Protein Binding of a Novel Platinum-Based Anticancer Agent BP-C1 Containing a Lignin-Derived Polymeric Ligand
Abstract
:1. Introduction
2. Materials and Methods
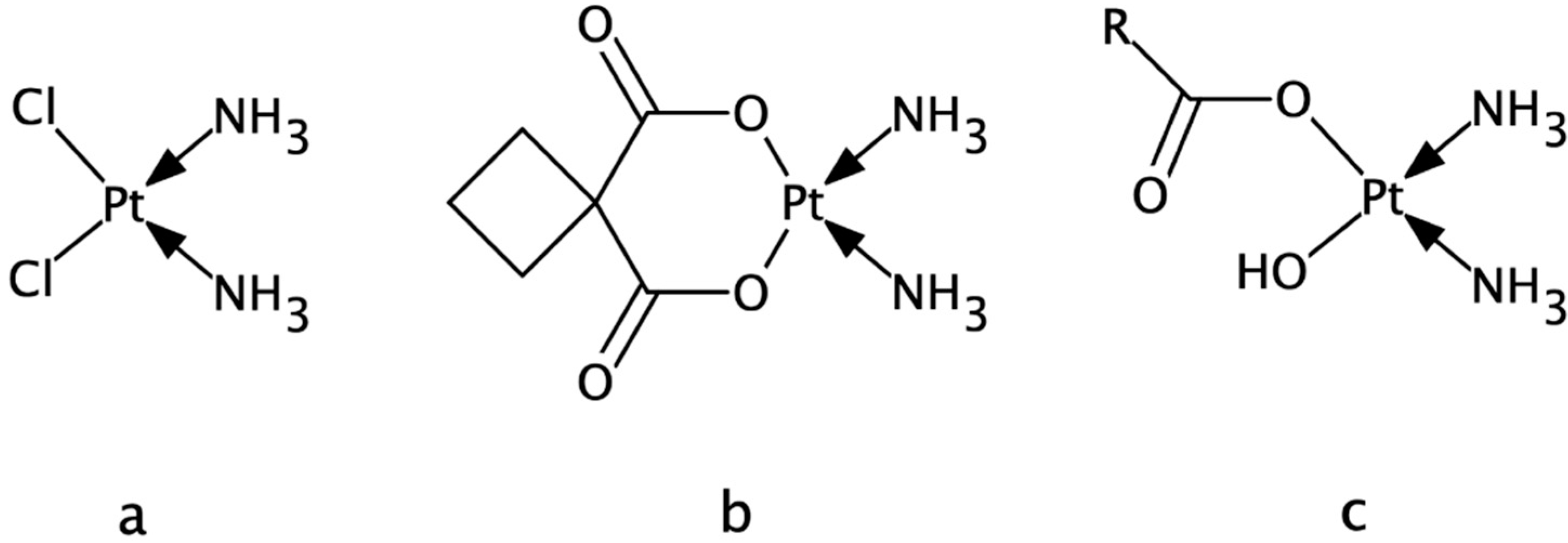
2.1. Tested Compound, Chemicals and Reagents
2.2. Equilibrium Dialysis
2.3. Platinum Quantification
2.4. Quality Assurance
2.5. Data Processing and Statistics
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michalke, B. Platinum speciation used for elucidating activation or inhibition of Pt-containing anti-cancer drugs. J. Trace Elem. Med. Biol. 2010, 24, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, B.; Vancamp, L.; Trosko, J.E.; Mansour, V.H. Platinum compounds—A new class of potent antitumour agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Cosaert, J.; Quoix, E. Platinum drugs in the treatment of non-small-cell lung cancer. Br. J. Cancer 2002, 87, 825–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccart, M.J.; Lamb, H.; Vermorken, J.B. Current and future potential roles of the platinum drugs in the treatment of ovarian cancer. Ann. Oncol. 2001, 12, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur J. Pharm. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, Y.-P.; Au-Yeung, S.C.F.; To, K.K.W. Platinum-based anticancer agents: Innovative design strategies and biological perspectives. Med. Res. Rev. 2003, 23, 633–655. [Google Scholar] [CrossRef] [PubMed]
- Solovyev, N.D.; Fedoros, E.I.; Drobyshev, E.J.; Ivanenko, N.B.; Pigarev, S.E.; Tyndyk, M.L.; Anisimov, V.N.; Vilpan, Y.A.; Panchenko, A.V. Anticancer activity and tissue distribution of platinum (II) complex with lignin-derived polymer of benzene-poly-carboxylic acids. J. Trace Elem. Med. Biol. 2017, 43, 72–79. [Google Scholar] [CrossRef]
- Fedoros, E.I.; Orlov, A.A.; Zherebker, A.; Gubareva, E.A.; Maydin, M.A.; Konstantinov, A.I.; Krasnov, K.A.; Karapetian, R.N.; Izotova, E.I.; Pigarev, S.E.; et al. Novel water-soluble lignin derivative BP-Cx-1: Identification of components and screening of potential targets in silico and in vitro. Oncotarget 2018, 9, 18578–18593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fedoros, E.I.; Baldueva, I.A.; Perminova, I.V.; Badun, G.A.; Chernysheva, M.G.; Grozdova, I.D.; Melik-Nubarov, N.S.; Danilova, A.B.; Nekhaeva, T.L.; Kuznetsova, A.I.; et al. Exploring bioactivity potential of polyphenolic water-soluble lignin derivative. Environ. Res. 2020, 191, 110049. [Google Scholar] [CrossRef]
- Butthongkomvong, K.; Raunroadroong, N.; Sorrarichingchai, S.; Sangsaikae, I.; Srimuninnimit, V.; Harling, H.; Larsen, S. Efficacy and tolerability of BP-C1 in metastatic breast cancer: A Phase II, randomized, double-blind, and placebo-controlled Thai multi-center study. Breast Cancer 2019, 11, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Lindkær-Jensen, S.; Larsen, S.; Habib-Lindkær-Jensen, N.; Efagertun, H.E. Positive effects on hematological and biochemical imbalances in patients with metastatic breast cancer stage IV, of BP-C1, a new anticancer substance. Drug Des. Devel. 2015, 9, 1481–1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheife, R.T. Protein binding: What does it mean? Dicp 1989, 23, S27–S31. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.; Jones, G.D.D.; Reid, H.J.; Shoeib, T.; Taylor, S.E.; Thomas, A.L.; Wood, J.P.; Sharp, B.L. Speciation of oxaliplatin adducts with DNA nucleotides. Metallomics 2011, 3, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, R.; Taheri-Kafrani, A.; Nabavizadeh, S.M.; Pouryasin, Z.; Shahsavani, M.B.; Khoshaman, K.; Rashidi, M. The binding assessment with human serum albumin of novel six-coordinate Pt(IV) complexes, containing bidentate nitrogen donor/methyl ligands. Mol. Biol. Res. Commun. 2015, 4, 167–179. [Google Scholar]
- Dabrowiak, J.C. Metals in Medicine; Wiley & Sons Ltd.: Weinheim, Germany, 2017. [Google Scholar]
- Larios, R.; Del Castillo Busto, M.E.; Garcia-Sar, D.; Ward-Deitrich, C.; Goenaga-Infante, H. Accurate quantification of carboplatin adducts with serum proteins by monolithic chromatography coupled to ICPMS with isotope dilution analysis. J. Anal. At. Spectrom. 2019, 34, 729–740. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The next generation of platinum drugs: Targeted pt(ii) agents, nanoparticle delivery, and Pt(IV) prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [Green Version]
- Wiglusz, K.; Trynda-Lemiesz, L. Platinum drugs binding to human serum albumin: Effect of non-steroidal anti-inflammatory drugs. J. Photochem. Photobiol. A: Chem. 2014, 289, 1–6. [Google Scholar] [CrossRef]
- Park, C.R.; Kim, H.Y.; Song, M.G.; Lee, Y.S.; Youn, H.; Chung, J.K.; Cheon, G.J.; Kang, K.W. Efficacy and Safety of Human Serum Albumin-Cisplatin Complex in U87MG Xenograft Mouse Models. Int. J. Mol. Sci. 2020, 21, 7932. [Google Scholar] [CrossRef]
- Shimada, M.; Itamochi, H.; Kigawa, J. Nedaplatin: A cisplatin derivative in cancer chemotherapy. Cancer Manag. Res. 2013, 5, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Greaves, E.D.; Angeli-Greaves, M.; Jaehde, U.; Drescher, A.; von Bohlen, A. Rapid determination of platinum plasma concentrations of chemotherapy patients using total reflection X-ray fluorescence. Spectrochim. Acta B 2006, 61, 1194–1200. [Google Scholar] [CrossRef]
- Piccolini, V.M.; Bottone, M.G.; Bottiroli, G.; De Pascali, S.A.; Fanizzi, F.P.; Bernocchi, G. Platinum drugs and neurotoxicity: Effects on intracellular calcium homeostasis. Cell Biol. Toxicol. 2013, 29, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Kireeva, G.; Kruglov, S.; Maydin, M.; Gubareva, E.; Fedoros, E.; Zubakina, E.; Ivanenko, N.; Bezruchko, M.; Solovyev, N. Modeling of Chemoperfusion vs. Intravenous Administration of Cisplatin in Wistar Rats: Adsorption and Tissue Distribution. Molecules 2020, 25, 4733. [Google Scholar] [CrossRef] [PubMed]
- Waters, N.J.; Jones, R.; Williams, G.; Sohal, B. Validation of a rapid equilibrium dialysis approach for the measurement of plasma protein binding. J. Pharm. Sci. 2008, 97, 4586–4595. [Google Scholar] [CrossRef] [PubMed]
- Navolotskii, D.V.; Ivanenko, N.B.; Solovyev, N.D.; Fedoros, E.I.; Panchenko, A.V. Pharmacokinetics and tissue distribution of novel platinum containing anticancer agent BP-C1 studied in rabbits using sector field inductively coupled plasma mass spectrometry. Drug Test. Anal. 2015, 7, 737–744. [Google Scholar] [CrossRef] [Green Version]
- Sigma-Aldrich. HPLC Analysis of Warfarin™ Anticoagulant on Ascentis® C18. Available online: https://www.sigmaaldrich.com/technical-documents/articles/analytical-applications/hplc/hplc-analysis-of-warfarin-anticoagulant-g002340.html (accessed on 4 April 2021).
- Levy, G. Protein binding of warfarin. Br. J. Clin. Pharm. 1995, 39, 211. [Google Scholar]
- Trainor, G.L. The importance of plasma protein binding in drug discovery. Expert Opin. Drug Discov. 2007, 2, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Musteata, F.M. Chapter 4—Clinical Utility of Free Drug Monitoring. In Therapeutic Drug Monitoring; Dasgupta, A., Ed.; Academic Press: Boston, MA, USA, 2012; pp. 75–101. [Google Scholar] [CrossRef]
- Tothill, P.; Matheson, L.M.; Smyth, J.F.; McKay, K. Inductively coupled plasma mass spectrometry for the determination of platinum in animal tissues and a comparison with atomic absorption spectrometry. J. Anal. At. Spectrom. 1990, 5, 619–622. [Google Scholar] [CrossRef]
- Solovyev, N.; Vinceti, M.; Grill, P.; Mandrioli, J.; Michalke, B. Redox speciation of iron, manganese, and copper in cerebrospinal fluid by strong cation exchange chromatography—Sector field inductively coupled plasma mass spectrometry. Anal. Chim. Acta 2017, 973, 25–33. [Google Scholar] [CrossRef]
- Solovyev, N.; Ala, A.; Schilsky, M.; Mills, C.; Willis, K.; Harrington, C.F. Biomedical copper speciation in relation to Wilson’s disease using strong anion exchange chromatography coupled to triple quadrupole inductively coupled plasma mass spectrometry. Anal. Chim. Acta 2020, 1098, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Vojtek, M.; Pinto, E.; Gonçalves-Monteiro, S.; Almeida, A.; Marques, M.P.M.; Mota-Filipe, H.; Ferreira, I.M.P.L.V.O.; Diniz, C. Fast and reliable ICP-MS quantification of palladium and platinum-based drugs in animal pharmacokinetic and biodistribution studies. Anal. Methods 2020, 12, 4806–4812. [Google Scholar] [CrossRef]
- Sooriyaarachchi, M.; Narendran, A.; Gailer, J. Comparative hydrolysis and plasma protein binding of cis-platin and carboplatin in human plasma in vitro. Metallomics 2011, 3, 49–55. [Google Scholar] [CrossRef] [PubMed]
- O’Dwyer, P.J.; Stevenson, J.P.; Johnson, S.W. Clinical pharmacokinetics and administration of established platinum drugs. Drugs 2000, 59, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Martinčič, A.; Cemazar, M.; Sersa, G.; Kovač, V.; Milačič, R.; Ščančar, J. A novel method for speciation of Pt in human serum incubated with cisplatin, oxaliplatin and carboplatin by conjoint liquid chromatography on monolithic disks with UV and ICP-MS detection. Talanta 2013, 116, 141–148. [Google Scholar] [CrossRef]
- Kato, R.; Sato, T.; Iwamoto, A.; Yamazaki, T.; Nakashiro, S.; Yoshikai, S.; Fujimoto, A.; Imano, H.; Ijiri, Y.; Mino, Y.; et al. Interaction of platinum agents, cisplatin, carboplatin and oxaliplatin against albumin in vivo rats and in vitro study using inductively coupled plasma-mass spectrometory. Biopharm. Drug Dispos. 2019, 40, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Fernández, D.; Verdaguer, J.M.; Ramírez-Camacho, R.; Palacios, M.A.; Gómez-Gómez, M.M. Accumulation, fractionation, and analysis of platinum in toxicologically affected tissues after cisplatin, oxaliplatin, and carboplatin administration. J. Anal. Toxicol. 2008, 32, 140–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
 ) stand for initial cell after the establishment of equilibrium; red diamonds (
) stand for initial cell after the establishment of equilibrium; red diamonds (  ) stand for target cell after crossing the dialysis membrane; green circles (
) stand for target cell after crossing the dialysis membrane; green circles (  ) stand for total Pt in both cells (mass balance). Error bars represent ± standard deviation (n = 6).
) stand for total Pt in both cells (mass balance). Error bars represent ± standard deviation (n = 6).
 ) stand for initial cell after the establishment of equilibrium; red diamonds (
) stand for initial cell after the establishment of equilibrium; red diamonds (  ) stand for target cell after crossing the dialysis membrane; green circles (
) stand for target cell after crossing the dialysis membrane; green circles (  ) stand for total Pt in both cells (mass balance). Error bars represent ± standard deviation (n = 6).
) stand for total Pt in both cells (mass balance). Error bars represent ± standard deviation (n = 6).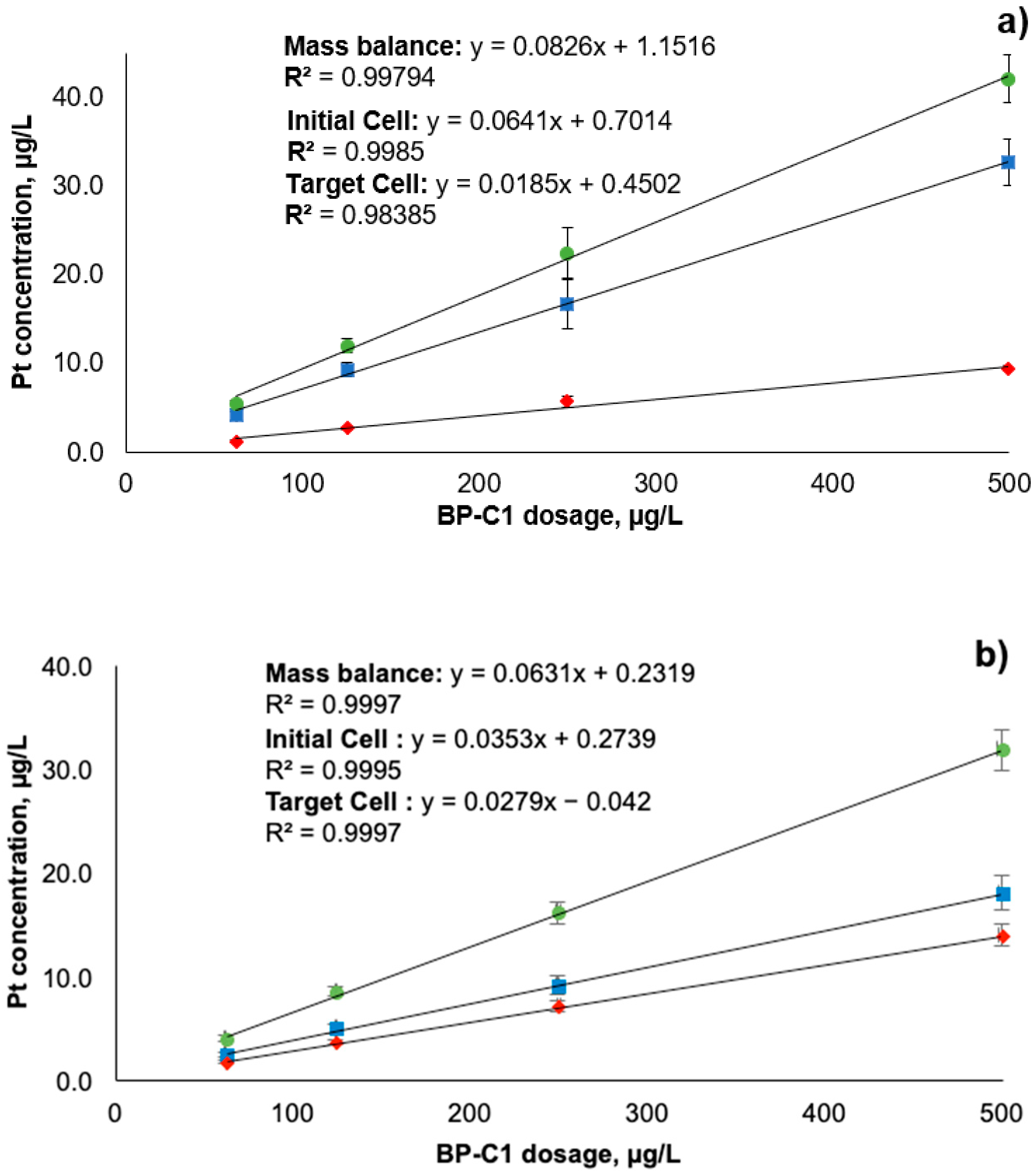
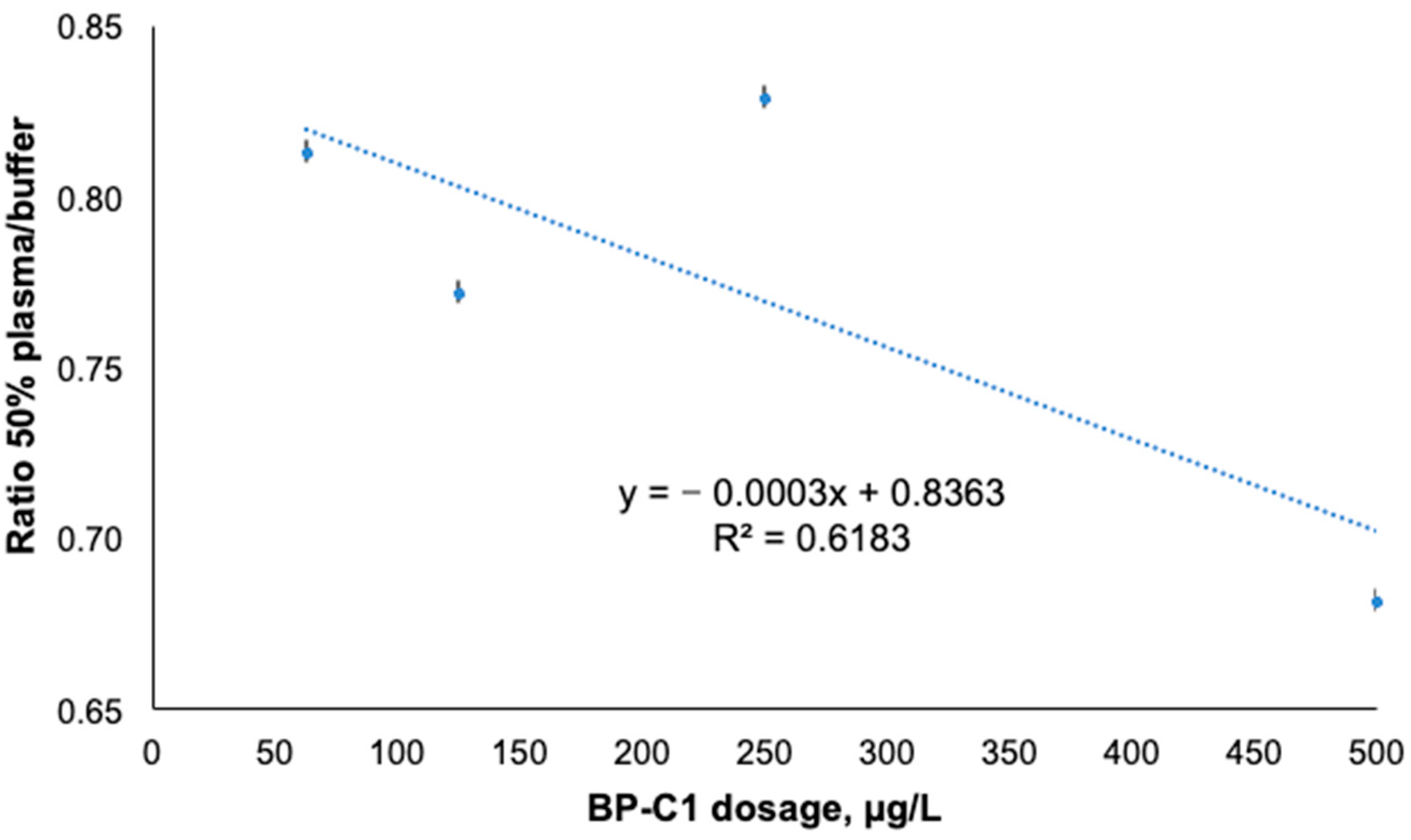
| Sample Name | Average Pt Concentration, µg/L | RSD, % | Relative Retention% | SD of Relative Retention% | p-Value * |
|---|---|---|---|---|---|
| 50% plasma + 500 µg/L BP-C1 retained fraction | 32.7 | 8.1 | 77.6 | 6.3 | 0.004 |
| 50% plasma + 500 µg/L BP-C1 dialysis fraction | 9.4 | 3.4 | |||
| Control buffer + 500 µg/L BP-C1 retained fraction | 17.9 | 9.0 | 56.4 | 5.1 | |
| Control buffer + 500 µg/L BP-C1 dialysis fraction | 13.8 | 7.2 | |||
| 50% plasma + 250 µg/L BP-C1 retained fraction | 16.7 | 1.3 | 74.4 | 1.0 | <0.001 |
| 50% plasma + 250 µg/L BP-C1 dialysis fraction | 5.8 | 10.6 | |||
| Control buffer + 250 µg/L BP-C1 retained fraction | 9.0 | 9.5 | 56.3 | 5.4 | |
| Control buffer + 250 µg/L BP-C1 dialysis fraction | 7.0 | 6.9 | |||
| 50% plasma + 125 µg/L BP-C1 retained fraction | 9.4 | 8.6 | 77.8 | 6.7 | 0.002 |
| 50% plasma + 125 µg/L BP-C1 dialysis fraction | 2.7 | 6.6 | |||
| Control buffer + 125 µg/L BP-C1 retained fraction | 4.9 | 7.9 | 58.3 | 4.6 | |
| Control buffer + 125 µg/L BP-C1 dialysis fraction | 3.5 | 4.5 | |||
| 50% plasma + 62.5 µg/L BP-C1 retained fraction | 4.2 | 11.1 | 76.1 | 8.4 | 0.008 |
| 50% plasma + 62.5 µg/L BP-C1 dialysis fraction | 1.3 | 8.9 | |||
| Control buffer + 62.5 µg/L BP-C1 retained fraction | 2.3 | 9.1 | 59.3 | 5.4 | |
| Control buffer + 62.5 µg/L BP-C1 dialysis fraction | 1.6 | 5.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedoros, E.; Pigarev, S.; Ivanenko, N.; Westbury, M.; Solovyev, N. Protein Binding of a Novel Platinum-Based Anticancer Agent BP-C1 Containing a Lignin-Derived Polymeric Ligand. Appl. Sci. 2021, 11, 11008. https://doi.org/10.3390/app112211008
Fedoros E, Pigarev S, Ivanenko N, Westbury M, Solovyev N. Protein Binding of a Novel Platinum-Based Anticancer Agent BP-C1 Containing a Lignin-Derived Polymeric Ligand. Applied Sciences. 2021; 11(22):11008. https://doi.org/10.3390/app112211008
Chicago/Turabian StyleFedoros, Elena, Sergey Pigarev, Natalya Ivanenko, Megan Westbury, and Nikolay Solovyev. 2021. "Protein Binding of a Novel Platinum-Based Anticancer Agent BP-C1 Containing a Lignin-Derived Polymeric Ligand" Applied Sciences 11, no. 22: 11008. https://doi.org/10.3390/app112211008
APA StyleFedoros, E., Pigarev, S., Ivanenko, N., Westbury, M., & Solovyev, N. (2021). Protein Binding of a Novel Platinum-Based Anticancer Agent BP-C1 Containing a Lignin-Derived Polymeric Ligand. Applied Sciences, 11(22), 11008. https://doi.org/10.3390/app112211008






