Abstract
Bioactive compounds are comprised of small quantities of extra nutritional constituents providing both health benefits and enhanced nutritional value, based on their ability to modulate one or more metabolic processes. Plant-based diets are being thoroughly researched for their cardiovascular properties and effectiveness against cancer. Flavonoids, phytoestrogens, phenolic compounds, and carotenoids are some of the bioactive compounds that aim to work in prevention and treating the cardiovascular disease in a systemic manner, including hypertension, atherosclerosis, and heart failure. Their antioxidant and anti-inflammatory properties are the most important characteristics that make them favorable candidates for CVDs treatment. However, their low water solubility and stability results in low bioavailability, limited accessibility, and poor absorption. The oral delivery of bioactive compounds is constrained due to physiological barriers such as the pH, mucus layer, gastrointestinal enzymes, epithelium, etc. The present review aims to revise the main bioactive compounds with a significant role in CVDs in terms of preventive, diagnostic, and treatment measures. The advantages of nanoformulations and novel multifunctional nanomaterials development are described in order to overcome multiple obstacles, including the physiological ones, by summarizing the most recent preclinical data and clinical trials reported in the literature. Nanotechnologies will open a new window in the area of CVDs with the opportunity to achieve effective treatment, better prognosis, and less adverse effects on non-target tissues.
1. Introduction
Cardiovascular disease (CVD) is one of the major disorders leading to death. It is mostly seen in both technologically advanced countries as well as in the evolving world, and it is accountable for high economic healthcare expenses [1]. Smoking, hypertension, hyperlipidemia, malnutrition, inadequate physical exercise, and obesity can all increase the risk of CVD. Coronary artery disease (CAD) is a condition caused by atherosclerosis as a result of the narrowing or blockage of coronary arteries. Atherosclerosis is a result of cholesterol and fatty deposits (plaques) inside the arteries.
Congestive heart failure, heart rhythm complications, congenital heart disease (heart disease that develops at birth), and endocarditis are just a few CV disorders [2,3]. The worldwide CVD deaths escalated by 21% from 2007 to 2017 because of population growth and aging; most deaths occur in low- and middle-income countries [4]. The World Health Organization (WHO) expresses that by 2030, CVD would be the major reason for around 23.3 million deaths [5]. The progress of CVD is accelerated by certain threat elements (smoking, dyslipidemia, hypertension, diabetes, and overweight). Fortunately, by adding prevention strategies, these can be avoided [6,7]. A transition to a healthier diet is a major key factor in preventing CVD and thus a shift to an ailment-free time [8].
An unhealthy diet is indeed one of the primary causes of CVD death, with about 72% of all CVD deaths [9]. Recent epidemiological data show that plant-based intakes are proficient and effective against CVD and cancer [10]. The term “plant-based diet” refers to a broad range of eating habits that include fewer animal products (such as meat and dairy products) and much more plant-source foods [11]. According to the recent literature [12], there is no consensus to describe “bioactive compound”. However, it is broadly acknowledged that these “complexes have the proficiency and aptitude with one or more parts of active tissue by predicting most of the effects” [13]. They provide a beneficial health-boosting impact and are being investigated for deferent pathologies prevention: cancer, heart disease, diabetes mellitus, etc. Lycopene, resveratrol, lignan, tannins, and indoles are only a few examples of bioactive compounds [14]. Bioactive compounds are grouped according to their biochemical configuration and functions. These compounds have been shown to have a protective nature against certain pathologies correlated with the immune system, oxidative stress, and inflammation, and they also can lower LDL cholesterol oxidation and control endothelial nitric oxide amalgamation; some of them have poor estrogenic properties [15]. These compounds might contribute significantly to minimizing the onset of age-related chronic illnesses and to control the glucose metabolic rate [16].
Abundant epidemiologic studies show that enhancing vegetable and fruit intake is linked to a reduction in the rate of CVD [17]. Considerable data indicate the protective mechanism of fruits and vegetables, nuts and seeds, whole grains, and seafood in the prevention and treatment of various cancer and heart diseases [18,19]. The ocean’s ecosystem accounts for half including all diversity in the world, rendering aquatic microbes a potential long-term reservoir of unique bioactive metabolites [20,21]. These dietary oral nutrients, when combined along with a regular meal, will help people get more of the components that are thought to have therapeutic outcomes [22]. It is well known that tea and coffee, the most consumed drinks worldwide, have certain beneficial properties. The caffeine present in the coffee seed is a purine alkaloid, i.e., 1,3,7-trimethylxanthine, with its latent properties, which are of some debated topics [23]. Meanwhile, components obtained from Allium sativum, also identified as garlic, is an herb belonging to the Alliaceae family that is frequently used as a seasoning in Southeast Asia and Europe. It comprises a high concentration of organosulfur complexes and flavonoids along with some other combinations that work together to impart a range of health benefits [24]. In the field of dietary supplements and universal healthcare, the bioactive constituents derived using environmental extraction techniques are gaining preference. The extraction acquiesces and pharmacological activities during the extraction method are also pressing issues that must be addressed. Scholarly research is being conducted on an ever-growing inventory of bioactive compounds [25].
However, in everyday life, it is difficult to ingest all the necessary nutrients to assure the proper function of the body or to complementary assist a drug-based treatment in CVDs. This is the reason why encapsulation techniques of nutraceuticals emerged as an effective approach designed to protect the bioactive compounds during the fabrication and storage, avoiding deterioration under environmental factors such as temperature, light, and UV exposure. Moreover, the development of encapsulated nutraceuticals into different carriers, overcomes the main drawback regarding their low bioavailability (such is the case of polyphenols), which greatly depends on several parameters, including solubility, digestibility, absorption, and metabolism. In addition, most bioactive compounds are unstable in alkaline conditions of biological fluids. By encapsulation in an adequate matrix, the incorporated compounds can be released with a specific concentration and time profile at a desirable site of action. At the nano-scale, the advanced nano-delivery systems have been demonstrated to boost the bioavailability and efficacy of bioactive compounds for therapeutic purpose [26]. On the other hand, effective nano-delivery systems have optimal characteristics for medicative agent-controlled release, long storage life, and enhanced therapeutic efficacy with no or minimal side effects.
In this context, the aim of this narrative review is to revise the main bioactive compounds with a significant role in CVDs in terms of preventive, diagnostic, and treatment measures. A discussion of the current evidence showing the advantages of nanoformulations and novel multifunctional nanomaterials able to overcome multiple obstacles, including the physiological ones, is made by summarizing the most recent preclinical data and clinical trials reported in the literature, describing the newer methodology and nanoformulation technologies that influence adequate drug or nutraceutical delivery.
2. Plant Bioactive Compounds
The phytoactive biocompounds can be isolated from plants using different extraction techniques. Based on specific functional group positions, bioactive compounds are categorized into primary and secondary metabolites [27]. Several metabolites are derived from fungi and vegetation based on their functional phase, tissue arrangement, ecological circumstances, and some other emphasis. Metabolites play a vital role in regulating cell maturation by functioning as plant development compounds. The most commonly found bioactive compounds are ethylene, auxin, gibberellins, plant development-abscisic acid, phytohormones–cytokinins, polyhydroxy steroids, and polyamines [28]. Glycosylated or covalently linked forms of bioactive compounds are engaged in the protection process that enables the plant to produce and store them in a harmless state [29]. Secondary metabolites used in traditional medicine can be extracted using a variety of methods, including Soxhlet extraction, maceration, and hydrodistillation. To minimize the use of solvents and introduce smoother extraction yields, ultrasonography, radiation, electromagnetic waves, high atmospheric pressure, and the use of supercritical fluids have already been examined [30,31,32]. Concentrated remains are acquired as the water drops are eradicated, and hence, the acquired part is known as an essential oil. Bioactive compounds are organized into three major classes based on their metabolic derivation: (a) phenolics; (b) terpenes; and (c) compounds containing nitrogen.
Fruit and vegetables consist of a wide range of bioactive compounds such as anthocyanins, betalains, carotenoids, flavonoids, glucosinolates, plant sterols, and tannins [33,34,35,36]. The occurrence and therapeutic properties of phytochemicals (phenolics, flavonoids, and carotenoids) have been collected in Table 1.

Table 1.
Bioactive compounds in fruits and vegetables along with their therapeutic properties.
3. Types of Bioactive Compounds
To summarize, we will present the recent advances on bioactive compounds that are widely recognized as promising strategies in the prevention, adjuvant therapy, or even cure of different chronic diseases of the 21st century, with a focus on CVs. Their multi-facet features in terms of dietary lifestyles shows positive effects on treating and preventing CVDs being emphasized in the next sections.
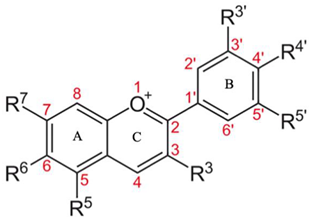
3.1. Flavonoids
Flavonoids are a copious and distinct cluster of bioactive compounds that occur as the core elements of polyphenols. They are divided into flavonols, flavones, flavanonols, flavanones, flavans (catechins, anthocyanins, and proanthocyanidins) and isoflavones and flavanonols [80]. Each flavonoid subclass and category have its own arrangement of herbal sources, roles, and therapeutic effects. As for their recognized antioxidant and anti-inflammatory effects, this arrangement of herbal bioactive compounds was revealed to have wellness benefits for individuals [81]. The main properties, source, and structure of flavonoids are briefly presented in Table 2, while a list containing the types of flavonoids, their functional unit, source, and therapeutic properties are presented in Table 3.

Table 2.
The main properties, source, and health benefits of flavonoids.

Table 3.
Types of flavonoids, their functional unit, plant source, and therapeutic properties.
3.2. Anthocyanins
Anthocyanin is one of the subclasses of phenolic phytochemicals; they are produced in cell sap and are hydrophilic in nature. They occur in higher plants tissue, such as fruits, flowers, leaves, and roots. Anthocyanins are the major cause for their specific coloration. In a brief manner, Table 4 presents the structure, biological source, and main therapeutic effects of anthocyanins [91].

Table 4.
The main source, benefits, and chemical structure of anthocyanins.
3.3. Tannins
Tannins are water-soluble polyphenols that are astringent and form bonds with proteins, as well as other organic compounds and macromolecules. Secondary metabolites easily get sequestered in plant cell vacuoles and protect different cell constituents. Table 5 contains the brief information related to chemical structure, biological source, and therapeutic benefits of tannins [92,93].

Table 5.
The chemical structure, therapeutic benefits, and biological source of tannins.
3.4. Betalains
Betalains are nitrogen (N)-containing vacuole pigments, similar to anthocyanins and flavonoids in appearance, yet pigments are red and yellow; they are capable of dissolving in water, as they contain nitrogen, and they can be found almost exclusively in families of the Caryophyllales. They are commonly used as color additives in food, being toxicologically safe. In Table 6, a brief presentation of the chemical structure, biological source, and therapeutic effects of betalains are presented [94,95].

Table 6.
Structure, biological source, and therapeutic benefits of betalains.
3.5. Carotenoids
Carotenoids are usually found in chloroplast in the form of red, yellow, and orange pigments. Carotenoids are in the category of lipid-soluble hydrocarbons. Xanthophylls are oxygenated derivatives of carotenoids. The red color of carrots denotes their name, but they are also present in green leaves, yellow fruits and red fruits, several fungi, and rhizomes. They are responsible for the color of egg yolk and some fish. Table 7 presents the main features related to carotenoids in a brief manner [96,97,98,99].

Table 7.
The structure, biological source, and therapeutic benefits of carotenoids.
Carotenoids are further classified into following categories according to Table 8.

Table 8.
Classification of carotenoids.
3.6. Plant Sterols
Plant sterols, also called phytosterols, are plant-derived fatty compounds. They are found in non-esterified and esterified forms of cinnamic/fatty acids. Plant sterol esters reduce coronary heart disease. Table 9 presents the data related to plant sterols in a brief manner [106,107].

Table 9.
The structure, biological source, and therapeutic benefits of plant sterols.
3.7. Glucosinolates
Glucosinolates are natural, anionic plant secondary metabolites. These are sulfur rich and belong to the order Brassicaceae. Table 10 presents the structure, biological source, and therapeutic benefits of glucosinolates [108,109,110,111].

Table 10.
Structure, biological source, and therapeutic benefits of glucosinolates.
4. Therapeutic Effect of Bioactive Compounds on Cardiovascular System
The advancement of disorders such as atherosclerosis and CVD is facilitated by oxidative stress. Diverse bioactive compounds’ anti-oxidative, anti-inflammatory, and metabolic effects are linked to their defense against atherosclerosis and CVD [112].
4.1. Carotenoids
Carotenoids are found in high amount in fruits and vegetables. Their sub-categorization is as per the chemical structure they have; i.e., as carotenes and xanthophylls. Carotenoids have antioxidant properties that are beneficial for health. Human organs and tissues have carotenoids. In tissues, the level of carotenoids is high as they have high levels of low-density lipoprotein receptors. Carotenoids help in the prevention of chronic cardiovascular diseases such as stroke and coronary heart disease. They have a multifaceted metabolism and react to systemic forces. As an antioxidant, they improve superoxide dismutase, glutamate dehydrogenase, catalase, and glucan particles. Carotenoids also inhibit IGF-1 activity and have been proved to be effective as an anticancer agent [113,114,115,116].
4.2. Polyphenols (Anthocyanins)
Berries are high in polyphenols, especially anthocyanins, as well as micronutrients and fiber. The consumption of berries results in an improvement in heart health. Chokeberries, cranberries, blueberries, and strawberries contain distilled anthocyanin derivatives. They showed substantial progress in LDL oxidation, lipid peroxidation, total plasma antioxidant potential, dyslipidemia, and glucose metabolism. It causes the activation of endothelial nitric oxide synthase, the lowered activity of carbohydrate digestive enzymes, and reduced oxidative stress by increasing the endothelial functions and the plasma lipid profiles. This led to a reduction of abnormal platelet aggregation. The research recommends berries (anthocyanins) as an important fruit group in a heart-healthy diet [117].
Pomegranate juice contains anthocyanins, catechins, quercetin, rutin, and ellagitannins, and it results in reducing high blood pressure, which is a result of the ACE activity antioxidant activity due to the radical scavenging effect of anthocyanins and hydrolyzable tannins [118,119,120,121].
4.3. Lycopene
Lycopene is a hydrocarbon carotenoid that is oxygenated and has quite a similar structure to β-carotene. Lycopene has an antioxidant property due to the presence of conjugated double bonds. This fat-soluble pigment imparts red color to a good variety of food items such as tomato, guava, watermelon, and others. Smoking is a major CVD risk factor. Smoke introduces free radicals into the human body, causing LDL oxidation, foam formation, and leading to atherogenesis. The severity of atherosclerosis is linked with an increase in LDL oxidation inclination. Lycopene prevents the oxidation of LDL and protects humans from coronary heart disease (CHD) [122].
Lycopene and plasma levels in cardiovascular disease have been investigated by researchers. The findings were analyzed. The higher intake of lycopene was compared with reduced levels of estone. Here, a 17% of reduction of CVD was linked with lycopene. It works by several mechanism such as the reduction of oxidation of biomolecules, the antiangiogenic effects, the reduction of cholesterol levels, the stimulation of apoptosis, and the reduction of inflammation [123].
4.4. Flavonoids
Randomized trials and many cohort studies have shown that flavonoids reduce CVD risk. Flavonoids produce a suitable response to LDL cholesterol, sensitiveness to insulin, and endothelial function [123]. A comprehensive evaluation of many investigations has indicated that the nutritional consumption of different groups of flavonoids, specifically as flavonols, anthocyanidins, proanthocyanidins, flavones, flavanones, and flavan-3-ols, minimize any chances of CVD drastically [124]. The quality of flavonoid subcategories in foods will be more essential than total flavonoids. Furthermore, the inverse correlation between flavonoid consumption and CVD risk is more pronounced in females than males. Even so, owing to the complementary nature of randomized clinical trials, there is indeed a lot of variance in the evidence [125].
Flavonoid research has exploded in popularity since its inception [126]. Understanding the availability of flavonoids, both natural and synthetic, and finding ways to improve their bioavailability were two of the most difficult tasks [127]. The scarcity of flavonoids is well known, but recently, these problems have been addressed. According to new research, the gut and its microbiome play a significant function in the development of prebiotic and microbiota enhancers as phenolic metabolites [128].
Flavonoids have been shown to reduce gastric and intestinal inflammation, as the metabolites act as enhancers of gut immune function [129]. As a result, attempts to increase flavonoids’ bioavailability aiming primarily on increasing their intestinal absorption. Borneol and methanol combination are traditional drug absorption enhancers [130]. Both borneol and methanol are toxic, and methanol causes blindness [131,132].
Effect on CV System
Regarding antiatherosclerotic effects, the pathogenesis of atherosclerosis begins with the oxidative alteration of low-density lipoproteins (LDL) by free radicals. Foam cells develop when oxidatively modified LDL is rapidly absorbed through a scavenger receptor. They act as antioxidant-chain breaking, in which flavonoids are radical species [133]. The capacity of quercetin and quercetin glycosides to shield LDL from oxidative activation has been shown to be successful [134]. A Japanese study found an inverted relationship between the flavonoid consumption and total plasma cholesterol levels [135].
Regarding antithrombogenic effects, platelet aggregation is critical in the composition of thrombotic disorders. Activated platelets bind to the vascular endothelium and develop lipid peroxides and oxygen free radicals, which prevent the formation of prostacyclin and nitrous oxide (NO) in the endothelium. Tea pigment shows the prevention of platelet adhesion or aggregation, and it reduces blood coagulation as well as increases fibrinolysis [136]. Flavonoids such as quercetin, kaempferol, and myricetin have been shown to inhibit platelet aggregation in animals [137]. Flavonols are particularly antithrombotic because they scavenge free radicals, keeping endothelial prostacyclin and nitric oxide concentrations constant [138].
Regarding cardioprotective effects, from both advanced and emerging economies, CVD is now the major cause of death. Atherosclerosis, coronary heart disease, arterial hypertension, and heart failure are all cardiovascular system (CVS) disorders. Oxidative stress is the primary cause of CVS disorders. Endogenous oxidants and reactive oxygen/nitrogen species (RONS) are in equilibrium in oxidative stress, with free radicals predominating. A prolonged dispensing of flavonoids has been shown to diminish or threaten to degrade the rate of CVD and its effects. They have an elevated inclination to transfer electrons, chelate ferrous ions, and sift reactive oxygen species [139]. Flavonoids are potential protectors against the persistent cardiotoxicity triggered by cytostatic medication such as doxorubicin [140,141].
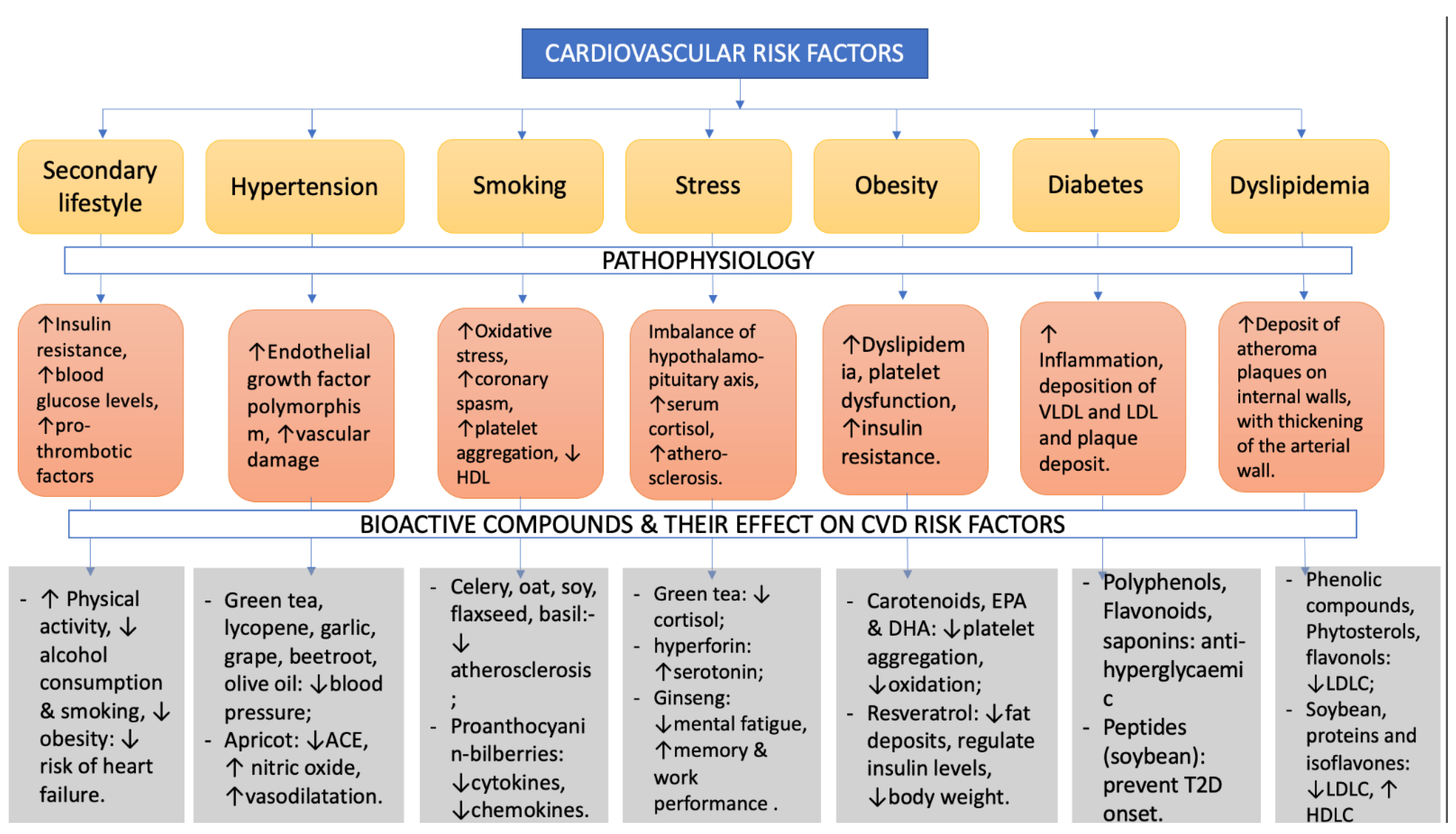
As a vasorelaxant agent, flavonoids help to reduce endothelial dysfunction (ED) by improving the vasorelaxation mechanism, which lowers arterial blood pressure [142,143]. ED is a crucial occurrence in the progression of CVD as well as a significant consequence involving arteriosclerosis as well as the occurrence of arterial thrombosis [144]. Flavonoids help to avoid a variety of CVD, such as high blood pressure and atherosclerosis [145,146]. Current laboratory studies show that these polyphenols can lower arterial pressure and improve the vasodilating system. Flavonoids have long been known to cause an endothelium-reliant response. Moreover, researchers discovered that anthocyanin delphinidin has a major endothelium-dependent vaso-relaxing effect [147,148]. Some of the cardiovascular risk factors, with a description of the pathophysiological effects they cause, and some bioactive compounds that may reduce the severity of the risk factors and also the positive changes they induce are described in Figure 1.
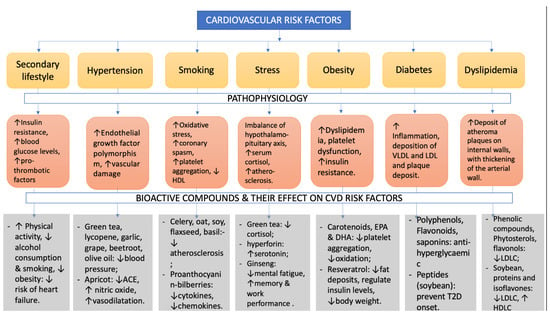
Figure 1.
CVD risk factors: pathophysiology and beneficial bioactive compounds to reduce the risk factors.
5. Nano-Delivery Systems of Bioactive Compounds
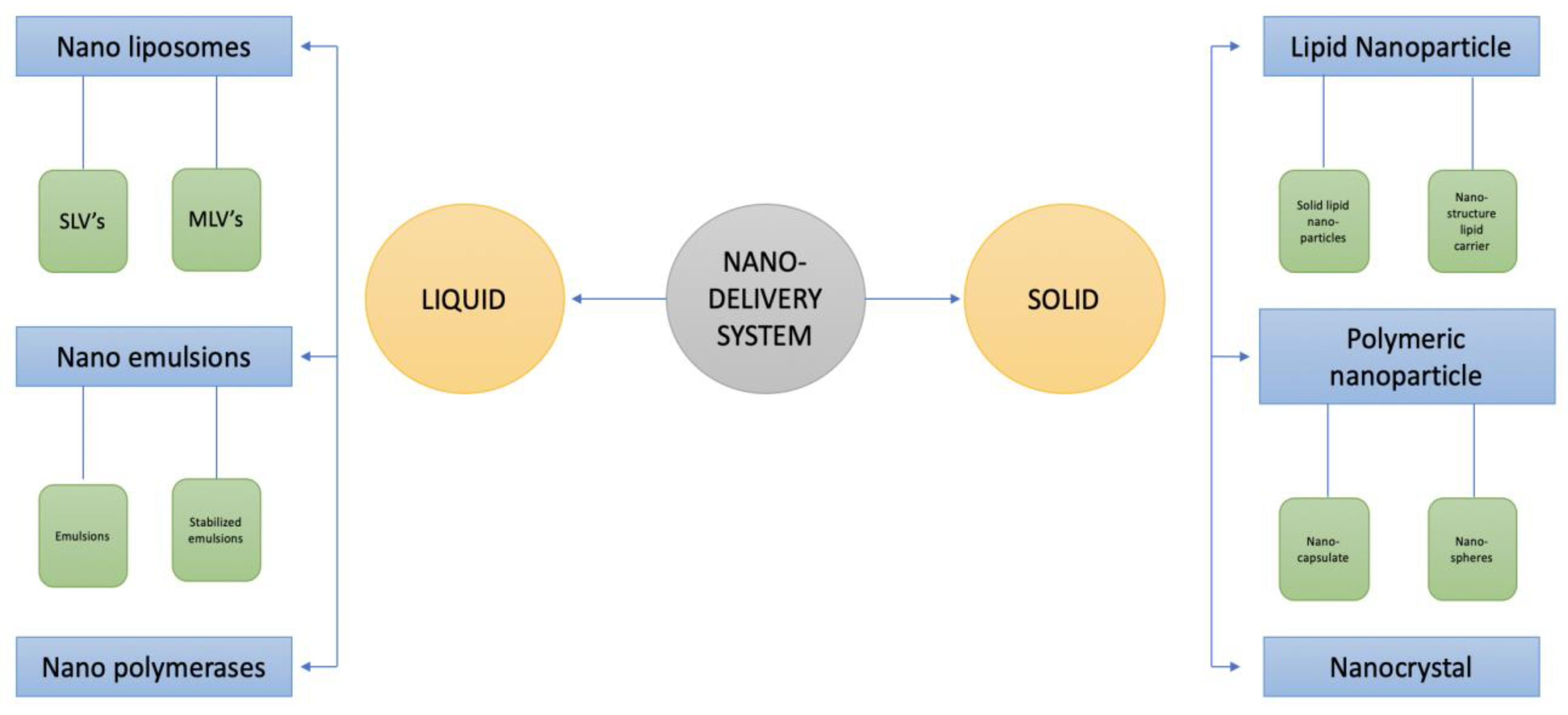
The nano-delivery method allows for the regulation of food bio-active components’ stability, solubility, and bioavailability, and it also maintains their targeted and controlled release. Food safety is a major issue because the usage of nano-delivery for food and drug delivery has grown in popularity [149,150,151,152]. Nano-delivery is carried out in two different ways: liquid and solid. Nano-emulsions, nano-liposomes, and nano-polymerases are the three different forms of liquid nano-delivery systems. Nano-liposomes are divided into SLVs (single lamellar vesicles) and MLVs (multilamellar large vesicles). Nanoparticles, polymeric nanoparticles, and nanocrystals are the three forms of solid nano-delivery systems. Solid lipid nanoparticles (SLNS) and nano-structured lipid carriers (NLCS) are two types of lipid particles. Polymeric nano-particles are of two different types, i.e., nano-spheres and nano-capsules. The types of nano-delivery system are presented in Figure 2.
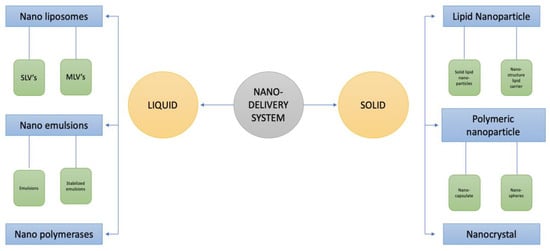
Figure 2.
Types of nano-delivery systems.
Due to chemical and enzymatic barriers and the poor solubility of compounds in the GI tract, a significant decrease in the quality of orally delivered drugs was noticed. It is well known that the action of bioactive compounds depends upon the extent of the bioaccessibility and bioavailability to the organism. There are several conventional drug carrier systems currently used for the effective delivery of cardioprotective drugs, either as oral tablets, parenteral/intravenous administration, or transdermal patches. However, some CVD treatments such as medications against angina pectoris (nitrates, calcium channel blockers, β blockers) produce significant adverse effects such as rash, constipation, nausea, drowsiness, edema, low blood pressure, or headache [153].
In this context, nanotechnology plays a determinant role by manipulating bioactive compounds encapsulated into nano-carriers with dimensions of 1–100 nm, providing a longer half-life, longer circulation time, longer mean residence time, and better pharmacokinetic clearance from the body [154]. These systems particularly depend on physicochemical factors that will influence their absorption, distribution, metabolism, and excretion, which are critical for administering bio-active compounds with improved in vivo results [155]. As the high surface to volume ratio of nanoparticles allows the conjugation, absorption, or encapsulation of bioactive molecules for delivery to the target site, drug delivery vehicles are successfully employed owing to their ability to deliver poorly soluble or highly toxic drugs to the target areas. Nanoparticles bind to the gut mucosa and epithelial cells, enter the bloodstream, and then are distributed to tissues and organs such as the liver, kidneys, spleen, heart, lungs, and brain. Nanoparticles and their metabolites are mainly excreted into the liver, kidneys, and colon [156,157].
Nowadays, conventional synthetic drugs are less present in practice owing to their costs and associated complications and side effects, while natural products have received much attention, as they are affordable for the majority of the population and possess multi-targeted effects with fewer side effects than synthetic drugs.
It is well known that polyphenols exhibit high antioxidant and anti-inflammatory properties, which are important for certain pathological conditions, including cardiovascular disease. Polyphenol consumption has been shown to improve endothelial function, blood pressure, and platelet function as well as the regulation of cellular processes such as inflammation and NO synthesis in vitro and in vivo [158]. The antioxidant mechanism is attributed to their ability to scavenge free oxygen and nitrogen species and to stimulate the expression of antioxidant enzymes (catalase, superoxide dismutase). When assessing the beneficial effects of polyphenols, it must be considered that only a small proportion of the ingested polyphenols are absorbed in the intestine, and therefore, a high quantity of polyphenols is required to achieve the expected improvement in terms of blood pressure lowering. The poor bioavailability is due not only to the low water solubility of polyphenols but also to the instability in alkaline conditions of biological fluids [159]. In order to overcome these drawbacks, nanoencapsulation technologies have emerged as a novel trend in drug carrier development, being nontoxic in nature, with the ability to escape from the host immune system, along with other advantages such as biodegradability, biocompatibility, and drug-targeting properties.
The encapsulation of active ingredients is nowadays a routine fabrication process and can be realized through different techniques: spray or freeze drying, coacervation, ionic gelation, extrusion, emulsion, electrospinning, electro-spraying, and liposomes formulation [160]. Encapsulation is also designed to protect the bioactive compounds during processing, storage, and transport from different undesirable factors such as temperature, light, and environmental oxidation. In this respect, biological materials such as polysaccharides, proteins, lipids, or low molecular surfactants are used [161], being highly biocompatible and nontoxic for human consumption. The release mechanisms might be related to diffusion, swelling, erosion, fragmentation, dissolution, or stimuli-controlled release. β-carotene, curcumin, quercetin, resveratrol, and epigallocatechin-3-gallate are only a few examples of nano-encapsulated bioactive compounds with improved bioavailability and metabolism when compared to non-encapsulated ones [26]. Based on literature research, Table 11 summarizes the most recent nanocarrier formulations developed for bioactive compounds with therapeutical indications in CVDs, along with experimental models (in vitro or in vivo) and the main outputs.

Table 11.
Nanocarrier formulations of different bioactive compounds.
5.1. Clinical Trials
Curcumin is probably the most studied polyphenol in treatment of CVD, being an inhibitor of p300 histone acetyltransferase activity, which is associated with heart failure. Highly bioavailable curcumin has been developed as a nanoformulation commercially known as Theracurmin® [171]. This revolutionary formulation is a submicron crystal solid dispersion of curcumin, consisting of 10 w/w% curcumin, 2% other curcuminoids (such as dimethoxy-curcumin and bisdemethoxycurcumin), 4% of gum ghatti, and 84% of water. In a clinical study performed with healthy participants, low (150 mg) and high (210 mg) doses of Theracurmin® were administrated in order to evaluate plasma curcumin levels in a dose-dependent manner [172]. The study evidenced that nanoformulation increases plasma curcumin levels in a dose-dependent manner without saturating the absorption system. Another clinical study revealed that the treatment of hypertensive patients using 60 mg/day of Theracurmin for 24 weeks significantly improved the parameters of diastolic function assessed by doppler echocardiography, which suggests that the nanoformulation improves left ventricular diastolic function without interfering with blood pressure in hypertensive patients [171].
However, it is difficult to interpret whether the observed effect is due to the nanoparticle formulation or to curcumin itself, as the authors did not include a non-encapsulated formulation of curcumin as a control group. A comparison between curcumin nanoformulation and powder curcumin was performed by Sasaki et al. [173] upon administration in a healthy participant, revealing that the bioavailability of Theracurmin® orally administrated was 27-fold higher than that of curcumin powder, even at a low dosage (30 mg). It was concluded that Theracurmin® shows higher bioavailability than currently available preparations (curcumin powder).
Despite the large number of reported preclinical studies related to polyphenol nanoformulations, their transition to the clinical sector been proven to have several limitations, as it is well known that the concentrations of polyphenols proved to be effective in vitro or in small animal models are much higher than the required levels in human subjects. Moreover, the effectiveness of nano-nutraceutical products strongly depends on preserving the bioavailability of the bioactive compounds.
5.2. Nanoparticles for Theranostic
The concept of a theranostic is derived from combined therapy and diagnostic tools assembled into a single platform [174], while the nano-theranostic embodies the most advanced technological approach with multifunctional attributes such as multimodal imaging, controlled, and localized drug targeting, allowing the development of personalized medicine. In this respect, engineered nanomaterials consisting of magnetic nanoparticles, liposomes, carbon-based nanomaterials, metal and/or polymeric based nanoparticles are good candidates for dual applications in terms of both diagnostics and therapeutic approaches [175,176,177]. For example, magnetic nanoparticles coated with natural compounds have proved their efficiency in the imaging of CVDs. Suzuki et al. [178] reported ultra-small superparamagnetic iron oxide nanoparticles coated with a specific polysaccharide (fucoidan) which have been successfully employed in MRI as contrast agents for arterial thrombus and elastase-induced vascular injury in a rat model. Another example is the case of gold nanoparticles used as contrasting agents, for the efficient detection and diagnosis of myocardial infarction. The method is based on engineered gold NPs conjugated with collagen, which demonstrated high-resolution detection of myocardial and ischemic injuries, along with adequate therapeutic tools [179]. Liposomal platforms have also proved to be a successful tool for diagnostic and therapeutic methods for platelet targeting in CVDs. The surface of liposomes with natural peptides was demonstrated to facilitate the drug delivery of active compounds and at the same time to target the cardiovascular tissues or damaged areas [180]. Many evidences for nano-based theranostics in terms of prognosis of atherosclerosis, myocardial infarction, aneurysms, angiogenesis, and other CVDs [175] have been reported, highlighting the benefit of sensitive detection of pathophysiological conditions combined with concomitant therapeutic measures. Although there has been reported success of preclinical studies related to theranostic nanoplatforms, the translational approach to clinical sectors remains unexplored, and moreover, the innate toxicity and stability of the nanoformulations are less studied. Most of the in vivo models were considered with small animals, and hence, huge differences might be detected by comparison with human anatomical features. On the other hand, the costs/benefits ratio must be considered, as there is a lack of studies reporting these aspects.
Developing innovative multifunctional nanomaterials with qualities that allow them to deliver specialized therapies through various physiological obstacles and to target specific cell types, tissues, and organs in the body is a major challenge for this project. Effective nano-delivery systems have ideal characteristics for controlled release of medicament, long storage life, and enhanced therapeutic efficacy with no or minimal side effects [181,182].
6. Conclusions
Several bioactive compounds are being diagnosed and tested to see if they have the potential to improve human health. They have antioxidant, anti-inflammatory, and anticarcinogenic factors, in addition to physiological and cellular benefits that protect against infectious diseases and metabolic illnesses such as diabetes, cardiovascular disorder, and cancer. They are derived from plants, and their consumption in diets has been related to tremendous fitness outcomes, making them ideal assets for the manufacturing of new nutritional dietary supplements with extensive shielding and preservative abilities.
Considering the high prevalence of CVDs worldwide, with a high level of morbidity and mortality, and excessive side effects of current therapies, the optimized alternative approaches are necessary for the prevention and/or treatment of these diseases. A large group of plant-derived bioactive compounds are used as alternative therapies for CVDs, which are summarized in this paper. They can be tailor-made to match the character and cultural preferences, to meet those lofty objectives, global and country-wide tasks to sell healthier, primarily plant-based diets. The role and significance of bioactive compounds for CVD treatment is still being explored, and its consequences must be established. Some polyphenols and flavonols used as bioactive compounds have been shown to reduce CVD risk factors. Their antioxidant and anti-inflammatory properties are the most important characteristics that make them favorable candidates for CVDs treatment. Along with the detailed properties of bioactive compounds used in CVDs, the current review provides an overview of the therapeutic advantages of nanoformulations along with recent advancements in this field.
The advantages of nano-delivery systems of bioactive compounds, recent preclinical studies, clinical trials, and nano-theranostic approaches are also discussed in this review, in order to offer a clearer understanding of the connection between bioactive compounds and CVD prevention, diagnostics, and treatment. Although the reported success of preclinical studies is related to the effectivity of nanoformulations and theranostic nanoplatforms, the translational approach to clinical sectors remains poorly explored, as the therapeutic applications of nano-phytomedicines in CVDs are still in their initial clinical phases. Nanotechnologies will provide a new window in the area of CVDs with the opportunity to achieve effective treatment, better prognosis, and fewer adverse effects on non-target tissues. However, further research is required for the development of low-cost nanoformulations and their effective usage, along with research in the toxicological approach.
Author Contributions
Conceptualization, R.K.S. and S.C.; data curation, R.K.S., A.G., E.A.Y.; writing—original draft preparation, R.K.S., A.G., E.A.Y.; writing—review and editing, R.K.S. and S.C.; funding acquisition, S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors R.K.S. and A.G. are grateful to Madhu Chitkara, Chancellor, Chitkara University, Rajpura, Patiala, India and Ashok Chitkara, Chancellor, Chitkara University, Rajpura, Patiala, India, for support and institutional facilities.
Conflicts of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- Martínez-Augustin, O.; Aguilera, C.M.; Gil-Campos, M.; Sánchez de Medina, F.; Gil, A. Bioactive anti-obesity food components. Int. J. Vitam. Nutr. Res. 2012, 82, 148–156. [Google Scholar] [CrossRef] [PubMed]
- NIH National Cancer Institute. What Is Cancer? Available online: https://www.cancer.gov/about-cancer/understanding/what-is-cancer (accessed on 5 May 2021).
- Felman, A.; Kohli, P. What to know about cardiovascular disease? Med. News Today 2019. Available online: https://www.medicalnewstoday.com/articles/257484 (accessed on 26 July 2019).
- Hemler, E.C.; Hu, F.B. Plant-Based Diets for Cardiovascular Disease Prevention: All Plant Foods Are Not Created Equal. Curr. Atheroscler. Rep. 2019, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Alwan, A.; World Health Organization. Global Status Report on Noncommunicable Diseases 2010; World Health Organization: Geneva, Switzerland, 2011; Volume 9, p. 162. Available online: https://www.who.int/nmh/publications/ncd_report_full_en.pdf (accessed on 5 May 2021).
- Gensini, G.F.; Comeglio, M.; Colella, A. Classical risk factors and emerging elements in the risk profile for coronary artery disease. Eur. Heart J. 1998, 19 (Suppl. A), A53–A61. [Google Scholar]
- Mahmood, S.S.; Levy, D.; Vasan, R.S.; Wang, T.J. The Framingham Heart Study and the epidemiology of cardiovascular disease: A historical perspective. Lancet 2014, 383, 999–1008. [Google Scholar] [CrossRef] [Green Version]
- Kris-Etherton, P.M.; Lefevre, M.; Beecher, G.R.; Gross, M.D.; Keen, C.L.; Etherton, T.D. Bioactive compounds in nutrition and health-research methodologies for establishing biological function: The antioxidant and anti-inflammatory effects of flavonoids on atherosclerosis. Annu. Rev. Nutr. 2004, 24, 511–538. [Google Scholar] [CrossRef]
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef]
- Bowen, K.J.; Sullivan, V.K.; Kris-Etherton, P.M.; Petersen, K.S. Nutrition and Cardiovascular Disease-an Update. Curr. Atheroscler. Rep. 2018, 20, 8. [Google Scholar] [CrossRef] [PubMed]
- Satija, A.; Hu, F.B. Plant-based diets and cardiovascular health. Trends Cardiovasc. Med. 2018, 28, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Guaadaoui, A.; Benaicha, S.; Elmajdoub, N.; Bellaoui, M.; Hamal, A. What is a Bioactive Compound? A Combined Definition for a Preliminary Consensus. Int. J. Nutr. Food Sci. 2014, 3, 174–179. [Google Scholar] [CrossRef]
- González, S. Dietary Bioactive Compounds and Human Health and Disease. Nutrients 2020, 12, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NCI Dictionary of Cancer Terms. Bioactive Compounds. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/bioactive-compound (accessed on 24 February 2021).
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Conti, M.V.; Guzzetti, L.; Panzeri, D.; De Giuseppe, R.; Coccetti, P.; Labra, M.; Cena, H. Bioactive compounds in legumes: Implications for sustainable nutrition and health in the elderly population. Trends Food Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Bazzano, L.A.; He, J.; Ogden, L.G.; Loria, C.M.; Vupputuri, S.; Myers, L.; Whelton, P.K. Fruit and vegetable intake and risk of cardiovascular disease in US adults: The first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Am. J. Clin. Nutr. 2002, 76, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Food and Nutrition Board of the Institute of Medicine, The National Academies. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Jimeno, J.; Faircloth, G.; Sousa-Faro, J.M.F.; Scheuer, P.; Rinehart, K. New Marine Derived Anticancer Therapeutics—A Journey from the Sea to Clinical Trials. Mar. Drugs. 2004, 2, 14–29. [Google Scholar] [CrossRef] [Green Version]
- Vignesh, S.; Raja, A.; James, A.R. Marine drugs: Implication and Future Studies. Int. J. Pharmacol. 2011, 7, 22–30. [Google Scholar] [CrossRef]
- Food and Drug Administration. FDA Basics—Dietary Supplements; Office of Dietary Supplements Programs, HFS-810 (FDA). 2019, USA. Available online: https://www.fda.gov/food/dietary-supplements (accessed on 5 May 2021).
- Scottish Intercollegiate Guidelines Network (SIGN). Risk Estimation and the Prevention of Cardiovascular Disease: A National Clinical Guideline. Edinburgh (Scotland): Scottish Intercollegiate Guidelines Network; 2017. (SIGN publication no. 149); 1–118. Available online: http://www.sign.ac.uk (accessed on 5 May 2021).
- Rahman, K.; Lowe, G.M. Garlic and cardiovascular disease: A critical review. J. Nutr. 2006, 136 (Suppl. 3), 736S–740S. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Gonçalves, R.F.S.; Martins, J.T.; Duarte, C.M.M.; Vicente, A.A.; Pinheiro, A.C. Advances in nutraceutical delivery systems: From formulation design for bioavailability enhancement to efficacy and safety evaluation. Trends Food Sci. Technol. 2018, 78, 270–291. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Koul, A.; Sharma, D.; Kaul, S.; Swamy, M.; Dhar, M.K. Metabolic engineering strategies for enhancing the production of bio-active compounds from medicinal plants. In Natural Bio-Active Compounds; Akhtar, M., Swamy, M., Eds.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Depuydt, S.; Van Praet, S.; Nelissen, H.; Vanholme, B.; Vereecke, D. How plant hormones and their interactions affect cell growth. In Molecular Cell Biology of the Growth and Differentiation of Plant Cells; Rose, R.J., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 174–195. [Google Scholar]
- Chaves Lobón, N.; Ferrer de la Cruz, I.; Alías Gallego, J.C. Autotoxicity of Diterpenes Present in Leaves of Cistus ladanifer L. Plants 2019, 8, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Ahmed, B.; Mohamed, S.; Adel, A.-R. Antioxidant Activities and Potential Impacts to Reduce Aflatoxins Utilizing Jojoba and Jatropha Oils and Extracts. Int. J. Pharmacol. 2017, 13, 1103–1114. [Google Scholar]
- Loi, M.; Paciolla, C.; Logrieco, A.F.; Mulè, G. Plant Bioactive Compounds in Pre- and Postharvest Management for Aflatoxins Reduction. Front. Microbiol. 2020, 11, 243. [Google Scholar] [CrossRef] [PubMed]
- Walden, R.; Tomlinson, B. Cardiovascular Disease. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011; Chapter 16. Available online: www.ncbi.nlm.nih.gov/books/NBK92767/ (accessed on 5 May 2021).
- Ciumărnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, Ș.C.; Răchișan, A.L.; Negrean, V.; Perné, M.G.; Donca, V.I.; Alexescu, T.G.; Para, I.; et al. The Effects of Flavonoids in Cardiovascular Diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef]
- McCullough, M.L.; Peterson, J.J.; Patel, R.; Jacques, P.F.; Shah, R.; Dwyer, J.T. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am. J. Clin. Nutr. 2012, 95, 454–464. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Novellino, E.; Souto, E.B.; Daliu, P.; Santini, A. Abelmoschus esculentus L.: Bioactive Components’ Beneficial Properties-Focused on Antidiabetic Role-For Sustainable Health Applications. Molecules 2018, 24, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saida, M.; Lamjed, B.; Amina, B.; Rym, E.; Soumaya, H.; Salem, E. Biological activities, and phytocompounds of northwest Algeria Ajuga iva (L) extracts: Partial identification of the antibacterial fraction. Microb. Pathog. 2018, 121, 173–178. [Google Scholar]
- Wang, S.; Zhao, Y.; Song, J.; Wang, R.; Gao, L.; Zhang, L.; Fang, L.; Lu, Y.; Du, G. Total flavonoids from Anchusa italica Retz. Improve cardiac function and attenuate cardiac remodeling post myocardial infarction in mice. J. Ethnopharmacol. 2020, 257, 112887. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Bondonno, C.P.; Blekkenhorst, L.C.; Considine, M.J.; Maghzal, G.; Stocker, R.; Woodman, R.J.; Ward, N.C.; Hodgson, J.M.; Croft, K.D. Flavonoid-Rich Apple Improves Endothelial Function in Individuals at Risk for Cardiovascular Disease: A Randomized Controlled Clinical Trial. Mol. Nutr. Food Res. 2018, 62, 1700674. [Google Scholar] [CrossRef]
- Panthi, M.; Subba, R.K.; Raut, B.; Khanal, D.P.; Koirala, N. Bioactivity evaluations of leaf extract fractions from young barley grass and correlation with their phytochemical profiles. BMC Complement. Med. Ther. 2020, 20, 64. [Google Scholar] [CrossRef] [Green Version]
- Pereyra, K.V.; Andrade, D.C.; Toledo, C.; Schwarz, K.G.; Uribe-Ojeda, A.; RÃ-os-Gallardo, A.P.; Quintanilla, R.A.; Contreras, S.A.; Del Rio, R. Dietary supplementation of a sulforaphane-enriched broccoli extract protects the heart from acute cardiac stress. J. Funct. Foods 2020, 75, 104267. [Google Scholar] [CrossRef]
- Zaini, R.G.; Brandt, K.; Clench, M.R.; Le Maitre, C.L. Effects of bioactive compounds from carrots (Daucus carota L.), polyacetylenes, beta-carotene and lutein on human lymphoid leukaemia cells. Anticancer Agents Med. Chem. 2012, 12, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Bartłomiej, S.; Justyna, R.K.; Ewa, N. Bioactive compounds in cereal grains—Occurrence, structure, technological significance and nutritional benefits—A review. Food Sci. Technol. Int. 2012, 18, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.Á.; Ramos, S. Impact of cocoa flavanols on human health. Food Chem. Toxicol. 2021, 151, 112121. [Google Scholar] [CrossRef]
- Neetu, K.; Choudhary, S.B.; Sharma, H.K.; Singh, B.K.; Kumar, A.A. Health-promoting properties of Corchorus leaves: A review. J. Herb. Med. 2019, 15, 100240. [Google Scholar]
- Haidar, B.; Ferdous, M.; Fatema, B.; Ferdous, A.S.; Islam, M.R.; Khan, H. Population diversity of bacterial endophytes from jute (Corchorus olitorius) and evaluation of their potential role as bioinoculants. Microbiol. Res. 2018, 208, 43–53. [Google Scholar] [CrossRef]
- Polley, K.R.; Oswell, N.J.; Pegg, R.B.; Paton, C.M.; Cooper, J.A. A 5-day high-fat diet rich in cottonseed oil improves cholesterol profiles and triglycerides compared to olive oil in healthy men. Nutr. Res. 2018, 60, 43–53. [Google Scholar] [CrossRef]
- Simha, P.; Mathew, M.; Ganesapillai, M. Empirical modeling of drying kinetics and microwave assisted extraction of bioactive compounds from Adathoda vasica and Cymbopogon citratus. Alex. Eng. J. 2016, 55, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Orgah, J.O.; He, S.; Wang, Y.; Jiang, M.; Wang, Y.; Orgah, E.A.; Duan, Y.; Zhao, B.; Zhang, B.; Han, J.; et al. Pharmacological potential of the combination of Salvia miltiorrhiza (Danshen) and Carthamus tinctorius (Honghua) for diabetes mellitus and its cardiovascular complications. Pharmacol. Res. 2020, 153, 104654. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Liu, Q.; Zhang, H.; Wang, S.; Liu, Q.; Zheng, H.; Liu, X.; Wang, X.; Shen, T.; et al. Dracomolphin AE, new lignans from Dracocephalum moldavica. Fitoterapia 2021, 150, 104841. [Google Scholar] [CrossRef]
- Bayang, J.P.; Laya, A.; Kolla, M.C.; Koubala, B.B. Variation of physical properties, nutritional value and bioactive nutrients in dry and fresh wild edible fruits of twenty-three species from Far North region of Cameroon. J. Agric. Food Res. 2021, 4, 100146. [Google Scholar] [CrossRef]
- Miao, S.M.; Zhang, Q.; Bi, X.B.; Cui, J.L.; Wang, M.L. A review of the phytochemistry and pharmacological activities of Ephedra herb. Chin. J. Nat. Med. 2020, 18, 321–344. [Google Scholar] [CrossRef]
- Graefe, E.U.; Veit, M. Urinary metabolites of flavonoids and hydroxycinnamic acids in humans after application of a crude extract from Equisetum arvense. Phytomedicine 1999, 6, 239–246. [Google Scholar] [CrossRef]
- Shim, Y.Y.; Gui, B.; Arnison, P.G.; Shim, Y.Y.; Gui, B.; Arnison, P.G.; Wang, Y.; Reaney, M.J.T.; Jiang, M.; Wang, Y.; et al. Flaxseed (Linum usitatissimum L.) bioactive compounds and peptide nomenclature: A review. Trends Food Sci. Technol. 2014, 38, 5–20. [Google Scholar] [CrossRef] [Green Version]
- Al-Snafi, A. Phytochemical constituents and medicinal properties of digitalis lanata and digitalis purpurea—A Review. IAJPS 2017, 4, 225–234. [Google Scholar]
- Martins, N.; Petropoulos, S.; Ferreira, I.C. Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre- and post-harvest conditions: A review. Food Chem. 2016, 211, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, I.; Ibrahim, W.; Yusuf, F.M.; Ahmad, S.A.; Ahmad, S. Biochemical Constituent of Ginkgo biloba (Seed) 80% Methanol Extract Inhibits Cholinesterase Enzymes in Javanese Medaka (Oryzias javanicus) Model. J. Toxicol. 2020, 2020, 8815313. [Google Scholar] [CrossRef]
- Yu, H.; Zhao, J.; You, J.; Li, J.; Ma, H.; Chen, X. Factors influencing cultivated ginseng (Panax ginseng CA Meyer) bioactive compounds. PLoS ONE 2019, 14, e0223763. [Google Scholar] [CrossRef]
- Rojas, R.; Castro-lópez, C.; Sánchez-Alejo, E.J.; Niño-Medina, G.; Martínez-Ávila, C.G. Phenolic compound recovery from grape fruit and by- products: An overview of extraction methods. Grape Wine Biotechnol. 2016. [Google Scholar] [CrossRef]
- Rababa’h, A.M.; Al Yacoub, O.N.; El-Elimat, T.; Rabab’ah, M.; Altarabsheh, S.; Deo, S.; Al-Azayzih, A.; Zayed, A.; Alazzam, S.; Alzoubi, K.H. The effect of hawthorn flower and leaf extract (Crataegus Spp.) on cardiac hemostasis and oxidative parameters in Sprague Dawley rats. Heliyon 2020, 6, e04617. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.S.N.; Machado, L.L.; Pessoa, O.D.; Braz-Filho, R.; Overk, C.R.; Yao, P.; Lemos, T.L. Pyrrolizidine alkaloids from heliotropium indicum. J. Braz. Chem. Soc. 2005, 16, 1410–1414. [Google Scholar] [CrossRef] [Green Version]
- De Angelis, D.; Pasqualone, A.; Costantini, M.; Ricciardi, L.; Lotti, C.; Pavan, S.; Summo, C. Data on the proximate composition, bioactive compounds, physicochemical and functional properties of a collection of faba beans (Vicia faba L.) and lentils (Lens culinaris Medik.). Data Brief 2020, 34, 106660. [Google Scholar] [CrossRef]
- Lu, J.; He, R.; Sun, P.; Zhang, F.; Linhardt, R.J.; Zhang, A. Molecular mechanisms of bioactive polysaccharides from Ganoderma lucidum (Lingzhi), a review. Int. J. Biol. Macromol. 2020, 150, 765–774. [Google Scholar] [CrossRef]
- Sudheer, S.; Alzorqi, I.; Manickam, S. Bioactive Compounds of the Wonder Medicinal Mushroom “Ganoderma lucidum”. In Bioactive Molecules in Food. Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Moringa oleifera Seeds and Oil: Characteristics and Uses for Human Health. Int. J. Mol. Sci. 2016, 17, 2141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Y.; Deng, F. Phytochemistry and biological activity of mustard (Brassica juncea): A review. CyTA-J. Food 2020, 18, 704–718. [Google Scholar] [CrossRef]
- Wu, J.R.; Leu, H.B.; Yin, W.H. The benefit of secondary prevention with oat fiber in reducing future cardiovascular event among CAD patients after coronary intervention. Sci. Rep. 2019, 9, 3091. [Google Scholar] [CrossRef] [PubMed]
- Boskou, D. Table Olives as Sources of Bioactive Compounds. In Olive and Olive Oil Bioactive Constituents. J. Agric. Food Chem. 2015, 217–259. [Google Scholar] [CrossRef]
- Marrelli, M.; Amodeo, V.; Statti, G.; Conforti, F. Biological Properties and Bioactive Components of Allium cepa L.: Focus on Potential Benefits in the Treatment of Obesity and Related Comorbidities. Molecules 2018, 24, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alper, C.M.; Mattes, R.D. Peanut consumption improves indices of cardiovascular disease risk in healthy adults. J. Am. Coll. Nutr. 2003, 22, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Boughalleb, F.; Bouhemda, T.; Abdellaoui, R.; Nasri, N. Unexploited polygonum equisetiforme seeds: Potential source of useful natural bioactive products. Ind. Crop. Prod. 2018, 122, 349–357. [Google Scholar] [CrossRef]
- Shah, A.R.; Sharma, P.; Gour, V.S.; Kothari, S.L.; Dar, K.B.; Ganie, S.A.; Shah, Y.R. Antioxidant, Nutritional, Structural, Thermal and Physico-Chemical Properties of Psyllium (Plantago Ovata) Seeds. Curr. Res. Nutr. Food Sci. 2020, 8. [Google Scholar] [CrossRef]
- Ferraz da Costa, D.C.; Pereira Rangel, L.; Quarti, J.; Santos, R.A.; Silva, J.L.; Fialho, E. Bioactive compounds and metabolites from grapes and red wine in breast cancer chemoprevention and therapy. Molecules 2020, 25, 3531. [Google Scholar] [CrossRef] [PubMed]
- Salehi, E.; Sardarodiyan, M. Bioactive phytochemicals in rice bran: Processing and functional properties. Biochem. Ind. J. 2016, 10, 101. [Google Scholar]
- Zampelas, A. The Effects of Soy and its Components on Risk Factors and End Points of Cardiovascular Diseases. Nutrients 2019, 11, 2621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, H.M.; Al-Zubaidy, A.M.A. Exploring natural essential oil components and antibacterial activity of solvent extracts from twelve Perilla frutescens L. Genotypes. Arab. J. Chem. 2020, 13, 7390–7402. [Google Scholar] [CrossRef]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.; Siervo, M.; Lara, J. Tomato and lycopene supplementation and cardiovascular risk factors: A systematic review and meta-analysis. Atherosclerosis 2017, 257, 100–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Xu, T.; Akoh, C. Effect of roasting on the volatile constituents of trichosanthes kirilowii seeds. J. Food Drug Anal. 2014, 22, 310–317. [Google Scholar] [CrossRef]
- Melini, V.; Acquistucci, R. Health-promoting compounds in pigmented thai and wild rice. Foods 2017, 6, 9. [Google Scholar] [CrossRef] [Green Version]
- Dai, W.; Zhang, Z.; Zhao, S. Baseline levels of serum high sensitivity C reactive protein and lipids in predicting the residual risk of cardiovascular events in Chinese population with stable coronary artery disease: A prospective cohort study. Lipids Health Dis. 2018, 17, 273. [Google Scholar] [CrossRef] [Green Version]
- Ververidis, F.; Trantas, E.; Douglas, C.; Vollmer, G.; Kretzschmar, G.; Panopoulos, N. Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I: Chemical diversity, impacts on plant biology and human health. Biotechnol. J. 2007, 2, 1214–1234. [Google Scholar] [CrossRef] [PubMed]
- Lotito, S.B.; Frei, B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: Cause, consequence, or epiphenomenon? Free Radic. Biol. Med. 2006, 41, 1727–1746. [Google Scholar] [CrossRef] [PubMed]
- Izzi, V.; Masuelli, L.; Tresoldi, I.; Sacchetti, P.; Modesti, A.; Galvano, F.; Bei, R. The effects of dietary flavonoids on the regulation of redox inflammatory networks. Front. Biosci. 2012, 17, 2396–2418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.F.; Cho, S.; Wang, J. Epicatechin protects hemorrhagic brain via synergistic Nrf2 pathways. Ann. Clin. Transl. Neurol. 2014, 1, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Nishiumi, S.; Miyamoto, S.; Kawabata, K.; Ohnishi, K.; Mukai, R.; Murakami, A.; Ashida, H.; Terao, J. Dietary flavonoids as cancer-preventive and therapeutic biofactors. Front. Biosci. 2011, 3, 1332–1362. [Google Scholar] [CrossRef]
- McKay, D.L.; Chen, C.Y.; Saltzman, E.; Blumberg, J.B. Hibiscus sabdariffa L. tea (tisane) lowers blood pressure in prehypertensive and mildly hypertensive adults. J. Nutr. 2010, 140, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.L.; Zahradka, P.; Taylor, C.G. Efficacy of flavonoids in the management of high blood pressure. Nutr. Rev. 2015, 73, 799–822. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Hernández Bautista, R.J.; Sandhu, M.A.; Hussein, O.E. Beneficial Effects of Citrus Flavonoids on Cardiovascular and Metabolic Health. Oxid. Med. Cell. Longev. 2019, 2019, 5484138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, J.; Xu, H.-T.; Yu, J.-J.; Gao, J.; Lei, J.; Yin, Q.S.; Li, B.; Pang, M.X.; Su, M.X.; Mi, W.J.; et al. Luteolin ameliorates hypertensive vascular remodeling through inhibiting the proliferation and migration of vascular smooth muscle cells. Evid. Based Complement. Altern. Med. 2015, 2015, 364876. [Google Scholar] [CrossRef] [Green Version]
- Thamcharoen, N.; Susantitaphong, P.; Wongrakpanich, S.; Chongsathidkiet, P.; Tantrachoti, P.; Pitukweerakul, S.; Avihingsanon, Y.; Praditpornsilpa, K.; Jaber, B.L.; Eiam-Ong, S. Effect of N- and T-type calcium channel blocker on proteinuria, blood pressure and kidney function in hypertensive patients: A meta-analysis. Hypertens. Res. 2015, 38, 902. [Google Scholar] [CrossRef] [Green Version]
- Jiang, R.W.; Lau, K.M.; Lam, H.M.; Yam, W.S.; Leung, L.K.; Choi, K.L.; Waye, M.M.; Mak, T.C.; Woo, K.S.; Fung, K.P. A comparative study on aqueous root extracts of Pueraria thomsonii and Pueraria lobata by antioxidant assay and HPLC fingerprint analysis. J. Ethnopharmacol. 2005, 96, 133–138. [Google Scholar] [CrossRef]
- Tsuda, T. Dietary anthocyanin-rich plants: Biochemical basis and recent progress in health benefits studies. Mol. Nutr. Food Res. 2012, 56, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Srilakshami, B. Food Science, 7th ed.; New Age International Publishers: New Delhi, India, 2018. [Google Scholar]
- Celestino, S.B.; Augustin, S. Proanthocyanidins and tannin-like compounds—Nature, occurrence, dietary intake and effects on nutrition and health. J. Sci. Food Agric. 2000, 80, 1094–1117. [Google Scholar]
- Salisbury, F.; Ross, C. Plant Physiology, 4th ed.; Wadsworth: Belmont, CA, USA, 1991; pp. 325–326. [Google Scholar]
- Rahimi, P.; Abedimanesh, S.; Mesbah-Namin, S.A.; Ostadrahimi, A. Betalains, the nature-inspired pigments, in health and diseases. Crit. Rev. Food Sci. Nutr. 2019, 59, 2949–2978. [Google Scholar] [CrossRef]
- Antonescu, A.-I.; Miere, F.; Fritea, L.; Ganea, M.; Zdrinca, M.; Dobjanschi, L.; Antonescu, A.; Vicas, S.I.; Bodog, F.; Sindhu, R.K.; et al. Perspectives on the Combined Effects of Ocimum basilicum and Trifolium pratense Extracts in Terms of Phytochemical Profile and Pharmacological Effects. Plants 2021, 10, 1390. [Google Scholar] [CrossRef] [PubMed]
- Leoncini, E.; Nedovic, D.; Panic, N.; Pastorino, R.; Edefonti, V.; Boccia, S. Carotenoid Intake from Natural Sources and Head and Neck Cancer: A Systematic Review and Meta-analysis of Epidemiological Studies. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1003–1011. [Google Scholar] [CrossRef] [Green Version]
- Voutilainen, S.; Nurmi, T.; Mursu, J.; Rissanen, T.H. Carotenoids and cardiovascular health. Am. J. Clin. Nutr. 2006, 83, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Granado-Lorencio, F.; Lagarda, M.J.; Garcia-López, F.J.; Sánchez-Siles, L.M.; Blanco-Navarro, I.; Alegría, A.; Pérez-Sacristán, B.; Garcia-Llatas, G.; Donoso-Navarro, E.; Silvestre-Mardomingo, R.A.; et al. Effect of β-cryptoxanthin plus phytosterols on cardiovascular risk and bone turnover markers in post-menopausal women: A randomized crossover trial. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Osganian, S.K.; Stampfer, M.J.; Rimm, E.; Spiegelman, D.; Manson, J.E.; Willett, W.C. Dietary carotenoids and risk of coronary artery disease in women. Am. J. Clin. Nutr. 2003, 77, 1390–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, A.C.; Zolfaghari, R.; Weisz, J. Vitamin A: Recent advances in the biotransformation, transport, and metabolism of retinoids. Curr. Opin. Gastroenterol. 2001, 17, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Holden, J.M.; Eldridge, A.L.; Beecher, G.R.; Buzzard, I.M.; Bhagwat, S.; Davis, C.S.; Douglass, L.W.; Gebhardt, S.; Haytowiz, D.; Schakel, S. Carotenoid content of US foods: An update of the database. J. Food Comp. Anal. 1999, 12, 169–196. [Google Scholar] [CrossRef] [Green Version]
- Stahl, W.; Sies, H. Lycopene: A biologically important carotenoid for humans? Arch. Biochem. Biophys. 1996, 336, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sommerburg, O.; Keunen, J.E.; Bird, A.; Van Kuijk, F. J G M. Fruits and vegetables that are sources for lutein and zeaxanthin: The macular pigment in human eyes. Br. J. Ophthalmol. 1998, 82, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Jialal, I.; Norkus, E.P.; Cristol, L.; Grundy, S.M. beta-Carotene inhibits the oxidative modification of low-density lipoprotein. Biochim. Biophys. Acta 1991, 1086, 134–138. [Google Scholar] [CrossRef]
- Ciccone, M.M.; Cortese, F.; Gesualdo, M.; Carbonara, S.; Zito, A.; Ricci, G.; De Pascalis, F.; Scicchitano, P.; Riccioni, G. Dietary intake of carotenoids and their antioxidant and anti-inflammatory effects in cardiovascular care. Mediat. Inflamm. 2013, 2013, 782137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trautwein, E.A.; Vermeer, M.A.; Hiemstra, H.; Ras, R.T. LDL-Cholesterol Lowering of Plant Sterols and Stanols-Which Factors Influence Their Efficacy? Nutrients 2018, 10, 1262. [Google Scholar] [CrossRef] [Green Version]
- Radojčić Redovniković, I.; Glivetić, T.; Delonga, K.; Vorkapic-Furac, J. Glucosinolates and their potential role in plant. Period. Biol. 2008, 110, 297–309. [Google Scholar]
- Tse, G.; Eslick, G.D. Cruciferous vegetables and risk of colorectal neoplasms: A systematic review and meta-analysis. Nutr. Cancer 2014, 66, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, P.; Kim, J.S. Anti-Carcinogenic Glucosinolates in Cruciferous Vegetables and Their Antagonistic Effects on Prevention of Cancers. Molecules 2018, 23, 2983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loef, M.; Walach, H. Fruit, vegetables and prevention of cognitive decline or dementia: A systematic review of cohort studies. J. Nutr. Health Aging 2012, 16, 626–630. [Google Scholar] [CrossRef]
- Walia, A.; Gupta, A.K.; Sharma, V. Role of Bioactive Compounds in Human Health. Acta Sci. Med. Sci. 2019, 3, 25–33. [Google Scholar]
- Greenberg, E.R.; Baron, J.A.; Karagas, M.R.; Greenberg, E.R.; Baron, J.A.; Karagas, M.R.; Stukel, T.A.; Nierenberg, D.W.; Stevens, M.M.; Mandel, J.S.; et al. Mortality associated with low plasma concentration of beta carotene and the effect of oral supplementation. JAMA 1996, 275, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet 2002, 360, 23–33. [Google Scholar] [CrossRef]
- Liu, S.; Lee, I.M.; Ajani, U.; Cole, S.R.; Buring, J.E.; Manson, J.E. Physicians’ Health Study. Intake of vegetables rich in carotenoids and risk of coronary heart disease in men: The Physicians’ Health Study. Int. J. Epidemiol. 2001, 30, 130–135. [Google Scholar] [CrossRef] [Green Version]
- Hennekens, C.H.; Buring, J.E.; Manson, J.E.; Stampfer, M.; Rosner, B.; Cook, N.R.; Belanger, C.; LaMotte, F.; Gaziano, J.M.; Ridker, P.M.; et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1145–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, A.; Rhone, M.; Lyons, T.J. Berries: Emerging impact on cardiovascular health. Nutr. Rev. 2010, 68, 168–177. [Google Scholar] [CrossRef] [Green Version]
- Basu, A.; Du, M.; Leyva, M.J.; Sanchez, K.; Betts, N.M.; Wu, M.; Aston, C.E.; Lyons, T.J. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J. Nutr. 2010, 140, 1582–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goszcz, K.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Bioactive polyphenols and cardiovascular disease: Chemical antagonists, pharmacological agents or xenobiotics that drive an adaptive response? Br. J. Pharmacol. 2017, 174, 1209–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asgary, S.; Sahebkar, A.; Afshani, M.R.; Keshvari, M.; Haghjooyjavanmard, S.; Rafieian-Kopaei, M. Clinical evaluation of blood pressure lowering, endothelial function improving, hypolipidemic and anti-inflammatory effects of pomegranate juice in hypertensive subjects. Phytother. Res. 2014, 28, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Dohadwala, M.M.; Holbrook, M.; Hamburg, N.M.; Shenouda, S.M.; Chung, W.B.; Titas, M.; Kluge, M.A.; Wang, N.; Palmisano, J.; Milbury, P.E.; et al. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am. J. Clin. Nutr. 2011, 93, 934–940. [Google Scholar] [CrossRef]
- Arab, L.; Steck, S. Lycopene and cardiovascular disease. Am. J. Clin. Nutr. 2000, 71 (Suppl. 6), 1691S–1697S. [Google Scholar] [CrossRef] [Green Version]
- Rees, A.; Dodd, G.F.; Spencer, J.P.E. The Effects of Flavonoids on Cardiovascular Health: A Review of Human Intervention Trials and Implications for Cerebrovascular Function. Nutrients 2018, 10, 1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G. Flavonoid intake and risk of CVD: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. 2014, 111, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A global concern for food safety, human health and their management. Front. Microbial. 2017, 7, 2170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, G.; Kay, C.D.; Crozier, A. The bioavailability, transport, and bioactivity of dietary flavonoids: A review from a historical perspective. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1054–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thilakarathna, S.H.; Rupasinghe, H.P. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef] [Green Version]
- Pei, R.; Liu, X.; Bolling, B. Flavonoids and gut health. Curr. Opin. Biotechnol. 2020, 61, 153–159. [Google Scholar] [CrossRef]
- Cinta-Pinzaru, S.; Cavalu, S.; Leopold, N.; Petry, R.; Kiefer, W. Raman and surface-enhanced Raman spectroscopy of tempyo spin labelled ovalbumin. J. Mol. Struct. 2001, 565, 225–229. [Google Scholar] [CrossRef]
- Arriagada, F.; Günther, G.; Morales, J. Nanoantioxidant-Based Silica Particles as Flavonoid Carrier for Drug Delivery Applications. Pharmaceutics 2020, 12, 302. [Google Scholar] [CrossRef] [Green Version]
- Zuo, L.; Ao, X.; Guo, Y. Study on the synthesis of dual-chain ionic liquids and their application in the extraction of flavonoids. J. Chromatogr. A 2020, 1628, 461446. [Google Scholar] [CrossRef]
- De Whalley, C.V.; Rankin, S.M.; Hoult, J.R.; Jessup, W.; Leake, D.S. Flavonoids inhibit the oxidative modification of low-density lipoproteins by macrophages. Biochem. Pharmacol. 1990, 39, 1743–1750. [Google Scholar] [CrossRef]
- Fuhrman, B.; Lavy, A.; Aviram, M. Consumption of red wine with meals reduces the susceptibility of human plasma and low-density lipoprotein to lipid peroxidation. Am. J. Clin. Nutr. 1995, 61, 549–554. [Google Scholar] [CrossRef]
- Arai, Y.; Watanabe, S.; Kimira, M.; Shimoi, K.; Mochizuki, R.; Kinae, N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J. Nutr. 2000, 130, 2243–2250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, F.Q.; Zhang, M.F.; Zhang, X.G.; Liu, J.M.; Yuan, W.L. A study on tea-pigment in prevention of atherosclerosis. Chin. Med. J. 1989, 102, 579–583. [Google Scholar] [PubMed]
- Osman, H.E.; Maalej, N.; Shanmuganayagam, D.; Folts, J.D. Grape juice but not orange or grapefruit juice inhibits platelet activity in dogs and monkeys. J. Nutr. 1998, 128, 2307–2312. [Google Scholar] [CrossRef] [Green Version]
- Gryglewski, R.J.; Korbut, R.; Robak, J.; Swies, J. On the mechanism of antithrombotic action of flavonoids. Biochem. Pharmacol. 1987, 36, 317–322. [Google Scholar] [CrossRef]
- Kandaswami, C.; Middleton, E., Jr. Free radical scavenging and antioxidant activity of plant flavonoids. Adv. Exp. Med. Biol. 1994, 366, 351–376. [Google Scholar] [PubMed]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J. Dietary Flavonoids as Cancer Chemopreventive Agents: An Updated Review of Human Studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef] [Green Version]
- Bast, A.; Kaiserová, H.; den Hartog, G.J.; Haenen, G.R.; Van der Vijgh, W.J. Protectors against doxorubicin-induced cardiotoxicity: Flavonoids. Cell Biol. Toxicol. 2007, 23, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, B.; Aviram, M. Flavonoids protect LDL from oxidation and attenuate atherosclerosis. Curr. Opin. Lipidol. 2001, 12, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Bernátová, I.; Pechánová, O.; Babál, P.; Kyselá, S.; Stvrtina, S.; Andriantsitohaina, R. Wine polyphenols improve cardiovascular remodeling and vascular function in NO-deficient hypertension. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H942–H948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayakody, R.L.; Senaratne, M.P.; Thomson, A.B.; Kappagoda, C.T. Cholesterol feeding impairs endothelium-dependent relaxation of rabbit aorta. Can. J. Physiol. Pharmacol. 1985, 63, 1206–1209. [Google Scholar] [CrossRef] [PubMed]
- Hertog, M.G.; Feskens, E.J.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2013, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Andriambeloson, E.; Kleschyov, A.L.; Muller, B.; Beretz, A.; Stoclet, J.C.; Andriantsitohaina, R. Nitric oxide production and endothelium-dependent vasorelaxation induced by wine polyphenols in rat aorta. Br. J. Pharmacol. 1997, 120, 1053–1058. [Google Scholar] [CrossRef] [Green Version]
- Burns, J.; Gardner, P.T.; O’Neil, J.; Crawford, S.; Morecroft, I.; McPhail, D.B.; Lister, C.; Matthews, D.; MacLean, M.R.; Lean, M.E.; et al. Relationship among antioxidant activity, vasodilation capacity, and phenolic content of red wines. J. Agric. Food Chem. 2000, 48, 220–230. [Google Scholar] [CrossRef]
- Trombino, S.; Cassano, R.; Muzzalupo, R.; Pingitore, A.; Cione, E.; Picci, N. Stearyl ferulate-based solid lipid nanoparticles for the encapsulation and stabilization of beta-carotene and alpha-tocopherol. Colloids Surf. B Biointerfaces 2009, 72, 181–187. [Google Scholar] [CrossRef]
- Vicas, S.I.; Cavalu, S.; Laslo, V.; Tocai, M.; Costea, T.O.; Moldovan, L. Growth, Photosynthetic Pigments, Phenolic, Glucosinolates Content and Antioxidant Capacity of Broccoli Sprouts in Response to Nanoselenium Particles Supply. Not. Bot. Horti Agrobot. Cluj Napoca 2019, 47, 821–828. [Google Scholar] [CrossRef]
- Vega-Villa, K.R.; Takemoto, J.K.; Yáñez, J.A.; Remsberg, C.M.; Forrest, M.L.; Davies, N.M. Clinical toxicities of nanocarrier systems. Adv. Drug Deliv. Rev. 2008, 60, 929–938. [Google Scholar] [CrossRef]
- Pala, R.; Anju, V.T.; Dyavaiah, M.; Busi, S.; Nauli, S.M. Nanoparticle-Mediated Drug Delivery for the Treatment of Cardiovascular Diseases. Int. J. Nanomed. 2020, 15, 3741–3769. [Google Scholar] [CrossRef]
- Borel, T.; Sabliov, C.M. Nanodelivery of bioactive components for food applications: Types of delivery systems, properties, and their effect on ADME profiles and toxicity of nanoparticles. Annu. Rev. Food Sci. Technol. 2014, 5, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.; Koliada, A.; Zayachkivska, A.; Lushchak, O. Nanodelivery of Natural Antioxidants: An Anti-aging Perspective. Front. Bioeng. Biotechnol. 2020, 7, 447. [Google Scholar] [CrossRef] [Green Version]
- Naahidi, S.; Jafari, M.; Edalat, F.; Raymond, K.; Khademhosseini, A.; Chen, P. Biocompatibility of engineered nanoparticles for drug delivery. J. Control. Release 2013, 166, 182–194. [Google Scholar] [CrossRef]
- Bertrand, N.; Leroux, J.C. The journey of a drug-carrier in the body: An anatomo-physiological perspective. J. Control. Release 2012, 161, 152–163. [Google Scholar] [CrossRef]
- Tena, N.; Martín, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Dube, A.; Ng, K.; Nicolazzo, J.; Larson, I.C. Effective use of reducing agents and nanoparticle encapsulation in stabilizing catechins in alkaline solution. Food Chem. 2010, 122, 662–667. [Google Scholar] [CrossRef]
- Oliveira, G.; Volino, M.; Conte-Junior, C.A.; Alvares, T.S. Food-derived polyphenol compounds and cardiovascular health: A nano-technological perspective. Food Biosci. 2021, 41, 101033. [Google Scholar] [CrossRef]
- Cavalu, S.; Bisboaca, S.; Mates, I.M.; Pasca, P.; Vasile, L.; Costea, T.; Luminita, F.; Vicas, S.L. Novel Formulation Based on Chitosan-Arabic Gum Nanoparticles Entrapping Propolis Extract Production, physico-chemical and structural characterization. Rev. Chim. 2018, 69, 3756–3760. [Google Scholar] [CrossRef]
- Alotaibi, B.; Tousson, E.; El-Masry, T.A.; Altwaijry, N.; Saleh, A. Ehrlich ascites carcinoma as model for studying the cardiac protective effects of curcumin nanoparticles against cardiac damage in female mice. Environ. Toxicol. 2021, 36, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, Y.; Zhang, W.; Bao, C.; Xie, Z. Relief of oxidative stress and cardiomyocyte apoptosis by using curcumin nanoparticles. Colloids Surf. B Biointerfaces 2017, 153, 174–182. [Google Scholar] [CrossRef]
- Rachmawati, H.; Soraya, I.S.; Kurniati, N.F.; Rahma, A. In Vitro Study on Antihypertensive and Anti-hypercholesterolemic Effects of a Curcumin Nano emulsion. Sci. Pharm. 2016, 84, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Carlson, L.J.; Cote, B.; Alani, A.W.; Rao, D.A. Polymeric micellar co-delivery of resveratrol and curcumin to mitigate in vitro doxorubicin-induced cardiotoxicity. J. Pharm. Sci. 2014, 103, 2315–2322. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, K.; Zeng, H.; Zhang, J.; Pu, Y.; Wang, Z.; Zhang, T.; Wang, B. Resveratrol solid lipid nanoparticles to trigger credible inhibition of doxorubicin cardiotoxicity. Int. J. Nanomed. 2019, 14, 6061–6071. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Hua, Y.; Zhong, J.; Li, X.; Xu, W.; Cai, Y.; Mao, Y.; Lu, X. Resveratrol Delivery by Albumin Nanoparticles Improved Neurological Function and Neuronal Damage in Transient Middle Cerebral Artery Occlusion Rats. Front. Pharmacol. 2018, 9, 1403. [Google Scholar] [CrossRef]
- Lozano, O.; Lázaro-Alfaro, A.; Silva-Platas, C.; Oropeza-Almazán, Y.; Torres-Quintanilla, A.; Bernal-Ramírez, J.; Alves-Figueiredo, H.; García-Rivas, G. Nanoencapsulated Quercetin Improves Cardioprotection during Hypoxia-Reoxygenation Injury through Preservation of Mitochondrial Function. Oxid. Med. Cell. Longev. 2019, 2019, 7683051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.J.; Yao, L.; Hu, Y.M.; Zhao, B.T. Effect of Quercetin-Loaded Mesoporous Silica Nanoparticles on Myocardial Ischemia-Reperfusion Injury in Rats and Its Mechanism. Int. J. Nanomed. 2021, 16, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; Vecchione, R.; De Capua, A.; Lagreca, E.; Iaffaioli, R.V.; Botti, G.; Netti, P.A.; Maurea, N. Nano-Encapsulation of Coenzyme Q10 in Secondary and Tertiary Nano-Emulsions for Enhanced Cardioprotection and Hepatoprotection in Human Cardiomyocytes and Hepatocytes During Exposure to Anthracyclines and Trastuzumab. Int. J. Nanomed. 2020, 15, 4859–4876. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, A. Highly bioavailable curcumin (Theracurmin): Its development and clinical application. PharmaNutrition 2015, 3, 123–130. [Google Scholar] [CrossRef]
- Kanai, M.; Imaizumi, A.; Otsuka, Y.; Sasaki, H.; Hashiguchi, M.; Tsujiko, K.; Matsumoto, S.; Ishiguro, H.; Chiba, T. Dose-escalation and pharmacokinetic study of nanoparticle curcumin, a potential anticancer agent with improved bioavailability, in healthy human volunteers. Cancer Chemother. Pharmacol. 2012, 69, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, H.; Sunagawa, Y.; Takahashi, K.; Imaizumi, A.; Fukuda, H.; Hashimoto, T.; Wada, H.; Katanasaka, Y.; Kakeya, H.; Fujita, M.; et al. Innovative preparation of curcumin for improved oral bioavailability. Biol. Pharm. Bull. 2011, 34, 660–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthu, M.S.; Mei, L.; Feng, S.S. Nanotheranostics: Advanced nanomedicine for the integration of diagnosis and therapy. Nanomedicine 2014, 9, 1277–1280. [Google Scholar] [CrossRef] [PubMed]
- Pala, R.; Pattnaik, S.; Busi, S.; Nauli, S.M. Nanomaterials as Novel Cardiovascular Theranostics. Pharmaceutics 2021, 13, 348. [Google Scholar] [CrossRef]
- Cavalu, S.; Fritea, L.; Brocks, M.; Barbaro, K.; Murvai, G.; Costea, T.O.; Antoniac, I.; Verona, C.; Romani, M.; Latini, A.; et al. Novel Hybrid Composites Based on PVA/SeTiO2Nanoparticles and Natural Hydroxyapatite for Orthopedic Applications: Correlations between Structural, Morphological and Biocompatibility Properties. Materials 2020, 13, 2077. [Google Scholar] [CrossRef] [PubMed]
- Fritea, L.; Banica, F.; Costea, T.O.; Moldovan, L.; Iovan, C.; Cavalu, S. A gold-nanoparticles—Graphene based electrochemical sensor for sensitive determination of nitrazepam. J. Electroanal. Chem. 2018, 830, 63–71. [Google Scholar] [CrossRef]
- Suzuki, M.; Bachelet-Violette, L.; Rouzet, F.; Beilvert, A.; Autret, G.; Maire, M.; Menager, C.; Louedec, L.; Choqueux, C.; Saboural, P.; et al. Ultrasmall superparamagnetic iron oxide nanoparticles coated with fucoidan for molecular MRI of intraluminal thrombus. Nanomedicine 2015, 10, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Danila, D.; Johnson, E.; Kee, P. CT imaging of myocardial scars with collagen-targeting gold nanoparticles. Nanomedicine 2013, 9, 1067–1076. [Google Scholar] [CrossRef]
- Lestini, B.J.; Sagnella, S.M.; Xu, Z.; Shive, M.S.; Richter, N.J.; Jayaseharan, J.; Case, A.J.; Kottke-Marchant, K.; Anderson, J.M.; Marchant, R.E. Surface modification of liposomes for selective cell targeting in cardiovascular drug delivery. J. Control. Release 2002, 78, 235–247. [Google Scholar] [CrossRef]
- Bilia, A.R.; Piazzini, V.; Guccione, C.; Risaliti, L.; Asprea, M.; Capecchi, G.; Bergonzi, M.C. Improving on Nature: The Role of Nanomedicine in the Development of Clinical Natural Drugs. Planta Med. 2017, 83, 366–381. [Google Scholar] [CrossRef] [Green Version]
- Piazzini, V.; Lemmi, B.; D’Ambrosio, M.; Luceri, C.; Cinci, L.; Ghelardini, C.; Bilia, A.R.; Di Cesare Mannelli, L.; Bergonzi, M.C. Nanostructured Lipid Carriers as Promising Delivery Systems for Plant Extracts: The Case of Silymarin. Appl. Sci. 2018, 8, 1163. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).