Porous Electrodeposited Cu as a Potential Electrode for Electrochemical Reduction Reactions of CO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Fabrication
2.2. Sample Characterization
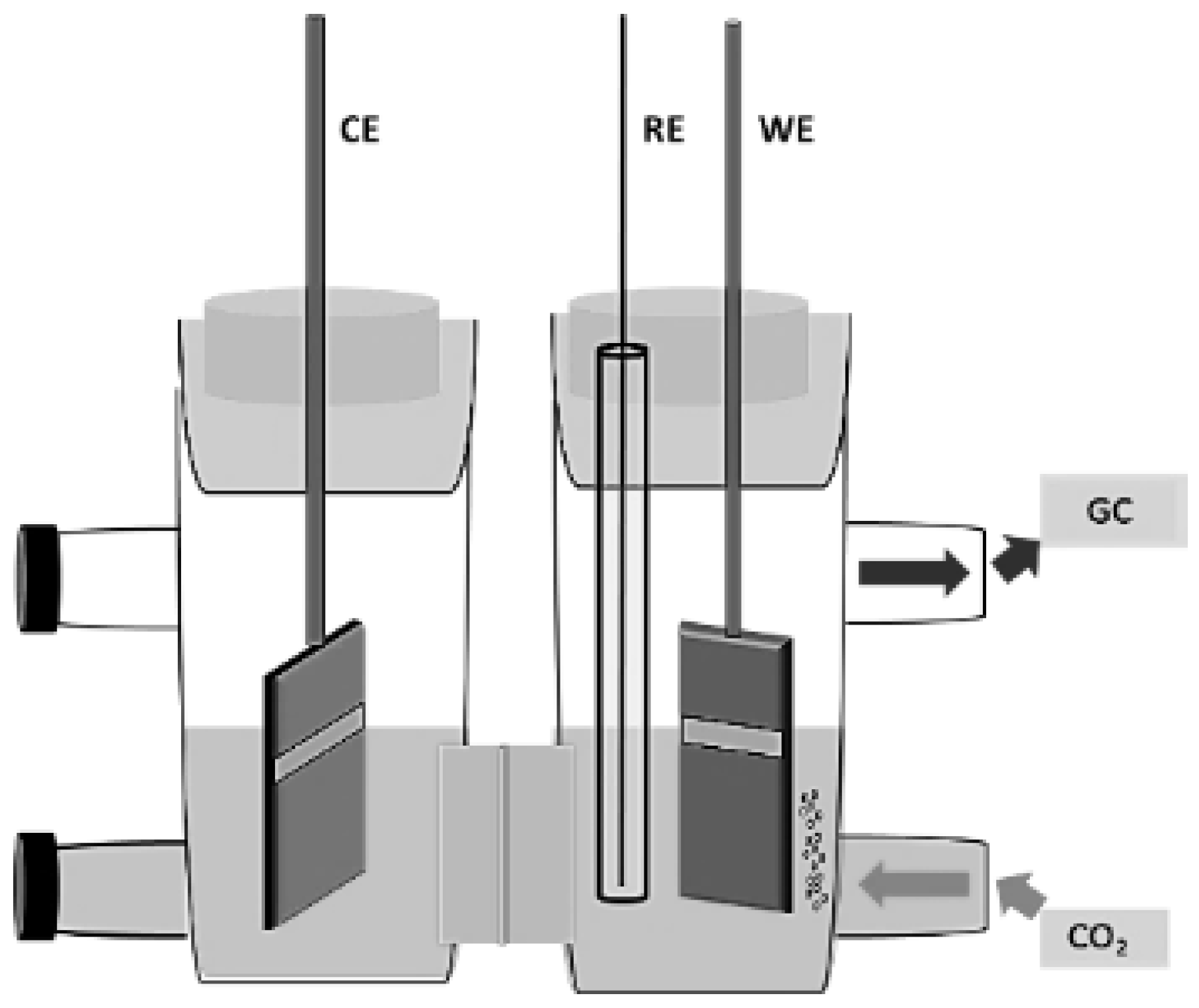
2.3. CO2RR Experiment
2.4. Product Analysis
3. Results and Discussions
3.1. Electrodeposition of Porous Copper
3.2. CO2RR Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, M.; Wang, J.; Li, P.; Chang, K.; Li, C.; Wang, T.; Jiang, B.; Zhang, H.; Liu, H.; Yamauchi, Y.; et al. Mesoporous palladium–copper bimetallic electrodes for selective electrocatalytic reduction of aqueous CO2 to CO. J. Mater. Chem. A 2016, 4, 4776–4782. [Google Scholar] [CrossRef]
- Dutta, A.; Rahaman, M.; Luedi, N.C.; Mohos, M.; Broekmann, P. Morphology Matters: Tuning the Product Distribution of CO2 Electroreduction on Oxide-Derived Cu Foam Catalysts. ACS Catal. 2016, 6, 3804–3814. [Google Scholar] [CrossRef]
- Pestryakov, A.; Petranovskii, V.; Pfänder, N.; Knop-Gericke, A. Supported foam–copper catalysts for methanol selective oxidation. Catal. Commun. 2004, 5, 777–781. [Google Scholar] [CrossRef]
- Shin, H.-C.; Liu, M. Copper Foam Structures with Highly Porous Nanostructured Walls. Chem. Mater. 2004, 16, 5460–5464. [Google Scholar] [CrossRef]
- Najdovski, I. The Electrochemical Fabrication of Porous Bimetallic Structures and Their Applications in Catalysis and Sensing. Ph.D. Thesis, RMIT University, Melbourne, Australia, 2013. [Google Scholar]
- Huang, Y.; Handoko, A.D.; Hirunsit, P.; Yeo, B.S. Electrochemical Reduction of CO2 Using Copper Single-Crystal Surfaces: Effects of CO* Coverage on the Selective Formation of Ethylene. ACS Catal. 2017, 7, 1749–1756. [Google Scholar] [CrossRef]
- Rasul, S.; Pugnant, A.; Xiang, H.; Fontmorin, J.-M.; Yu, E.H. Low cost and efficient alloy electrocatalysts for CO2 reduction to formate. J. CO2 Util. 2019, 32, 1–10. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.W.; Kanan, M.W. Aqueous CO2 Reduction at Very Low Overpotential on Oxide-Derived Au Nanoparticles. J. Am. Chem. Soc. 2012, 134, 19969–19972. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Yang, X.; Lu, A.-Y.; Tseng, C.-C.; Hedhili, M.N.; Li, L.-J.; Huang, K.-W. Low overpotential and high current CO2 reduction with surface reconstructed Cu foam electrodes. Nano Energy 2016, 27, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Sen, S.; Liu, D.; Palmore, G.T.R. Electrochemical Reduction of CO2 at Copper Nanofoams. ACS Catal. 2014, 4, 3091–3095. [Google Scholar] [CrossRef]
- Kas, R.; Hummadi, K.K.; Kortlever, R.; De Wit, P.; Milbrat, A.; Luiten-Olieman, M.W.J.; Benes, N.E.; Koper, R.K.M.T.M.; Mul, G. Three-dimensional porous hollow fibre copper electrodes for efficient and high-rate electrochemical carbon dioxide reduction. Nat. Commun. 2016, 7, 10748. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.; Jouny, M.; Luc, W.; Zhu, W.; Zhu, J.; Jiao, F. A Highly Porous Copper Electrocatalyst for Carbon Dioxide Reduction. Adv. Mater. 2018, 30, e1803111. [Google Scholar] [CrossRef]
- Zhu, P.; Wu, Z.; Zhao, Y. Hierarchical porous Cu with high surface area and fluid permeability. Scr. Mater. 2019, 172, 119–124. [Google Scholar] [CrossRef]
- Cao, C.; Cheng, J. Fabrication of robust surfaces with special wettability on porous copper substrates for various oil/water separations. Chem. Eng. J. 2018, 347, 585–594. [Google Scholar] [CrossRef]
- Michailidis, N.; Stergioudi, F.; Seventekidis, P.; Tsouknidas, A.; Sagris, D. Production of porous copper with high surface area for efficient water purification. CIRP J. Manuf. Sci. Technol. 2016, 13, 85–89. [Google Scholar] [CrossRef]
- Rehman, T.-U.; Ali, H.M. Experimental investigation on paraffin wax integrated with copper foam based heat sinks for electronic components thermal cooling. Int. Commun. Heat Mass Transf. 2018, 98, 155–162. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, W.; Lin, Y.; Chen, L.; Chu, X.; Zheng, T.; Wan, S.; Lin, J. Novel copper foam with ordered hole arrays as catalyst support for methanol steam reforming microreactor. Appl. Energy 2019, 246, 24–37. [Google Scholar] [CrossRef]
- Plowman, B.J.; Jones, L.A.; Bhargava, S.K. Building with bubbles: The formation of high surface area honeycomb-like films via hydrogen bubble templated electrodeposition. Chem. Commun. 2015, 51, 4331–4346. [Google Scholar] [CrossRef]
- Zhang, H.; Ye, Y.; Shen, R.; Ru, C.; Hu, Y. Effect of Bubble Behavior on the Morphology of Foamed Porous Copper Prepared via Electrodeposition. J. Electrochem. Soc. 2013, 160, D441–D445. [Google Scholar] [CrossRef]
- Nguyen, T.; Boudard, M.; Carmezim, M.; Montemor, F. Hydrogen bubbling-induced micro/nano porous MnO 2 films prepared by electrodeposition for pseudocapacitor electrodes. Electrochim. Acta 2016, 202, 166–174. [Google Scholar] [CrossRef]
- Berkesi, K. Electrodeposited iron-based nanofoams as precursors for transducers. J. Phys. Conf. Ser. 2017, 939, 012037. [Google Scholar] [CrossRef]
- Yang, K.D.; Ko, W.R.; Lee, J.H.; Kim, S.J.; Lee, H.; Lee, M.H.; Nam, K.T. Morphology-Directed Selective Production of Ethylene or Ethane from CO2 on a Cu Mesopore Electrode. Angew. Chem. Int. Ed. 2017, 56, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Padilla, M.; Baturina, O.; Gordon, J.P.; Artyushkova, K.; Atanassov, P.; Serov, A. Selective CO2 electroreduction to CH4 on porous Cu films synthesized by sacrificial support method. J. CO2 Util. 2017, 19, 137–145. [Google Scholar] [CrossRef]
- Jhong, H.-R.; Ma, S.; Kenis, P.J. Electrochemical conversion of CO2 to useful chemicals: Current status, remaining challenges, and future opportunities. Curr. Opin. Chem. Eng. 2013, 2, 191–199. [Google Scholar] [CrossRef]
- Hori, Y. Electrochemical CO2 Reduction on Metal Electrodes. In Modern Aspects of Electrochemistry; Vayenas, C.G., White, R.E., Gamboa-Aldeco, M.E., Eds.; Springer: New York, NY, USA, 2008; pp. 89–189. [Google Scholar]
- Kuhl, K.P.; Cave, E.R.; Abram, D.N.; Jaramillo, T.F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 2012, 5, 7050–7059. [Google Scholar] [CrossRef]
- Pérez-Fortes, M.; Schöneberger, J.C.; Boulamanti, A.; Tzimas, E. Methanol synthesis using captured CO2 as raw material: Techno-economic and environmental assessment. Appl. Energy 2016, 161, 718–732. [Google Scholar] [CrossRef]
- Hirunsit, P.; Soodsawang, W.; Limtrakul, J. CO2 Electrochemical Reduction to Methane and Methanol on Copper-Based Alloys: Theoretical Insight. J. Phys. Chem. C 2015, 119, 8238–8249. [Google Scholar] [CrossRef]
- Hatsukade, T.; Kuhl, K.P.; Cave, E.R.; Abram, D.N.; Jaramillo, T.F. Insights into the electrocatalytic reduction of CO2 on metallic silver surfaces. Phys. Chem. Chem. Phys. 2014, 16, 13814–13819. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jung, H.; Kim, N.-K.; Oh, H.-S.; Min, B.K.; Hwang, Y.J. Mixed Copper States in Anodized Cu Electrocatalyst for Stable and Selective Ethylene Production from CO2 Reduction. J. Am. Chem. Soc. 2018, 140, 8681–8689. [Google Scholar] [CrossRef]
- Wang, X.; Klingan, K.; Klingenhof, M.; Möller, T.; de Araújo, J.F.; Martens, I.; Bagger, A.; Jiang, S.; Rossmeisl, J.; Dau, H.; et al. Morphology and mechanism of highly selective Cu(II) oxide nanosheet catalysts for carbon dioxide electroreduction. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Li, C.; Kanan, M.W. CO2 Reduction at Low Overpotential on Cu Electrodes Resulting from the Reduction of Thick Cu2O Films. J. Am. Chem. Soc. 2012, 134, 7231–7234. [Google Scholar] [CrossRef]
- Ren, D.; Deng, Y.; Handoko, A.D.; Chen, C.S.; Malkhandi, S.; Yeo, B.S. Selective Electrochemical Reduction of Carbon Dioxide to Ethylene and Ethanol on Copper(I) Oxide Catalysts. ACS Catal. 2015, 5, 2814–2821. [Google Scholar] [CrossRef]
- Xiao, H.; Iii, W.A.G.; Cheng, T.; Liu, Y. Cu metal embedded in oxidized matrix catalyst to promote CO2 activation and CO dimerization for electrochemical reduction of CO2. Proc. Natl. Acad. Sci. USA 2017, 114, 6685. [Google Scholar] [PubMed] [Green Version]
- Daiyan, R.; Lu, X.; Ng, Y.H.; Amal, R. Highly Selective Conversion of CO2 to CO Achieved by a Three-Dimensional Porous Silver Electrocatalyst. ChemistrySelect 2017, 2, 879–884. [Google Scholar] [CrossRef]
- Niu, J.; Liu, X.; Xia, K.; Xu, L.; Xu, Y.; Fang, X.; Lu, W. Effect of electrodeposition parameters on the morphology of three-dimensional porous copper foams. Int. J. Electrochem. Sci. 2015, 10, 7331–7340. [Google Scholar]
- Nguyen-Phan, T.-D.; Wang, C.; Marin, C.M.; Zhou, Y.; Stavitski, E.; Popczun, E.J.; Yu, Y.; Xu, W.; Howard, B.H.; Stuckman, M.Y.; et al. Understanding three-dimensionally interconnected porous oxide-derived copper electrocatalyst for selective carbon dioxide reduction. J. Mater. Chem. A 2019, 7, 27576–27584. [Google Scholar] [CrossRef]
- Chou, T.-C.; Chang, C.-C.; Yu, H.-L.; Yu, W.-Y.; Dong, C.-L.; Velasco-Vélez, J.-J.; Chuang, C.-H.; Chen, L.-C.; Lee, J.-F.; Chen, J.-M.; et al. Controlling the Oxidation State of the Cu Electrode and Reaction Intermediates for Electrochemical CO2 Reduction to Ethylene. J. Am. Chem. Soc. 2020, 142, 2857–2867. [Google Scholar] [CrossRef]







| Sample Group | Bath Composition | Current Density (A/cm2) | Deposition Time (s) |
|---|---|---|---|
| A | 1.5M H2SO4 + 0.2M CuSO4 | 2, 3, 3.5 | 20, 40, 60 |
| B | 1.5M H2SO4 + 0.4M CuSO4 | 2, 3, 3.5 | 20, 40, 60 |
| C | 1.5M H2SO4 + 0.4M CuSO4 + 0.05M HCl | 2, 3, 3.5 | 20, 40, 60 |
| Characteristics | A | B | C |
|---|---|---|---|
| Apparent pore size (µm) | 58 | 52 | 340 |
| Apparent porosity (%) | 31 | 37 | 51 |
| BET surface area (m2/g) | 19.56 | 4.00 | 3.75 |
| Apparent density (g/cm3) | 0.34 | 0.47 | 0.26 |
| True porosity (%) | 96.26 | 94.77 | 97.07 |
| EDX Cu content (at.%) | 89.0 | 87.2 | 71.3 |
| EDX O content (at.%) | 10.5 | 12.8 | 27.8 |
| Sample | Reduction Current Density (mA/cm2) | Product (µmol) | ||||||
|---|---|---|---|---|---|---|---|---|
| Gas Product | Aqueous Product | |||||||
| H2 | CO | CH3CHO | CH3COO− | CH3COCH3 | CH5OH | HCOO− | ||
| Cu foil | −0.62 | 3.58 | - | - | - | 1.03 | 1.61 | 0.95 |
| A | −4.11 | 5.42 | 0.09 | - | 0.68 | 0.94 | 1.90 | 0.26 |
| B | −4.74 | 3.40 | 0.08 | 1.08 | 0.49 | 0.70 | 2.56 | 0.19 |
| C | −4.40 | 9.98 | 0.36 | - | 0.95 | 1.02 | 1.38 | 1.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darayen, J.; Chailapakul, O.; Praserthdam, P.; Panpranot, J.; Tungasmita, D.N.; Boonyongmaneerat, Y. Porous Electrodeposited Cu as a Potential Electrode for Electrochemical Reduction Reactions of CO2. Appl. Sci. 2021, 11, 11104. https://doi.org/10.3390/app112311104
Darayen J, Chailapakul O, Praserthdam P, Panpranot J, Tungasmita DN, Boonyongmaneerat Y. Porous Electrodeposited Cu as a Potential Electrode for Electrochemical Reduction Reactions of CO2. Applied Sciences. 2021; 11(23):11104. https://doi.org/10.3390/app112311104
Chicago/Turabian StyleDarayen, Jidsucha, Orawon Chailapakul, Piyasan Praserthdam, Joongjai Panpranot, Duangamol N. Tungasmita, and Yuttanant Boonyongmaneerat. 2021. "Porous Electrodeposited Cu as a Potential Electrode for Electrochemical Reduction Reactions of CO2" Applied Sciences 11, no. 23: 11104. https://doi.org/10.3390/app112311104







