Google Scholar] [CrossRef]
- Kalaydzhiev, H.; Georgiev, R.; Ivanova, P.; Stoyanova, M.; Silva, C.L.; Chalova, V.I. Enhanced solubility of rapeseed meal protein isolates prepared by sequential isoelectric precipitation. Foods 2020, 9, 703. [Google Scholar] [CrossRef]
- Georgiev, R.; Ivanov, I.G.; Ivanova, P.; Tumbarski, Y.; Kalaydzhiev, H.; Dincheva, I.N.; Badjakov, I.K.; Chalova, V.I. Phytochemical profile and bioactivity of industrial rapeseed meal ethanol-wash solutes. Waste Biomass Valor. 2021, 12, 5051–5063. [Google Scholar] [CrossRef]
- Gandova, V.; Ivanova, P.; Kalaydzhiev, H.; Perifanova-Nemska, M.; Chalova, V.I. Dissolution and surface tension properties of ethanol-wash solute obtained from industrial sunflower meal. Biointerface Res. Appl. Chem. 2021, 11, 11284–11292. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Hill, J.B.; Kessler, G. An automated determination of glucose utilizing a glucose oxidase-peroxidase system. J. Lab. Clin. Med. 1961, 57, 970–980. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M. A rapid and sensitive for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- International Association for Cereal Science and Technology. 104/1: Determination of Ash in Cereals and Cereal Products. Approved 1960, Revised 1990; International Association for Cereal Science and Technology: Vienna, Austria, 1990. [Google Scholar]
- Petkova, N.; Ivanov, I.; Denev, P.; Pavlov, A. Bioactive substance and free radical scavenging activities of flour from Jerusalem artichoke (Helianthus tuberosus L.) tubers—A comparative study. Turk. J. Agric. Nat. Sci. 2014, 1, 1773–1778. [Google Scholar]
- Kivrak, I.; Duru, M.E.; Öztürk, M.; Mercan, N.; Harmandar, M.; Topçu, G. Antioxidant, anticholinesterase and antimicrobial constituents from the essential oil and ethanol extract of Salvia Potentillifolia. Food Chem. 2009, 116, 470–479. [Google Scholar] [CrossRef]
- ISO 11885:2007. Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). Available online: http://www.iso.org/iso/catalogue_detail.htm?csnumber=36250 (accessed on 6 January 2019).
- BDS 11374. Available online: https://bds-bg.org/ (accessed on 16 February 2019).
- Roessner, U.; Wagner, C.; Kopka, J.; Trethewey, R.N.; Willmitzer, L. Simultaneous analysis of metabolites in potato tuber by gas chromatography-mass spectrometry. Plant J. 2000, 23, 131–142. [Google Scholar] [CrossRef]
- Ivanov, I.; Vrancheva, R.Z.; Marchev, A.S.; Petkova, N.T.; Aneva, I.Y.; Denev, P.P.; Georgieva, V.G.; Pavlov, A.I. Antioxidant activities and phenolic compounds in Bulgarian Fumaria species. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 296–306. [Google Scholar]
- Irshad, M.; Zafaryab, M.; Singh, M.; Rizvi, M. Comparative analysis of the antioxidant activity of Cassia fistula extracts. Int. J. Med. Chem. 2012, 2012, 15712. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Nagata, T.; Fan, X. Antioxidative activities in some common seaweeds. Plant Foods Hum. Nutr. 1998, 52, 253–262. [Google Scholar] [CrossRef]
- Roy, S.; Hazra, B.; Mandal, N.; Chaudhuri, T.K. Assessment of the antioxidant and free radical scavenging activities of methanolic extract of Diplazium esculentum. Int. J. Food Prop. 2013, 16, 1351–1370. [Google Scholar] [CrossRef] [Green Version]
- Aljohi, A.; Matou-Nasri, S.; Ahmed, N. Antiglycation and antioxidant properties of Momordica charantia. PLoS ONE 2016, 11, e0159985. [Google Scholar] [CrossRef]
- Tumbarski, Y.; Deseva, I.; Mihaylova, D.; Stoyanova, M.; Krastev, L.; Nikolova, R.; Yanakieva, V.; Ivanov, I. Isolation, characterization and amino acid composition of a bacteriocin produced by Bacillus methylotrophicus strain BM47. Food Technol. Biotechnol. 2018, 56, 546–552. [Google Scholar] [CrossRef]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef]
- Singh, Y.; Farooq, A.; Raza, A.; Mahmood, M.A.; Jain, S. Sustainability of a non-edible vegetable oil-based bio-lubricants for automotive applications: A review. Process. Saf. Environ. Prot. 2017, 111, 701–713. [Google Scholar] [CrossRef]
- Bai, Y.; Zhai, Y.; Ji, C.; Zhang, T.; Chen, W.; Shen, X.; Hong, J. Environmental sustainability challenges of China’s edible vegetable oil industry: From farm to factory. Resour. Conserv. Recycl. 2021, 170, 105606. [Google Scholar] [CrossRef]
- Baümler, E.R.; Carrín, M.E.; Carelli, A.A. Extraction of sunflower oil using ethanol as solvent. J. Food Eng. 2016, 178, 190–197. [Google Scholar] [CrossRef]
- Balto, A.S.; Lapis, T.J.; Silver, R.K.; Ferreira, A.J.; Beaudry, C.M.; Lim, J.; Penner, M.H. On the use of differential solubility in aqueous ethanol solutions to narrow the DP range of food-grade starch hydrolysis products. Food Chem. 2016, 197, 872–880. [Google Scholar] [CrossRef] [Green Version]
- Nyyssölä, A.; Ellilä, S.; Nordlund, E.; Poutanen, K. Reduction of FODMAP content by bioprocessing. Trends Food Sci. Technol. 2020, 99, 257–272. [Google Scholar] [CrossRef]
- Bonestroo, M.H.; Kusters, B.J.M.; De Wit, J.C.; Rombouts, F.M. Glucose and sucrose fermenting capacity of homofermentative lactic acid bacteria used as starters in fermented salads. Int. J. Food Microbiol. 1992, 15, 365–376. [Google Scholar] [CrossRef]
- Naher, U.A.; Radziah, O.; Halimi, M.S.; Shamsuddin, Z.H.; Razi, I.M. Specific growth rate and carbon sugar consumption of diazotrophs isolated from rice rhizosphere. J. Biol. Sci. 2008, 8, 1008–1014. [Google Scholar] [CrossRef] [Green Version]
- Molina-Ramírez, C.; Castro, M.; Osorio, M.; Torres-Taborda, M.; Gómez, B.; Zuluaga, R.; Gómez, C.; Gañán, P.; Rojas, O.J.; Castro, C. Effect of different carbon sources on bacterial nanocellulose production and structure using the low pH resistant strain Komagataeibacter medellinensis. Materials 2017, 10, 639. [Google Scholar] [CrossRef]
- Gao, M.; Ploessl, D.; Shao, Z. Enhancing the co-utilization of biomass-derived mixed sugars by yeasts. Front. Microbiol. 2019, 9, 3264. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Hassan, S.E.-D.; Alrefaey, H.M.A.; Elsakhawy, T. Efficient co-utilization of biomass-derived mixed sugars for lactic acid production by Bacillus coagulans Azu-10. Fermentation 2021, 7, 28. [Google Scholar] [CrossRef]
- Msomi, N.Z.; Erukainure, O.L.; Islam, M. Suitability of sugar alcohols as antidiabetic supplements: A review. J. Food Drug Anal. 2021, 29, 1–14. [Google Scholar] [CrossRef]
- Ibrahim, O.O. Sugars alcohols: Chemical structures, manufacturing, properties and applications. EC Nutr. 2016, 6, 817–824. [Google Scholar] [CrossRef]
- Wölnerhanssen, B.K.; Meyer-Gerspach, A.C.; Beglinger, C.; Islam, M.S. Metabolic effects of the natural sweeteners xylitol and erythritol: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1986–1998. [Google Scholar] [CrossRef]
- Rice, T.; Zannini, E.; Arendt, E.K.; Coffey, A. A review of polyols–biotechnological production, food applications, regulation, labeling and health effects. Crit. Rev. Food Sci. Nutr. 2020, 60, 2034–2051. [Google Scholar] [CrossRef]
- Kõljalg, S.; Smidt, I.; Chakrabarti, A.; Bosscher, D.; Mändar, R. Exploration of singular and synergistic effect of xylitol and erythritol on causative agents of dental caries. Sci. Rep. 2020, 10, 6297. [Google Scholar] [CrossRef]
- Citeau, M.; Regis, J.; Carré, P.; Fine, F. Value of hydroalcoholic treatment of rapeseed for oil extraction and protein enrichment. OCL 2019, 26, 1. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, L.M.; Fernández, M.B.; Pérez, E.E.; Crapiste, G.H. Performance of green solvents in the extraction of sunflower oil from enzyme-treated collets. Eur. J. Lipid Sci. Technol. 2021, 123, 2000132. [Google Scholar] [CrossRef]
- Harun, M. Fatty acid composition of sunflower in 31 inbreed and 28 hybrids. Biomed. J. Sci. Tech. Res. 2019, 16, 12032–12038. [Google Scholar] [CrossRef]
- Virsangbhai, C.K.; Goyal, A.; Tanwar, B.; Sihag, M.K. Potential health benefits of conjugated linoleic acid: An important functional dairy ingredient. Eur. J. Nutr. Food Saf. 2020, 11, 200–213. [Google Scholar] [CrossRef]
- Khanra, A.; Srivastava, M.; Rai, M.P.; Prakash, R. Application of unsaturated fatty acid molecules derived from microalgae toward mild steel corrosion inhibition in HCl solution: A novel approach for metal–inhibitor association. ACS Omega 2018, 3, 12369–12382. [Google Scholar] [CrossRef] [Green Version]
- Hassabo, A.G.; Sharaawy, S.; Mohamed, A.L. Unsaturated fatty acids based materials as auxiliaries for printing and finishing of cellulosic fabrics. Biointerface Res. Appl. Chem. 2019, 9, 4284–4291. [Google Scholar] [CrossRef]
- Žilić, S.; Barać, M.; Pešić, M.; Crevar, M.; Stanojević, S.; Nišavić, A.; Saratlić, G.; Tolimir, M. Characterization of sunflower seed and kernel proteins. Helia 2010, 33, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Holeček, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taha, F.S.; Mohamed, G.F.; Mohamed, S.H.; Mohamed, S.S.; Kamil, M.M. Optimization of the extraction of total phenolic compounds from sunflower meal and evaluation of the bioactivities of chosen extracts. Am. J. Food Technol. 2011, 6, 1002–1020. [Google Scholar] [CrossRef]
- Laguna, O.; Odinot, E.; Bisotto, A.; Barea, B.; Villeneuve, P.; Sigoillot, J.C.; Record, E.; Faulds, C.B.; Fine, F.; Lesage-Meessen, L.; et al. Release of phenolic acids from sunflower and rapeseed meals using different carboxylic esters hydrolases from Aspergillus niger. Ind. Crops Prod. 2019, 139, 111579. [Google Scholar] [CrossRef]
- Jia, W.; Kyriakopoulou, K.; Roelofs, B.; Ndiaye, M.; Vincken, J.P.; Keppler, J.K.; van Der Goot, A.J. Removal of phenolic compounds from de-oiled sunflower kernels by aqueous ethanol washing. Food Chem. 2021, 362, 130204. [Google Scholar] [CrossRef] [PubMed]
- Selamoglu, Z.; Sevindik, M.; Bal, C.; Ozaltun, B.; Sen, I.; Pasdaran, A. Antioxidant, antimicrobial and DNA protection activities of phenolic content of Tricholoma virgatum (Fr.) P. Kumm. Biointerface Res. Appl. Chem. 2020, 10, 5500–5506. [Google Scholar] [CrossRef]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of chlorogenic acid on regulating glucose and lipids metabolism: A review. Evid. Based Complement. Alternat. Med. 2013, 2013, 801457. [Google Scholar] [CrossRef]
- Govindharaj, D.; Nachimuthu, S.; Gonsalves, D.F.; Kothandan, R.; Dhurai, B.; Rajamani, L.; Ramakrishana, S. Molecular docking analysis of chlorogenic acid against matrix metalloproteinases (MMPs). Biointerface Res. Appl. Chem. 2020, 10, 6865–6873. [Google Scholar] [CrossRef]
- Drinceanu, D.; Luca, I.; Julean, C.; Ştef, L.; Mihai, A.; Simiz, E.; Gherasim, V.; Sofian, D. Mineral content of the main feed ingredients used in poultry biological farms. Sci. Papers Anim. Sci. Biotech. 2010, 43, 42–46. [Google Scholar]
- Chalova, V.I.; Ricke, S.C. Organic animal nutrition and feed supplementations. In Organic Meat Production and Processing; Ricke, S.C., van Loo, E.J., Johnson, M.G., O’Bryan, C.A., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2012; pp. 157–1758. ISBN 9780813821269. [Google Scholar] [CrossRef]
- Ligas, B.; Izydorczyk, G.; Mikula, K.; Skrzypczak, D.; Konkol, D.; Korczyński, M.; Witek-Krowiak, A.; Chojnacka, K. Valorization of post-extraction residues-analysis of the influence of new feed additives with micronutrients on eggs quality parameters. Poult. Sci. 2021, 100, 101416. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. Int. Sch. Res. Notices 2011, 2011, 402647. [Google Scholar] [CrossRef] [Green Version]
- D’Amato, R.; Regni, L.; Falcinelli, B.; Mattioli, S.; Benincasa, P.; Dal Bosco, A.; Pacheco, P.; Proietti, P.; Troni, E.; Santi, C.; et al. Current knowledge on selenium biofortification to improve the nutraceutical profile of food: A comprehensive review. J. Agric. Food Chem. 2020, 68, 4075–4097. [Google Scholar] [CrossRef]
- Kipp, A.P.; Strohm, D.; Brigelius-Flohé, R.; Schomburg, L.; Bechthold, A.E.; Leschik-Bonnet, E.; Heseker, H. Revised reference values for selenium intake. J. Trace Elem. Med. Biol. 2015, 32, 195–199. [Google Scholar] [CrossRef] [Green Version]
- Kebede, M.; Admassu, S. Application of antioxidants in food processing industry: Options to improve the extraction yields and market value of natural products. Adv. Food Tech. Nutr. Sci. 2019, 5, 38–49. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cisneros-Yupanqui, M.; Chalova, V.I.; Kalaydzhiev, H.R.; Mihaylova, D.; Krastanov, A.I.; Lante, A. Preliminary characterisation of wastes generated from the rapeseed and sunflower protein isolation process and their valorisation in delaying oil oxidation. Food Bioprocess Technol. 2021, 14, 1962–1971. [Google Scholar] [CrossRef]
- Klotz, L.O.; Kröncke, K.D.; Buchczyk, D.P.; Sies, H. Role of copper, zinc, selenium and tellurium in the cellular defense against oxidative and nitrosative stress. J. Nutr. 2003, 133, 1448S–1451S. [Google Scholar] [CrossRef] [PubMed]
- Wacewicz, M.; Socha, K.; Soroczyńska, J.; Niczyporuk, M.; Aleksiejczuk, P.; Ostrowska, J.; Borawska, M.H. Selenium, zinc, copper, Cu/Zn ratio and total antioxidant status in the serum of vitiligo patients treated by narrow-band ultraviolet-B phototherapy. J. Dermatol. Treat. 2018, 29, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Socha, K.; Klimiuk, K.; Naliwajko, S.K.; Soroczyńska, J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Kochanowicz, J. Dietary habits, selenium, copper, zinc and total antioxidant status in serum in relation to cognitive functions of patients with Alzheimer’s disease. Nutrients 2021, 13, 287. [Google Scholar] [CrossRef]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Yu, L. Effects of extraction solvent on wheat bran antioxidant activity estimation. Lebensm. Wiss. Technol. 2004, 37, 717–721. [Google Scholar] [CrossRef]
- Kerchev, P.; Ivanov, S. Influence of extraction techniques and solvents on the antioxidant capacity of plant material. Biotechnol. Biotechnol. Equip. 2008, 22, 556–559. [Google Scholar] [CrossRef] [Green Version]
- Center for Food Safety and Applied Nutrition (CFSAN). 2007. Available online: https://www.fda.gov/about-fda/fda-organization/center-food-safety-and-applied-nutrition-cfsan (accessed on 18 January 2008).
- Merghem, M.; Dahamna, S.; Khennouf, S. In vivo antioxidant activity of Ruta montana L. Extracts J. Mater. Environ. Sci. 2019, 10, 470–477. [Google Scholar]
- Moretton, C.; Gouttefangeas, C.; Dubois, C.; Tessier, F.J.; Fradin, C.; Prost-Camus, E.; Prost, M.; Haumont, M.; Nigay, H. Investigation of the antioxidant capacity of caramels: Combination of laboratory assays and C. elegans model. J. Funct. Foods 2021, 78, 104308. [Google Scholar] [CrossRef]
- Osdaghi, E.; Young, A.J.; Harveson, R.M. Bacterial wilt of dry beans caused by Curtobacterium flaccumfaciens pv. flaccumfaciens: A new threat from an old enemy. Mol. Plant Pathol. 2020, 21, 605–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, S.; Wu, C.; Wu, T.; Yu, H.; Yang, S.; Hu, Y. Preparation and characterisation of chlorogenic acid-gelatin: A type of biologically active film for coating preservation. Food Chem. 2017, 221, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Alexandrino, T.D.; da Silva, M.G.; Ferrari, R.A.; Ruiz, A.L.T.G.; Duarte, R.M.T.; Simabuco, F.M.; Bezerra, R.M.N.; Pacheco, M.T.B. Evaluation of some in vitro bioactivities of sunflower phenolic compounds. Curr. Res. Nutr. Food Sci. 2021, 4, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Bonev, B.; Hooper, J.; Parisot, J. Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. J. Antimicrob. Chemother. 2008, 61, 1295–1301. [Google Scholar] [CrossRef] [Green Version]
- Valgas, C.; Souza, S.M.D.; Smânia, E.F.; Smânia, A., Jr. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar] [CrossRef] [Green Version]
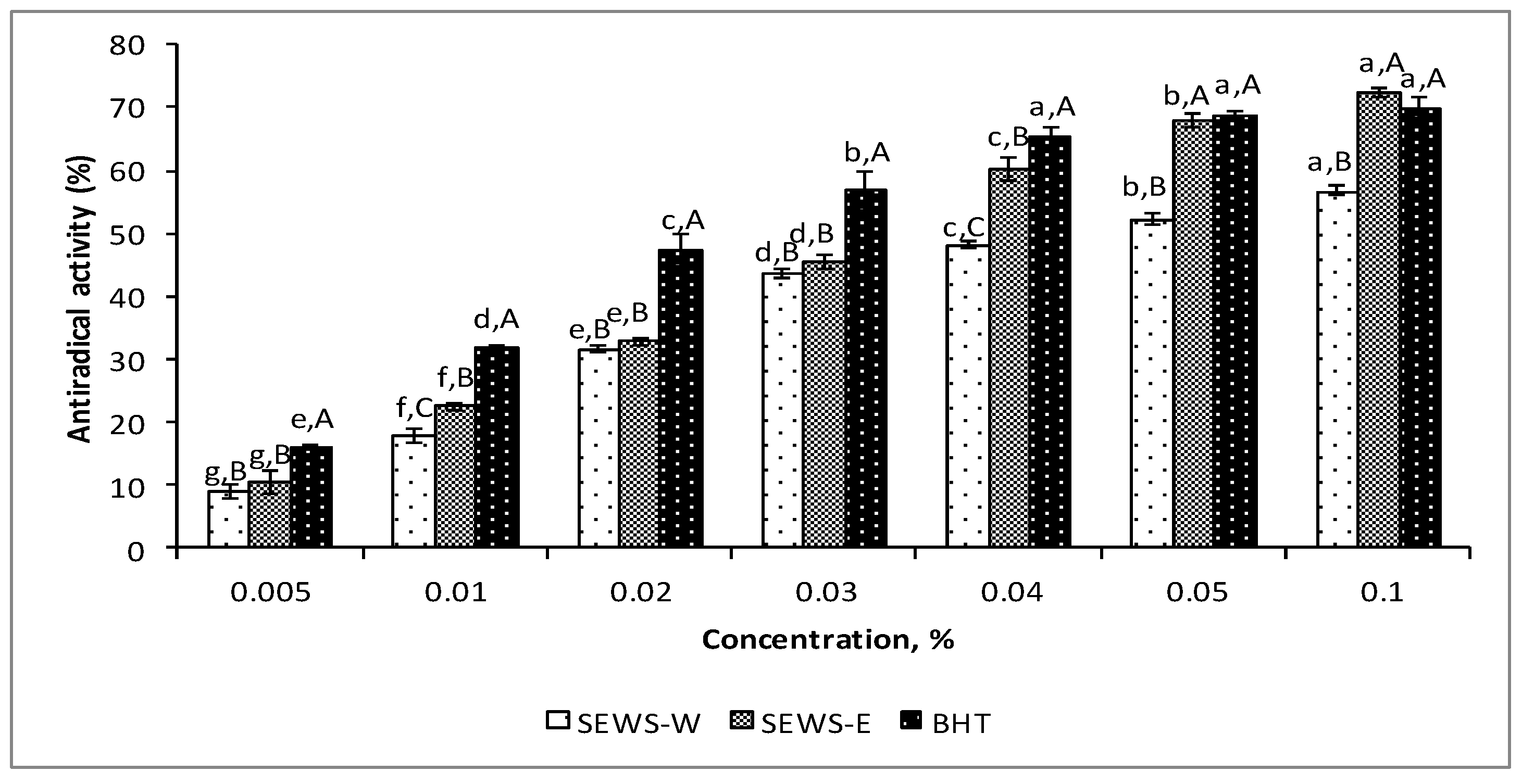

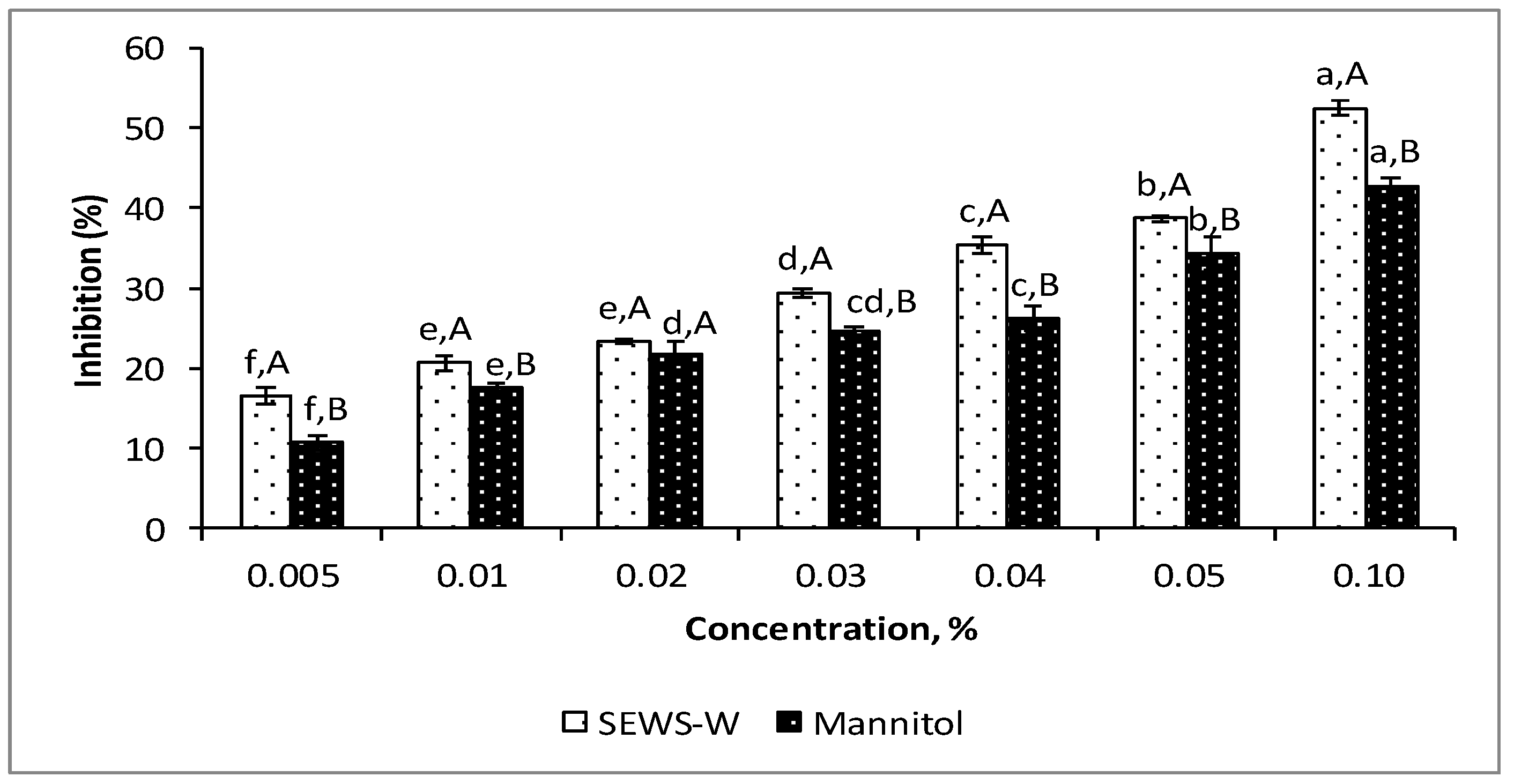
| Component | Content, % |
|---|---|
| Total carbohydrates | 62.14 ± 2.87 |
| Glucose | 3.18 ± 0.09 |
| Total lipids | 7.73 ± 0.18 |
| Protein | 1.99 ± 0.08 |
| Ash | 6.33 ± 0.16 |
| Total phenols | 16.38 ± 0.55 |
| Total flavonoids | 4.41 ± 0.17 |
| Compound | RT | RI | TIC, % | |
|---|---|---|---|---|
| A: Polar compounds | ||||
| Amino acids | ||||
| 1 | Alanine | 4.69 | 1097 | 0.64 |
| 2 | Valine | 4.90 | 1208 | 0.78 |
| 3 | Leucine | 6.03 | 1266 | 1.16 |
| 4 | Isoleucine | 6.23 | 1285 | 1.40 |
| 5 | Proline | 6.30 | 1293 | 0.97 |
| 6 | Glycine | 6.35 | 1299 | 0.84 |
| 7 | Serine | 6.65 | 1351 | 0.39 |
| 8 | Threonine | 6.78 | 1376 | 0.70 |
| 9 | Aspartic acid | 8.19 | 1509 | 1.52 |
| 10 | Methionine | 8.27 | 1512 | 0.45 |
| 11 | Glutamic acid | 10.60 | 1608 | 1.43 |
| 12 | Phenylalanine | 10.68 | 1635 | 0.89 |
| 13 | Asparagine | 10.74 | 1659 | 0.26 |
| 14 | Glutamine | 1160 | 1765 | 1.30 |
| 15 | Lysine | 13.38 | 1911 | 0.75 |
| 16 | Tyrosine | 13.52 | 1930 | 0.21 |
| 17 | Histidine | 19.55 | 2145 | 0.39 |
| Organic acids | ||||
| 18 | Succinic acid | 6.40 | 1305 | 1.12 |
| 19 | Fumaric acid | 6.61 | 1344 | 0.49 |
| 20 | Malic acid | 8.00 | 1477 | 0.86 |
| 21 | Pyroglutamic acid | 8.88 | 1518 | 4.31 |
| Sugar acids and alcohols | ||||
| 22 | Glycerol | 5.95 | 1264 | 4.77 |
| 23 | Glyceric acid | 6.52 | 1341 | 3.01 |
| 24 | Threitol | 8.61 | 1488 | 1.35 |
| 25 | Erythreol | 9.03 | 1493 | 0.37 |
| 26 | Threonic acid | 10.55 | 1569 | 1.48 |
| 27 | Xylitol | 10.80 | 1690 | 3.24 |
| 28 | Arabitol | 10.89 | 1699 | 4.11 |
| 29 | Ribonic acid | 11.67 | 1822 | 2.17 |
| 30 | Glucitol | 14.92 | 1942 | 5.34 |
| 31 | Dulcitol | 15.15 | 1961 | 2.31 |
| 32 | Gluconic acid | 16.04 | 1996 | 0.86 |
| 33 | Myo-inositol | 16.64 | 2090 | 2.18 |
| 34 | Galactinol | 28.72 | 2951 | 1.80 |
| Saccharides (mono-, di-) | ||||
| 35 | Xylose | 10.60 | 1661 | 1.05 |
| 36 | Arabinose | 10.68 | 1673 | 1.82 |
| 37 | Fructose | 12.03 | 1856 | 5.86 |
| 38 | Galactose | 14.57 | 1876 | 6.66 |
| 39 | Glucose | 14.62 | 1881 | 5.41 |
| 40 | Sucrose | 23.69 | 2620 | 14.01 |
| 41 | Melibiose | 25.36 | 2846 | 9.93 |
| B: Nonpolar compounds | ||||
| Fatty acids | ||||
| 1 | Myristic acid | 12.60 | 1837 | 0.84 |
| 2 | Palmitoleic acid | 15.34 | 1905 | 0.30 |
| 3 | Palmitelaidic acid | 15.44 | 1912 | 0.73 |
| 4 | Palmitic acid | 15.79 | 1926 | 6.18 |
| 5 | Linoleic acid | 18.63 | 2096 | 12.10 |
| 6 | Oleic acid | 18.71 | 2099 | 0.14 |
| 7 | Linolenic acid | 18.79 | 2102 | 1.72 |
| 8 | Stearic acid | 19.07 | 2247 | 4.30 |
| 9 | Arachidic acid | 20.65 | 2311 | 4.86 |
| Alkenes | ||||
| 10 | n-Eicosane | 17.01 | 2000 | 0.42 |
| 11 | n-Tricosane | 20.31 | 2300 | 2.11 |
| C: Phenolic compounds | ||||
| 1 | Salicylic acid | 8.60 | 1510 | 0.64 |
| 2 | Cinnamic acid | 8.91 | 1557 | 3.23 |
| 3 | p-Hydroxybenzoic acid | 10.46 | 1642 | 2.76 |
| 4 | Vanillic acid | 11.18 | 1775 | 0.63 |
| 5 | Syringic acid | 14.10 | 1884 | 1.85 |
| 6 | Ferulic acid | 16.01 | 2103 | 0.90 |
| 7 | Caffeic acid | 17.55 | 2140 | 0.81 |
| 8 | Sinapic acid | 19.21 | 2253 | 0.74 |
| 9 | Chlorogenic acid | 31.80 | 3111 | 85.41 |
| Component | Content, mg/kg |
|---|---|
| Copper (Cu) | 109.36 |
| Iron (Fe) | 259.02 |
| Manganese (Mn) | 5.10 |
| Selenium (Se) | 0.10 |
| Zinc (Zn) | 22.11 |
| Lead (Pb) | <0.10 |
| Cadmium (Cd) | <0.01 |
| Test Microorganism | Zone of Inhibition, mm | |||||
|---|---|---|---|---|---|---|
| SEWS Concentration, mg/mL | ||||||
| 5.0 | 2.5 | 1.25 | 0.625 | 0.313 | 0.156 | |
| Gram (+) bacteria | ||||||
| Bacillus subtilis ATCC 6633 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bacillus cereus | 0 | 0 | 0 | 0 | 0 | 0 |
| Bacillus amyloliquefaciens 4BCL-YT | 8 | 0 | 0 | 0 | 0 | 0 |
| Staphylococcus aureus ATCC 25923 | 0 | 0 | 0 | 0 | 0 | 0 |
| Listeria monocytogenes ATCC 8632 | 0 | 0 | 0 | 0 | 0 | 0 |
| Enterococcus faecalis | 0 | 0 | 0 | 0 | 0 | 0 |
| Micrococcus luteus 2YC-YT | 8 | 0 | 0 | 0 | 0 | 0 |
| Curtobacterium flaccumfaciens PM-YT | 12 | 12 | 10 | 10 | 8 | 0 |
| Gram (−) bacteria | ||||||
| Salmonella enteritidis | 0 | 0 | 0 | 0 | 0 | 0 |
| Escherichia coli ATCC 8739 | 9 | 8 | 0 | 0 | 0 | 0 |
| Proteus vulgaris ATCC 6380 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pseudomonas aeruginosa ATCC 9027 | 8 | 8 | 0 | 0 | 0 | 0 |
| Fungi | ||||||
| Candida albicans NBIMCC 74 | 0 | 0 | 0 | 0 | 0 | 0 |
| Saccharomyces cerevisiae | 0 | 0 | 0 | 0 | 0 | 0 |
| Aspergillus niger ATCC 1015 | 10 | 8 | 0 | 0 | 0 | 0 |
| Aspergillus flavus | 8 | 0 | 0 | 0 | 0 | 0 |
| Fusarium moniliforme ATCC 38932 | 12 | 10 | 8 | 8 | 0 | 0 |
| Test Microorganism | Minimum Inhibitory Concentration, mg/mL |
|---|---|
| Gram (+) bacteria | |
| Bacillus subtilis ATCC 6633 | N |
| Bacillus cereus | N |
| Bacillus amyloliquefaciens 4BCL-YT | 5.0 |
| Staphylococcus aureus ATCC 25923 | N |
| Listeria monocytogenes ATCC 8632 | N |
| Enterococcus faecalis | N |
| Micrococcus luteus 2YC-YT | 5.0 |
| Curtobacterium flaccumfaciens PM-YT | 0.313 |
| Gram (−) bacteria | |
| Salmonella enteritidis | N |
| Escherichia coli ATCC 8739 | 2.5 |
| Proteus vulgaris ATCC 6380 | N |
| Pseudomonas aeruginosa ATCC 9027 | 2.5 |
| Fungi | |
| Candida albicans NBIMCC 74 | N |
| Saccharomyces cerevisiae | N |
| Aspergillus niger ATCC 1015 | 2.5 |
| Aspergillus flavus | 5.0 |
| Fusarium moniliforme ATCC 38932 | 0.625 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, P.; Ivanov, I.G.; Tumbarski, Y.; Kalaydzhiev, H.; Dincheva, I.N.; Chalova, V.I. Bioactivity Potential of Industrial Sunflower Meal Ethanol-Wash Solute Obtained as Waste from Protein Isolation Process. Appl. Sci. 2021, 11, 11007. https://doi.org/10.3390/app112211007
Ivanova P, Ivanov IG, Tumbarski Y, Kalaydzhiev H, Dincheva IN, Chalova VI. Bioactivity Potential of Industrial Sunflower Meal Ethanol-Wash Solute Obtained as Waste from Protein Isolation Process. Applied Sciences. 2021; 11(22):11007. https://doi.org/10.3390/app112211007
Chicago/Turabian StyleIvanova, Petya, Ivan G. Ivanov, Yulian Tumbarski, Hristo Kalaydzhiev, Ivayla N. Dincheva, and Vesela I. Chalova. 2021. "Bioactivity Potential of Industrial Sunflower Meal Ethanol-Wash Solute Obtained as Waste from Protein Isolation Process" Applied Sciences 11, no. 22: 11007. https://doi.org/10.3390/app112211007
APA StyleIvanova, P., Ivanov, I. G., Tumbarski, Y., Kalaydzhiev, H., Dincheva, I. N., & Chalova, V. I. (2021). Bioactivity Potential of Industrial Sunflower Meal Ethanol-Wash Solute Obtained as Waste from Protein Isolation Process. Applied Sciences, 11(22), 11007. https://doi.org/10.3390/app112211007









