A Durable PVDF/PFOTES-SiO2 Superhydrophobic Coating on AZ31B Mg Alloy with Enhanced Abrasion Resistance Performance and Anti-Corrosion Properties
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
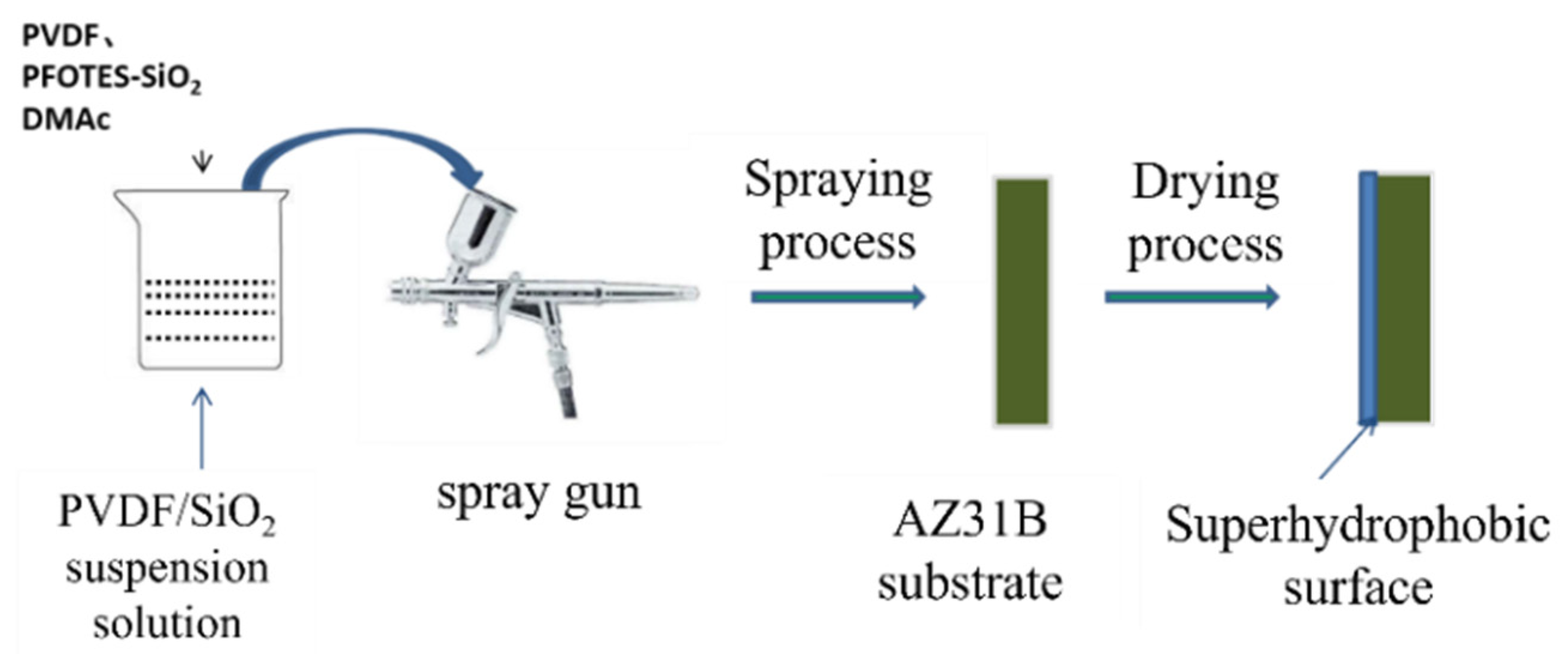
2.2. Preparation of the Superhydrophobic Surfaces
2.3. Characterization
2.4. Electrochemical Tests
2.5. Corrosion Tests
3. Results and Discussion
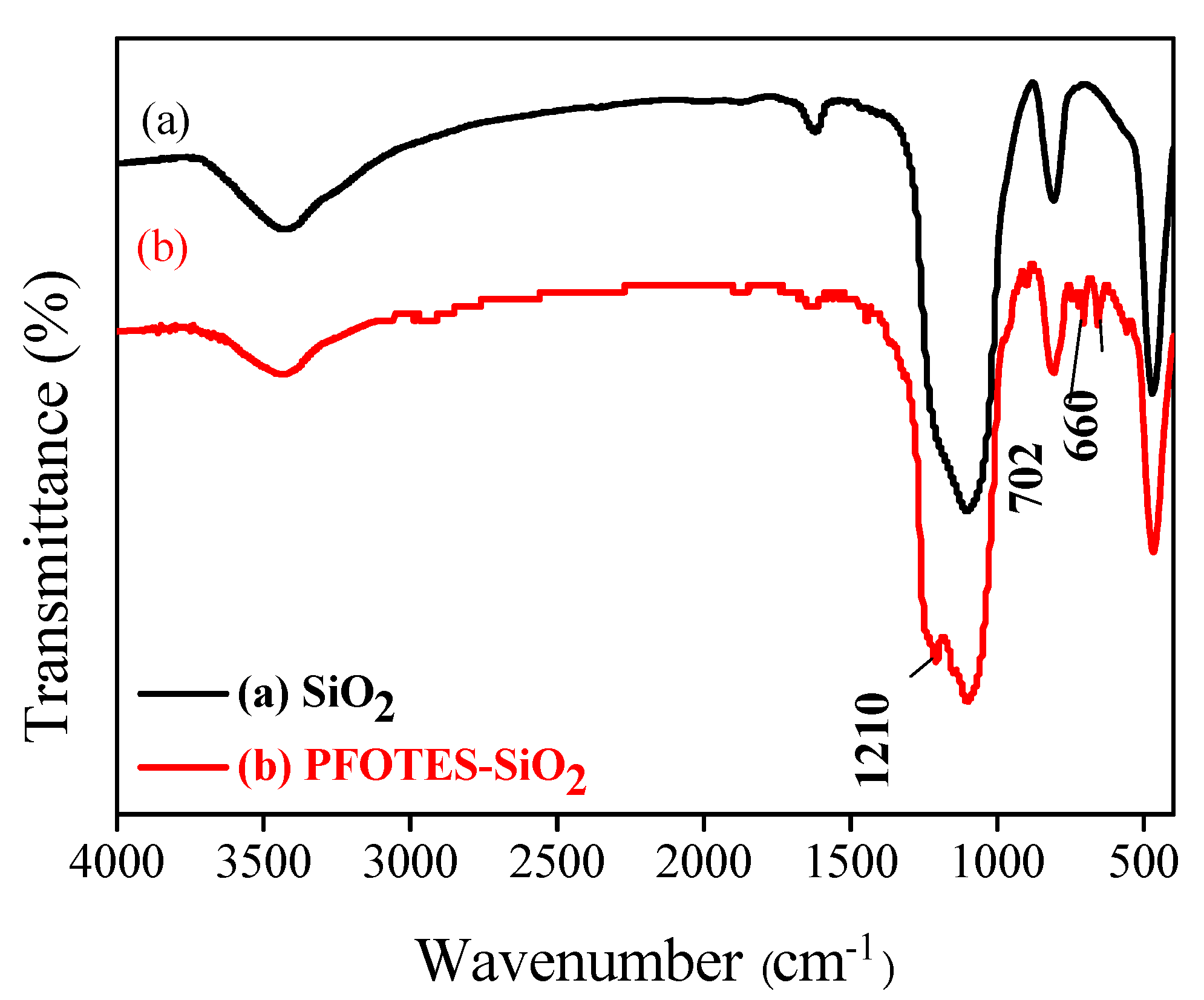
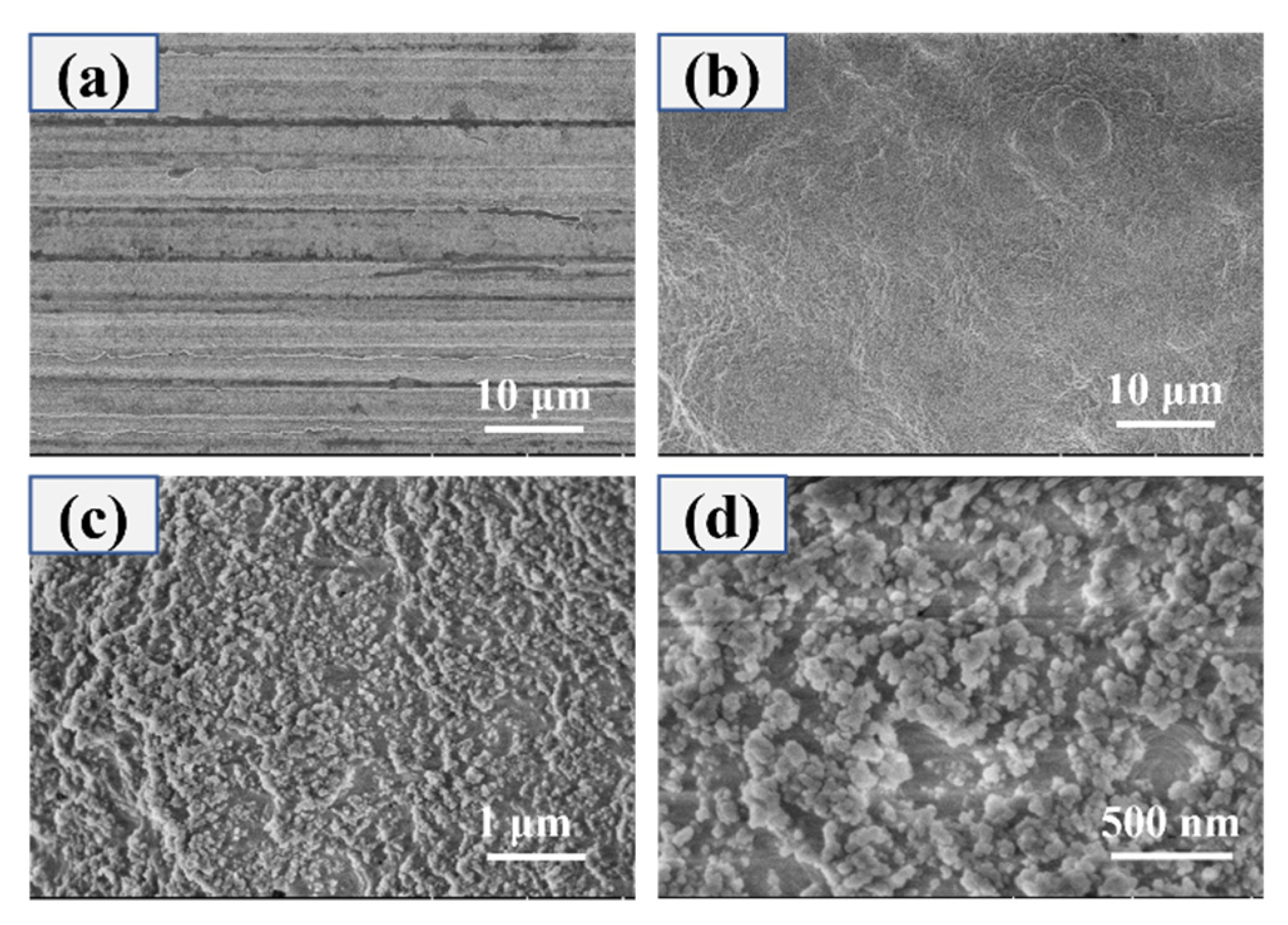
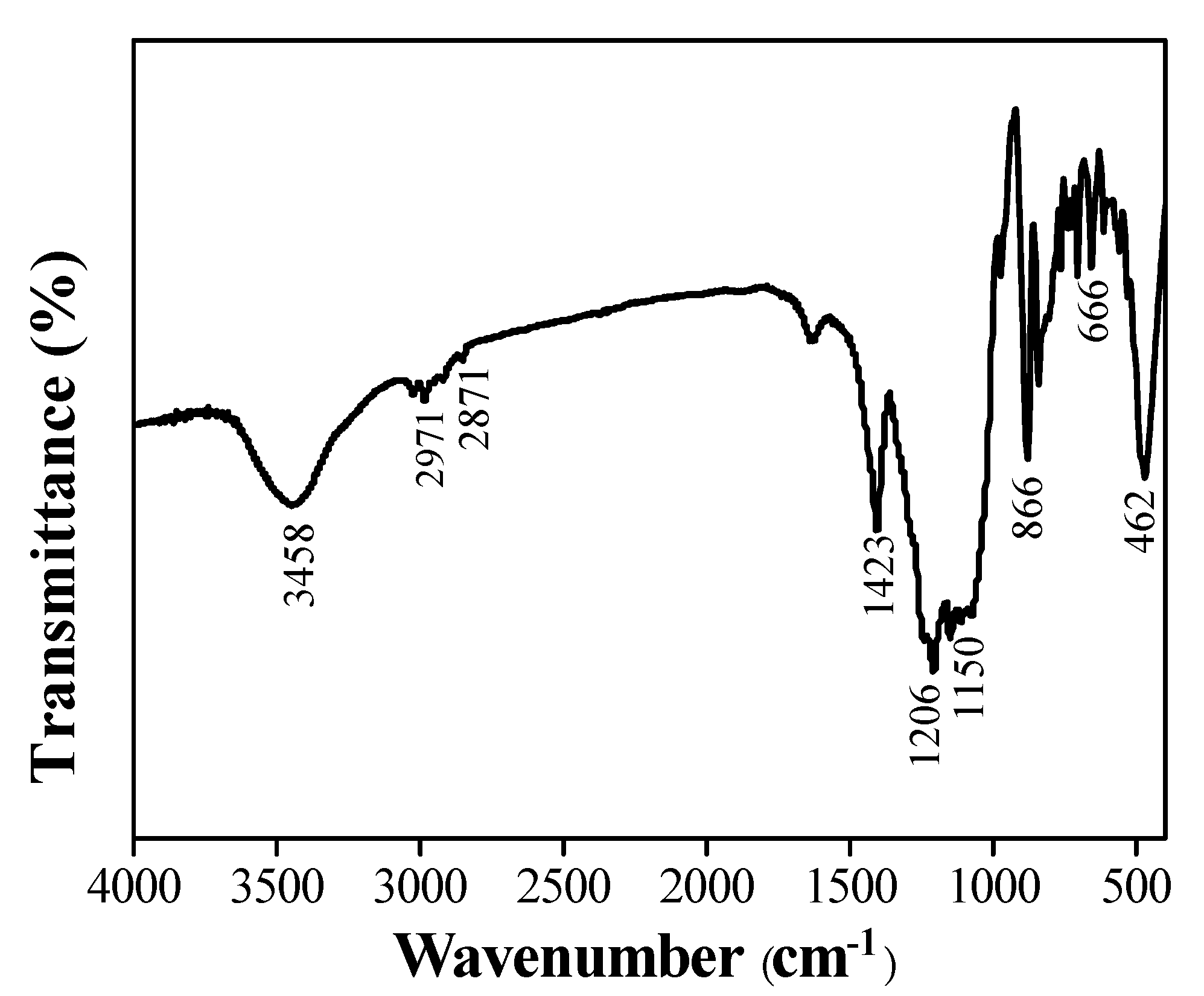
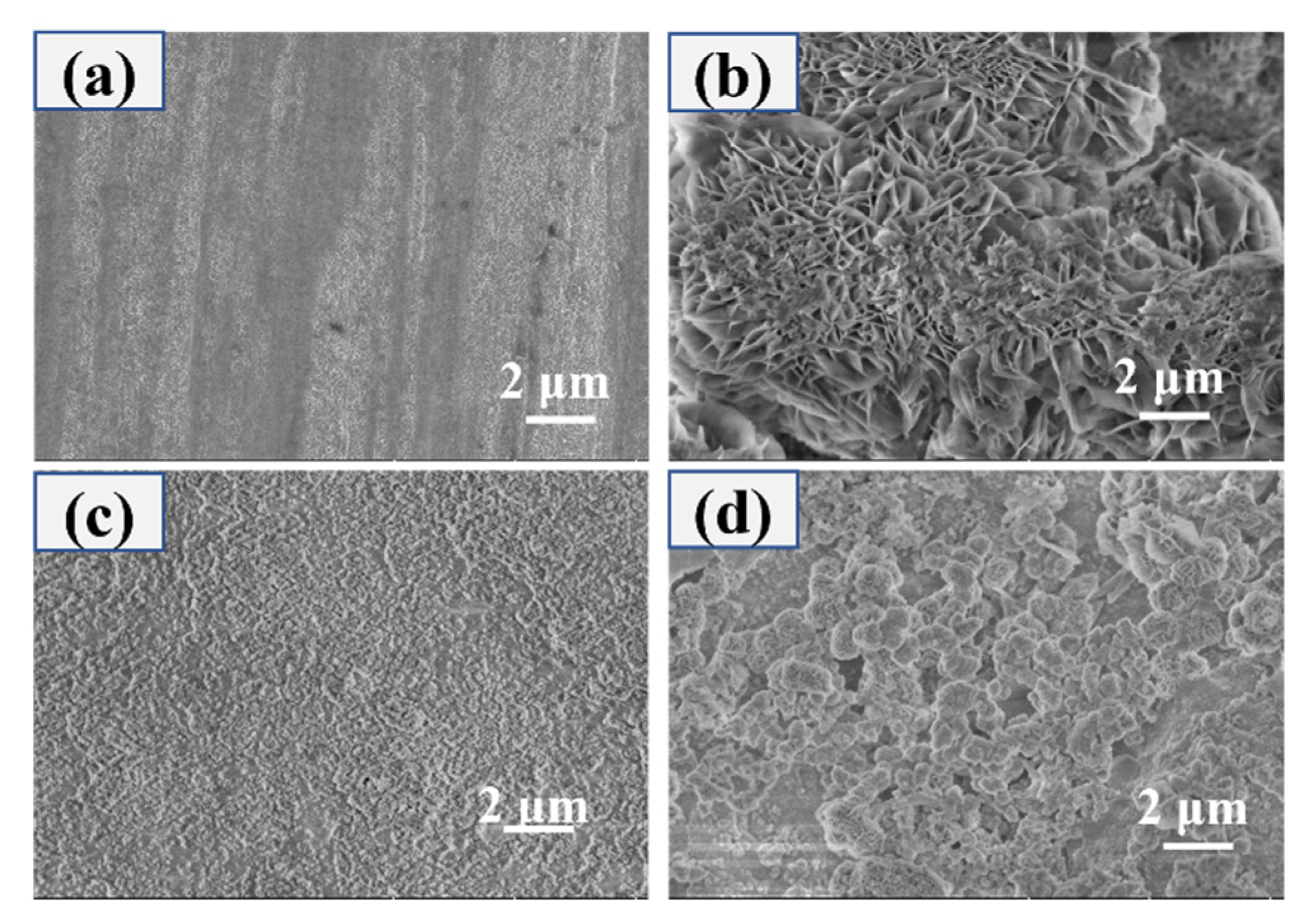
3.1. Surface Morphology and Composition
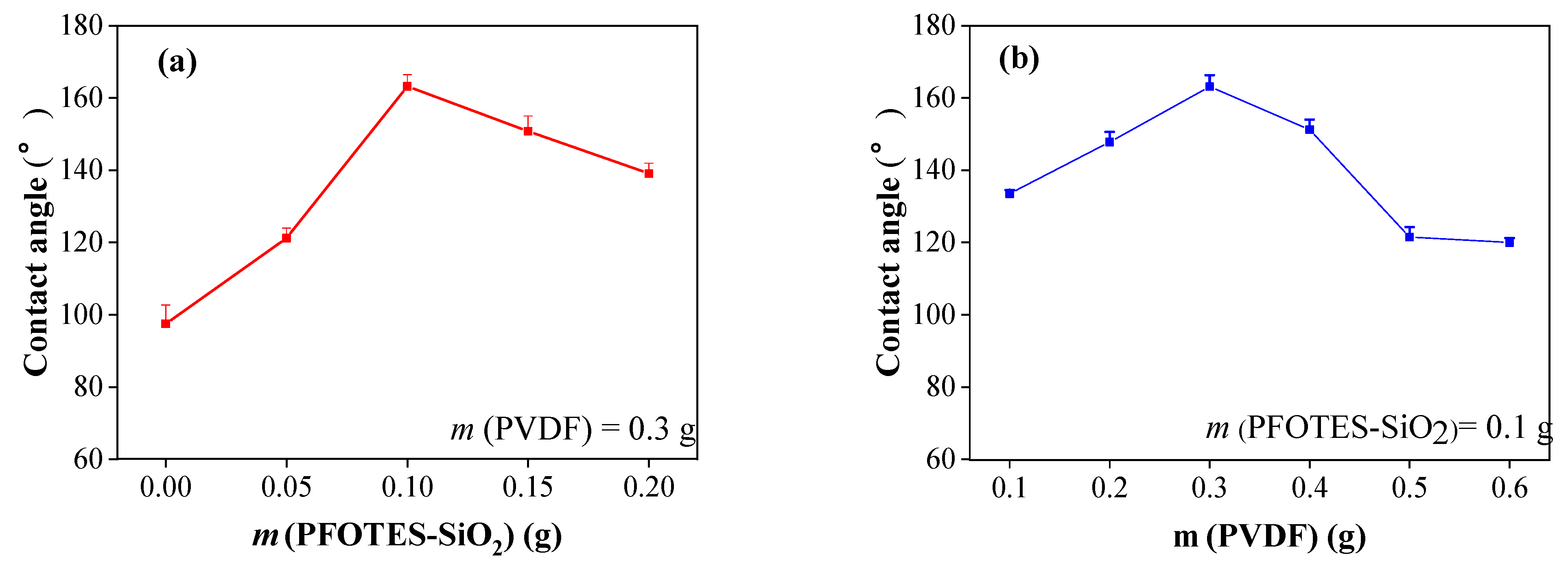
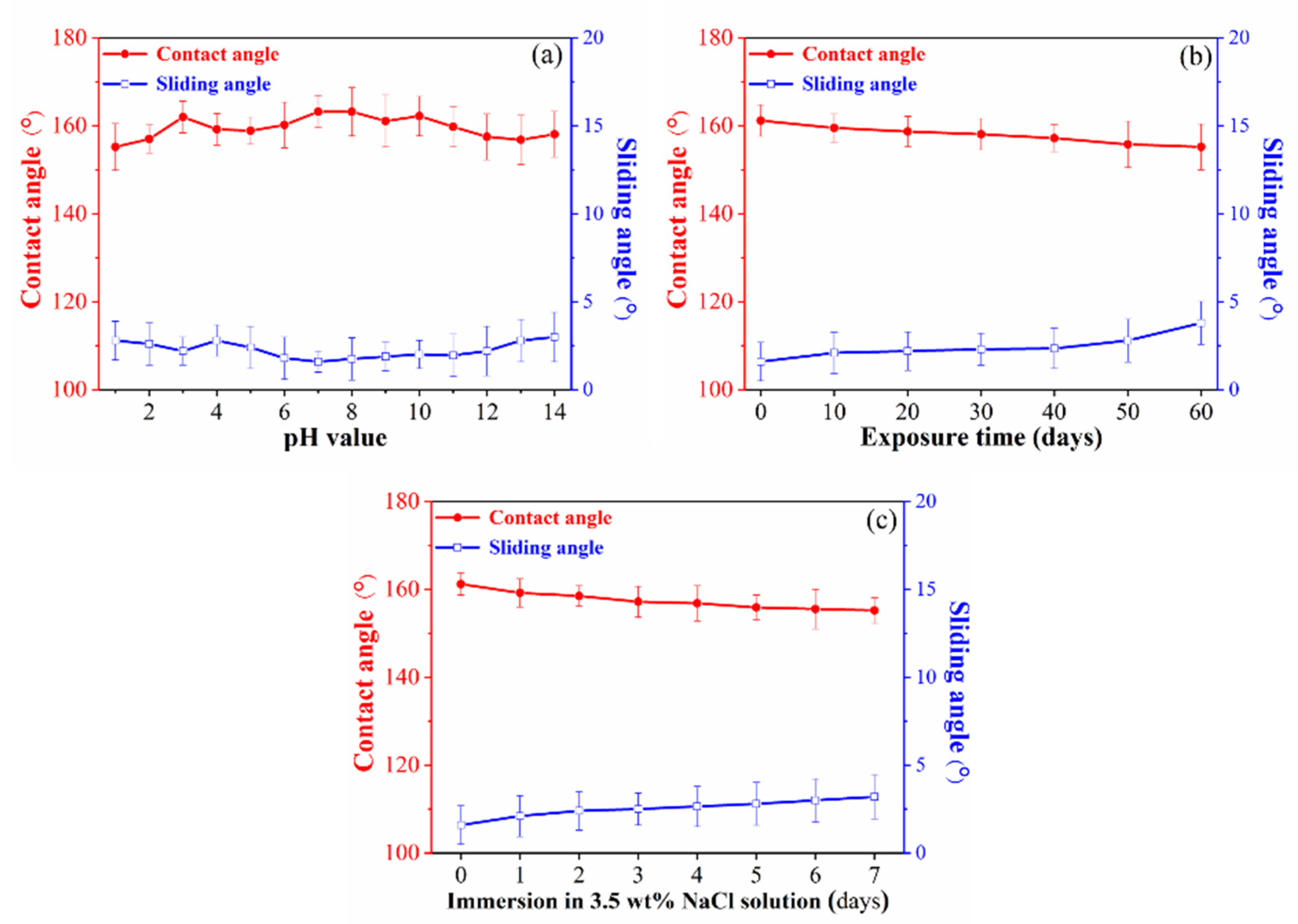
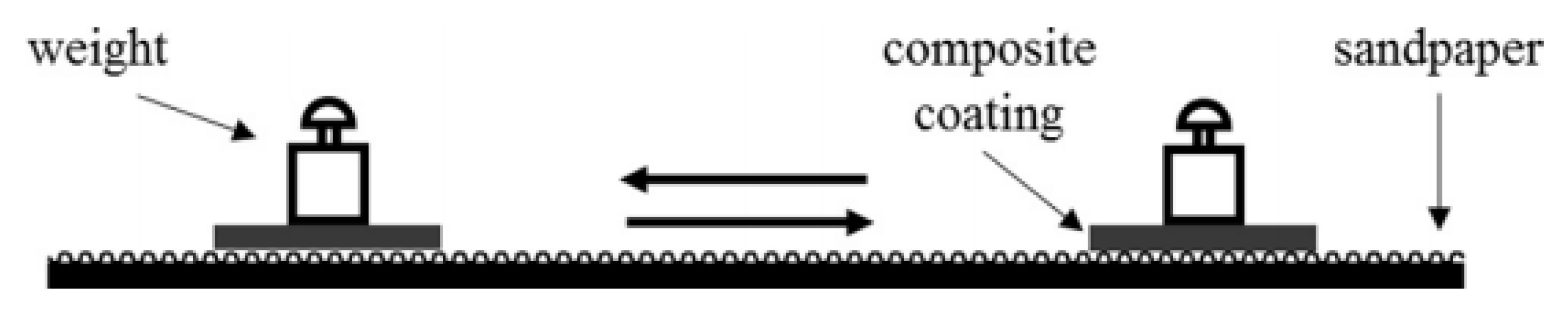
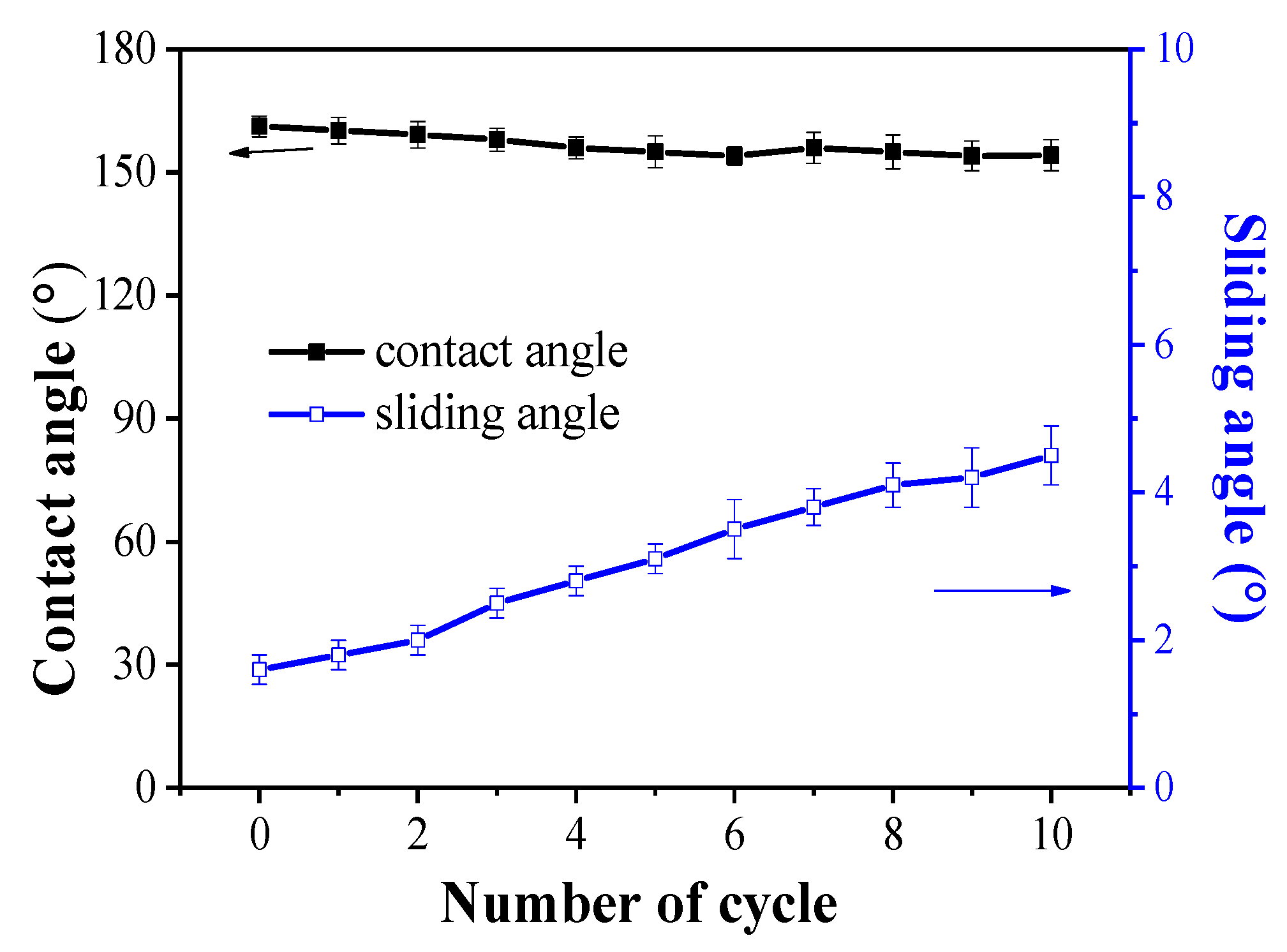
3.2. Stability and Wear Resistance of Superhydrophobic Surfaces
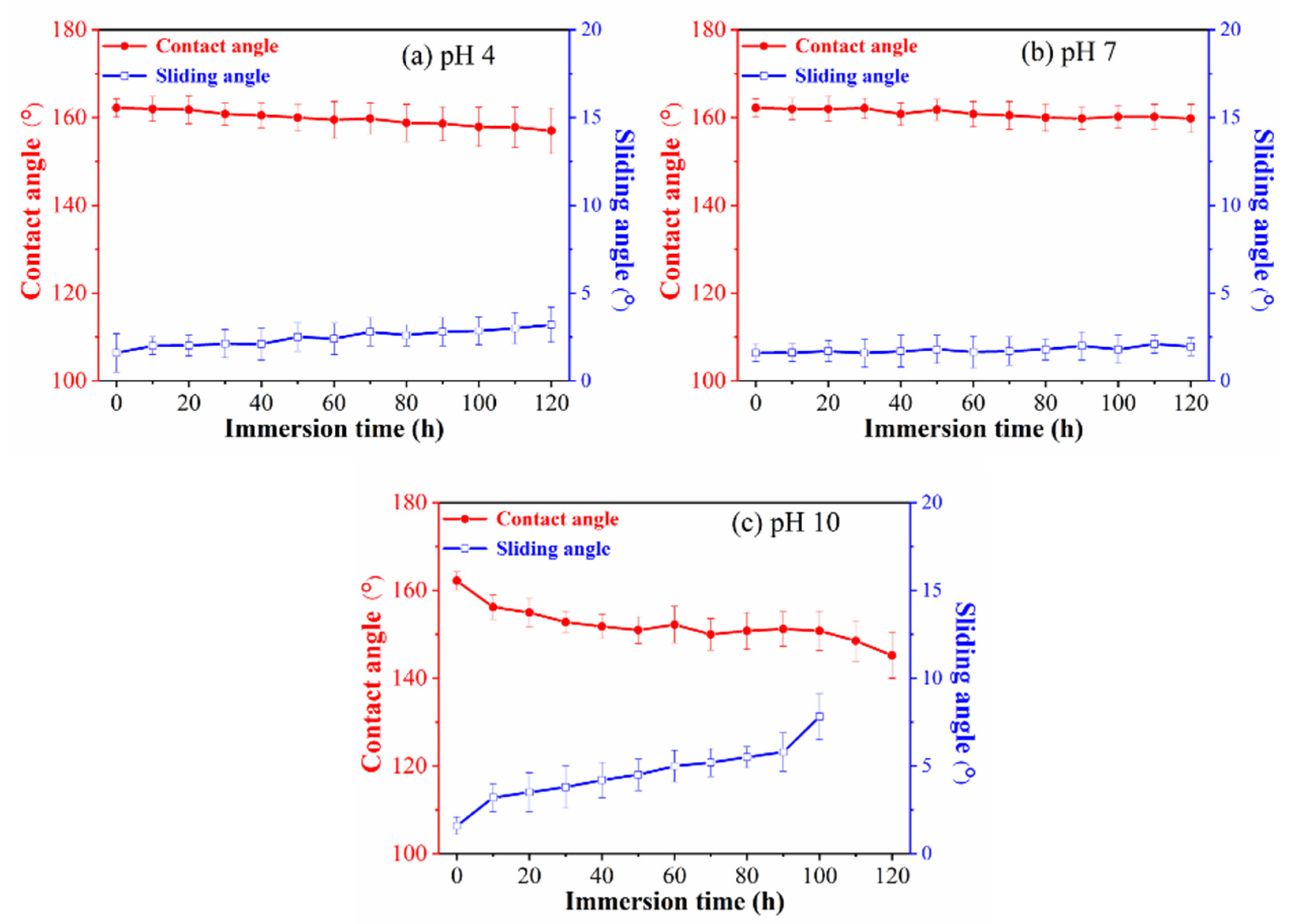
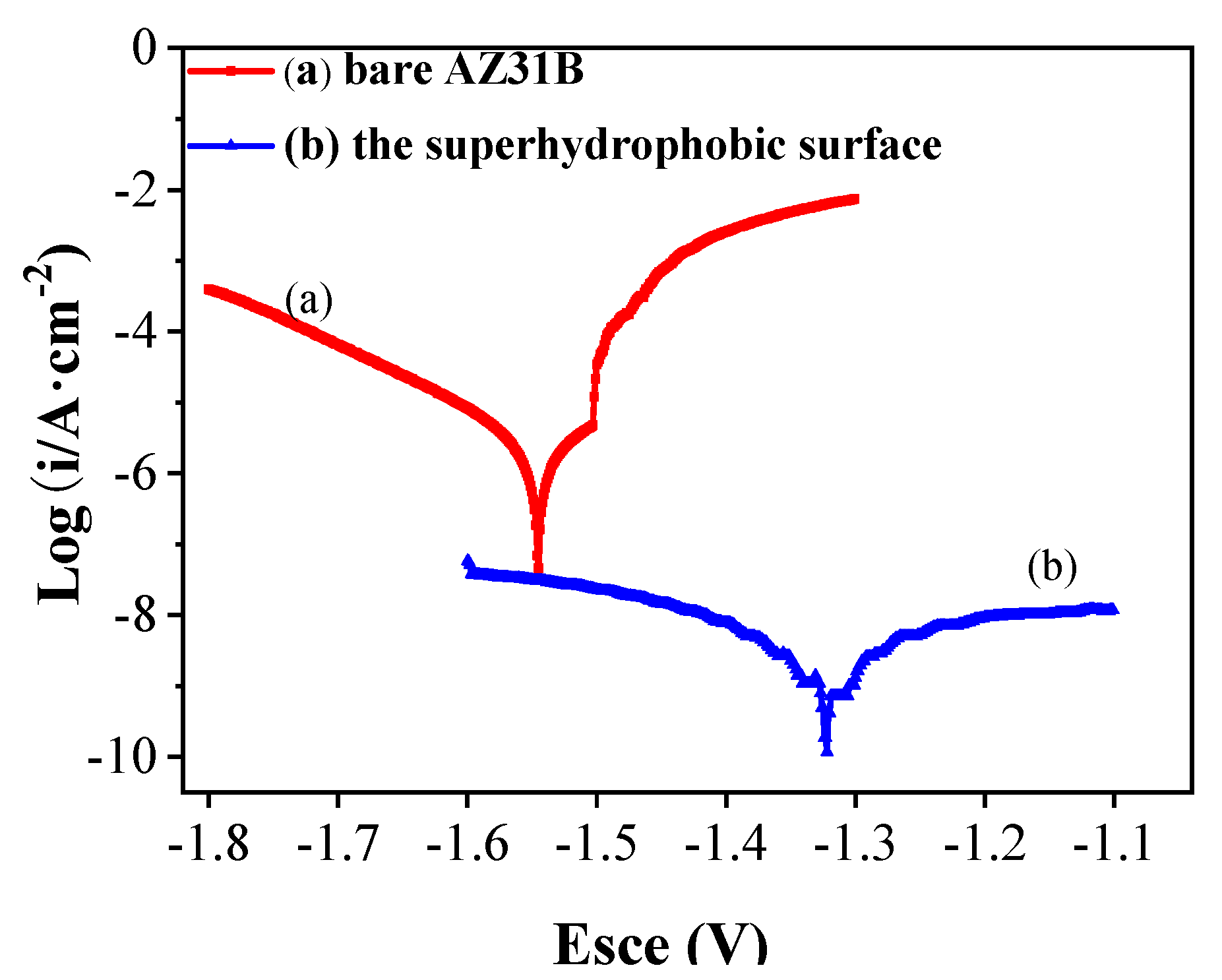
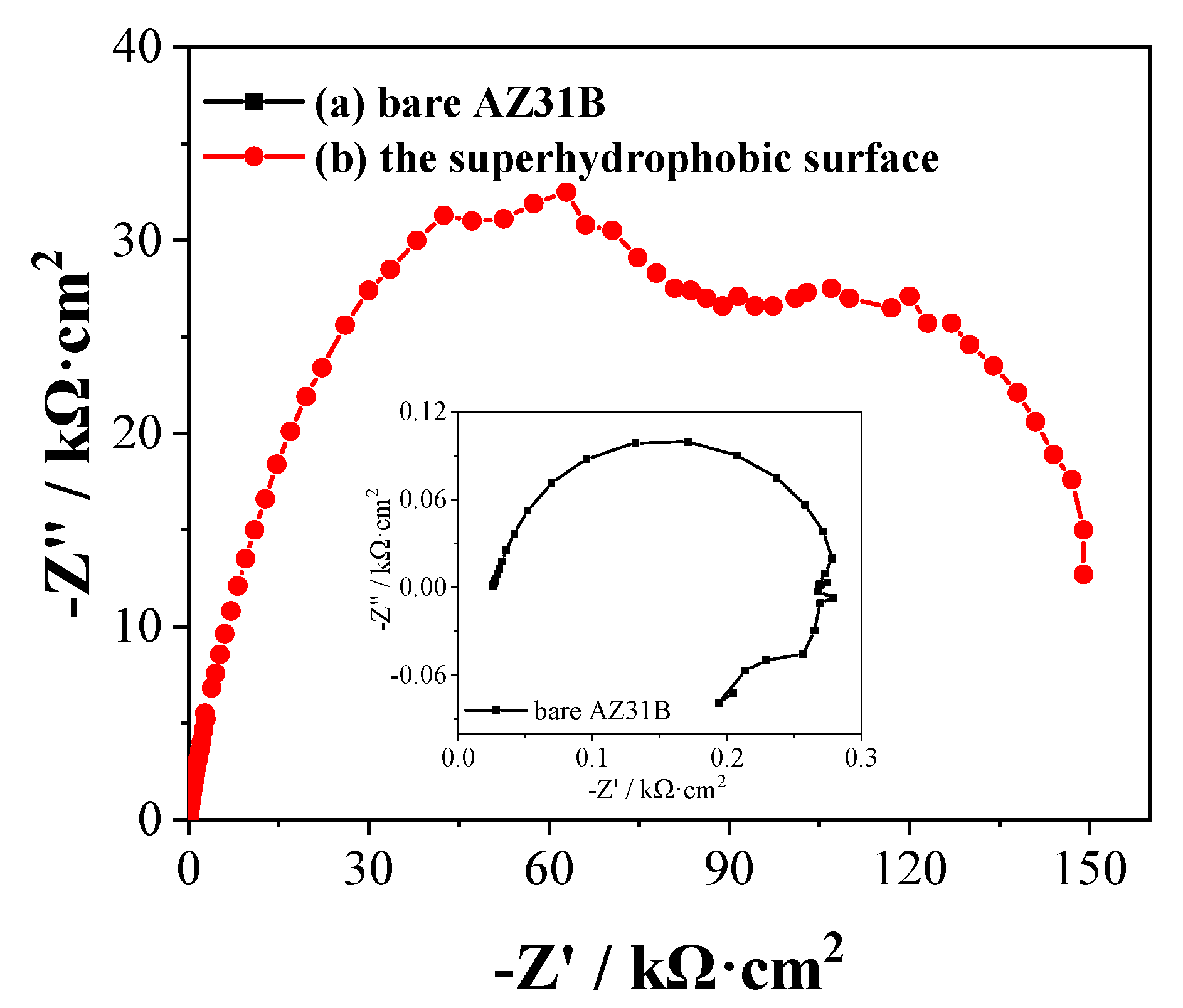
3.3. Corrosion Resistance Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, G.; Wu, L.; Tang, A.; Zhang, S.; Yuan, B.; Zheng, Z.; Pan, F. A Novel Approach to Fabricate Protective Layered Double Hydroxide Films on the Surface of Anodized Mg-Al Alloy. Adv. Mater. Interfaces 2017, 4, 1700163. [Google Scholar] [CrossRef]
- Chang, L.R.; Cao, F.H.; Cai, J.S.; Liu, W.J.; Zhang, Z.; Zhang, J.Q. Influence of electric parameters on MAO of AZ91D magnesium alloy using alternative square-wave power source. Trans. Nonferrous Met. Soc. China 2011, 21, 307–316. [Google Scholar] [CrossRef]
- Atrens, A.; Song, G.L.; Liu, M.; Shi, A.M.; Cao, F.Y.; Dargusch, M.S. Review of Recent Developments in the Field of Magnesium Corrosion. Adv. Eng. Mater. 2015, 17, 400–453. [Google Scholar] [CrossRef]
- Wang, H.Y.; Liu, Z.J.; Wang, E.Q.; Yuan, R.X.; Gao, D.; Zhang, X.G.; Zhu, Y.J. A robust superhydrophobic PVDF composite coating with wear/corrosion-resistance properties. Appl. Surf. Sci. 2015, 332, 518–524. [Google Scholar] [CrossRef]
- Gray-Munro, J.; Campbell, J. Mimicking the hierarchical surface topography and superhydrophobicity of the lotus leaf on magnesium alloy AZ31. Mater. Lett. 2017, 189, 271–274. [Google Scholar] [CrossRef]
- Sonmez, S.; Aksakal, B.; Dikicic, B. Influence of hydroxyapatite coating thickness and powder particle size on corrosion performance of MA8M magnesium alloy. J. Alloy Compd. 2014, 456, 125–131. [Google Scholar] [CrossRef]
- Qiu, X.; Wan, P.; Tan, L.L.; Fan, X.M.; Yang, K. Preliminary research on a novel bioactive silicon doped calcium phosphate coating on AZ31 magnesium alloy via electrodeposition. Mater. Sci. Eng. 2014, 36, 65–76. [Google Scholar] [CrossRef]
- Wu, Y.; Hang, T.; Yu, Z.; Xu, L.; Li, M. Lotus leaf-like dual-scale silver film applied as a superhydrophobic and self-cleaning substrate. Chem. Commun. 2014, 50, 8405–8407. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.Q.; Wang, S.D.; Ye, X.S.; Liu, Z.; Wu, Z.J. Corrosion resistance and wetting properties of silica-based superhydrophobic coatings on AZ31B Mg alloy surfaces. Appl. Surf. Sci. 2018, 453, 1–10. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Song, G.L.; Wu, P.P.; Huang, J.F.; Zheng, D.J. A protective superhydrophobic Mg-Zn-Al LDH film on Surface-Alloyed Magnesium. J. Alloys Compd. 2021, 855, 157550–157562. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.Y.; Zhu, Y.; Li, Z.H.; Shen, T.; Yang, D.Q. An environment-friendly fabrication of superhydrophobic surfaces on steel and magnesium alloy. Mater. Lett. 2016, 171, 297–299. [Google Scholar] [CrossRef]
- Wan, H.R.; Hu, X.F. One-step solve-thermal process for the construction of anticorrosion bionic superhydrophobic surfaces on magnesium alloy. Mater. Lett. 2016, 174, 209–212. [Google Scholar] [CrossRef]
- Li, J.H.; Liu, Q.; Wang, Y.L.; Chen, R.R.; Takahashi, K.; Li, R.M.; Liu, L.H.; Wang, J. Formation of a corrosion-resistant and anti-icing superhydrophobic surface on magnesium alloy via a single-step method. J. Electrochem. Soc. 2016, 163, 62–69. [Google Scholar] [CrossRef]
- Wei, J.F.; Li, B.C.; Jing, L.Y.; Tian, N.; Zhao, X.; Zhang, J.P. Efficient protection of Mg alloy enabled by combination of a conventional anti-corrosion coating and a superamphiphobic coating. Chem. Eng. J. 2020, 390, 124562–124575. [Google Scholar] [CrossRef]
- Emelyanenko, K.A.; Domantovsky, A.G.; Chulkova, E.V.; Emelyanenko, A.M.; Boinovich, L.B. Thermally induced gradient of properties on a superhydrophobic magnesium alloy surface. Metals 2021, 11, 41. [Google Scholar] [CrossRef]
- Viswanathan, S. Recent progress in superhydrophobic and superamphiphobic coatings for magnesium and its alloys. J. Magnes. Alloy. 2021, 9, 748–778. [Google Scholar]
- Wang, S.; Li, Y.; Fei, X.; Sun, M.; Zhang, C.; Li, Y.; Yang, Q.B.; Hong, X. Preparation of adurable superhydrophobic membrane by electrospinning poly(vinylidenefluoride)(PVDF) mixed with epoxy-siloxane modified SiO2 nanoparticles: A possible route to superhydrophobic surfaces with low water sliding angle and high water contact angle. J. Colloid Interface Sci. 2011, 359, 380–388. [Google Scholar]
- Liu, T.; Li, X.; Wang, D.; Huang, Q.; Liu, Z.; Li, N.; Xiao, C. Superhydrophobicity and regeneration of PVDF/SiO2 composite films. Appl. Surf. Sci. 2017, 396, 1443–1449. [Google Scholar] [CrossRef]
- Gao, J.; Wu, L.; Zheng, G. A hierarchical carbon nanotube/SiO2 nanoparticle network induced superhydrophobic and conductive coating for wearable strain sensors with superior sensitivity and ultra-low detection limit. J. Mater. Chem. C. 2019, 7, 4199–4209. [Google Scholar] [CrossRef]
- Lafuma, A.; Quéré, D. Superhydrophobic states. Nat. Mater. 2003, 2, 457–460. [Google Scholar] [CrossRef]
- Xu, W.; Song, J.; Sun, J.; Lu, Y.; Yu, Z. Rapid fabrication of large-area, corrosion-resistant superhydrophobic mg alloy surfaces. ACS Appl. Mater. Interf. 2011, 3, 4404–4414. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, U.; Cansoy, C.E. Applicability of Cassie-Baxter equation for superhydrophobic fluoropolymer-silica composite films. Appl. Surf. Sci. 2015, 335, 99–106. [Google Scholar] [CrossRef]
- Qing, Y.; Yang, C.; Yu, N.; Shang, Y.; Sun, Y.Z.; Wang, L.S.; Liu, C.S. Superhydrophobic TiO2/polyvinylidene fluoride composite surface with reversible wettability switching and corrosion resistance. Chem. Eng. J. 2016, 290, 37–44. [Google Scholar] [CrossRef]
- Oberli, L.; Caruso, D.; Hall, C.; Fabretto, M.; Murphy, P.J.; Evans, D. Condensation and freezing of droplets on superhydrophobic surfaces. Adv. Colloid Interf. 2013, 210, 47–57. [Google Scholar] [CrossRef]
- Kumar, D.; Li, L.; Chen, Z. Mechanically robust polyvinylidene fluoride (PVDF) based superhydrophobic coatings for self-cleaning applications. Prog. Org. Coat. 2016, 101, 385–390. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Si, X.; He, Z.J.; Qi, J.L.; Feng, J.F.; Cao, J. Mechanical durable ceria superhydrophobic coating fabricated by simple hot-press sintering. Appl. Surf. Sci. 2020, 529, 147113–147122. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, T.; Wu, M.; Wei, W.C. Durable superhydrophobic surface with hierarchical microstructures for efficient water collection. Surf. Coat. Technol. 2021, 419, 127279–127288. [Google Scholar] [CrossRef]
- Rabuni, M.F.; Sulaiman, N.M.N.; Aroua, M.K.; Hashim, N.A. Effects of alkaline environments at mild conditions on the stability of PVDF membrane: An experimental study. Ind. Eng. Chem. Res. 2013, 52, 15874–15882. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, H.; Yu, Y.; Chen, S.G. Corrosion protection of silane coatings modified by carbon nanotubes on stainless steel. Int. J. Electrochem. Sci. 2015, 10, 3497–3509. [Google Scholar]
- Liu, Q.; Chen, D.; Kang, Z. One-step electrodeposition process to fabricate corrosion-resistant superhydrophobic surface on magnesium alloy. ACS Appl. Mater. Interfaces 2015, 7, 1859–1867. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, X.; Zhang, J.; Han, Z.; Ren, L. A electro-deposition process for fabrication of biomimeticsuper-hydrophobic surface and its corrosion resistance on magnesium alloy. Electrochim. Acta 2014, 125, 395–403. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, W.G.; Yin, X.M.; Wang, H.Y.; Han, Z.W.; Ren, L.Q. Controlling wettability for improved corrosion inhibition on magnesium alloy as biomedical implant materials. Adv. Mater. Internsh. 2016, 3, 1500723–1500732. [Google Scholar] [CrossRef]
- Cai, J.; Wang, S.; Zhang, J.H.; Liu, Y.; Hang, T.; Ling, H.Q.; Li, M. Chemical grafting of the superhydrophobic surface on copper with hierarchical microstructure and its formation mechanism. Appl. Surf. Sci. 2018, 436, 950–956. [Google Scholar] [CrossRef]
- Zhou, S.; Zhu, X.; Yan, Q. One step electrochemical deposition to achieve superhydrophobic cobalt incorporated amorphous carbon-based film with self-cleaning and anti-corrosion. Surf. Interface Anal. 2017, 50, 290–296. [Google Scholar] [CrossRef]


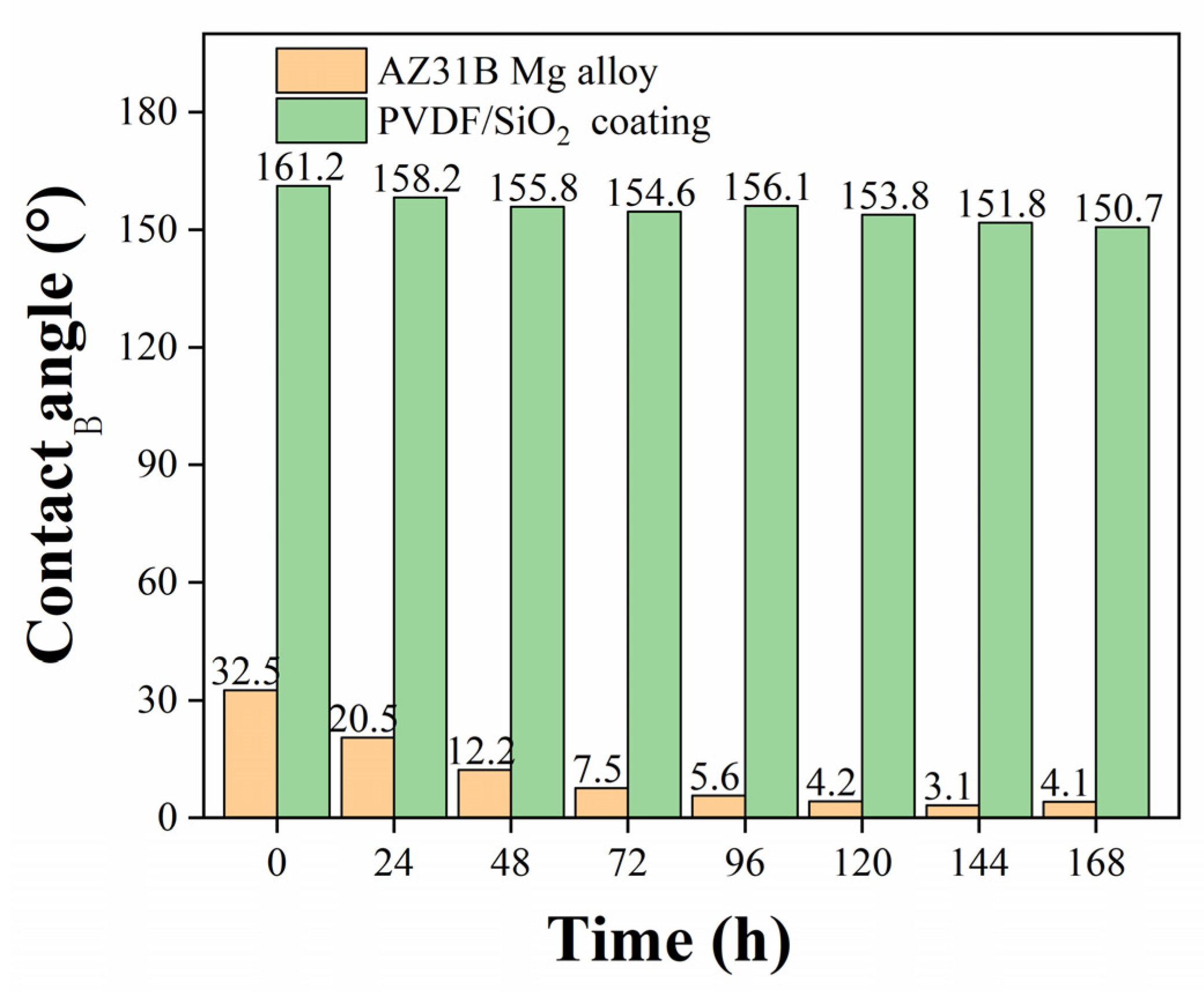
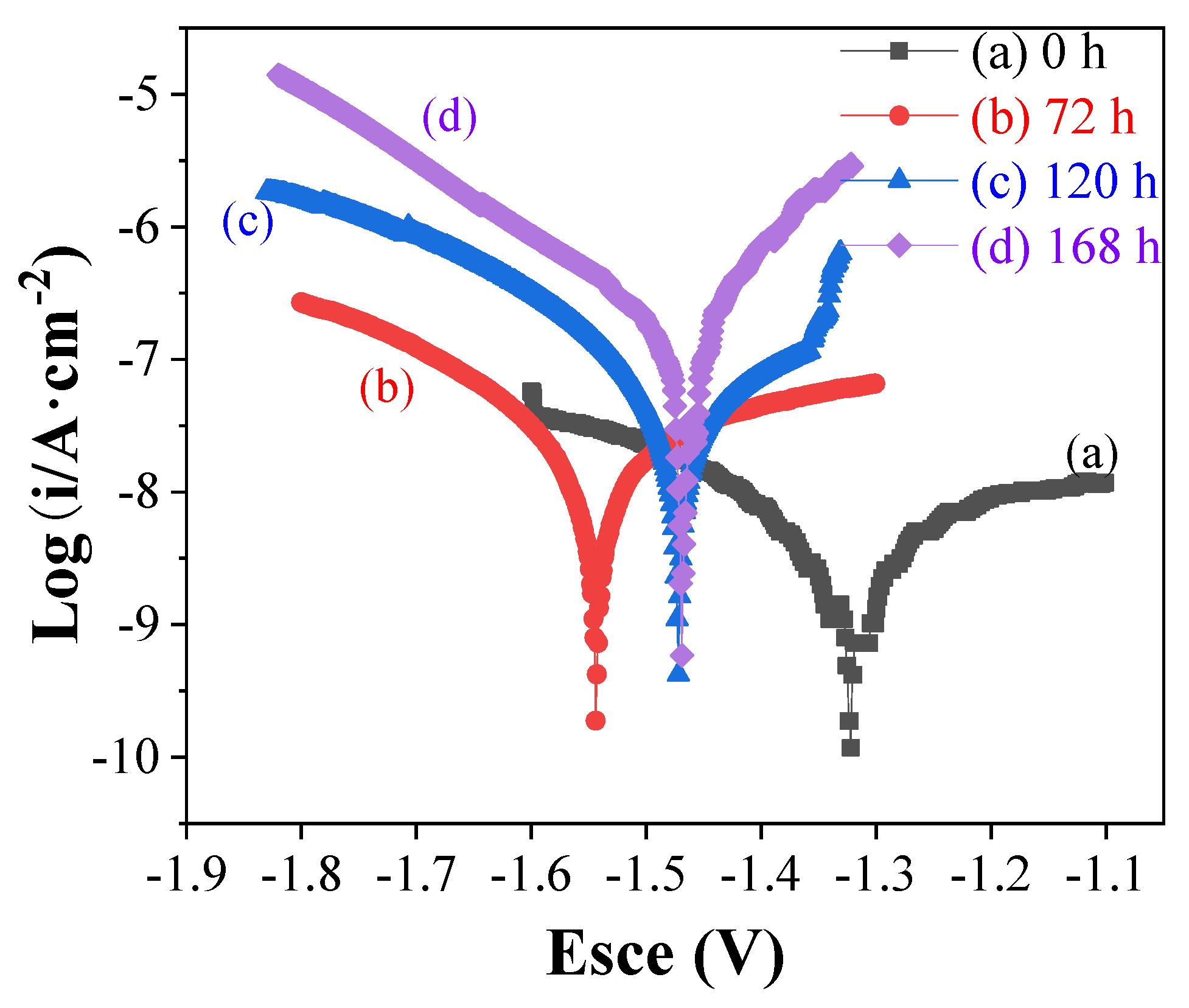
| Sample | Corrosion Potential | Corrosion Current Density |
|---|---|---|
| Ecorr (V) | Icorr (A·cm−2) | |
| 0 h | −1.32 ± 0.04 | (6.18 ± 2.34) × 10−9 |
| 72 h | −1.54 ± 0.07 | (2.18 ± 3.34) × 10−8 |
| 120 h | −1.47 ± 0.10 | (4.828 ± 2.95) × 10−8 |
| 144 h | −1.48 ± 0.06 | (5.58 ± 2.35) × 10−7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, Z.; Liu, Z.; Wang, S.; Ye, X.; Wu, Z. A Durable PVDF/PFOTES-SiO2 Superhydrophobic Coating on AZ31B Mg Alloy with Enhanced Abrasion Resistance Performance and Anti-Corrosion Properties. Appl. Sci. 2021, 11, 11172. https://doi.org/10.3390/app112311172
Qian Z, Liu Z, Wang S, Ye X, Wu Z. A Durable PVDF/PFOTES-SiO2 Superhydrophobic Coating on AZ31B Mg Alloy with Enhanced Abrasion Resistance Performance and Anti-Corrosion Properties. Applied Sciences. 2021; 11(23):11172. https://doi.org/10.3390/app112311172
Chicago/Turabian StyleQian, Zhiqiang, Zhong Liu, Shidong Wang, XiuShen Ye, and Zhijian Wu. 2021. "A Durable PVDF/PFOTES-SiO2 Superhydrophobic Coating on AZ31B Mg Alloy with Enhanced Abrasion Resistance Performance and Anti-Corrosion Properties" Applied Sciences 11, no. 23: 11172. https://doi.org/10.3390/app112311172
APA StyleQian, Z., Liu, Z., Wang, S., Ye, X., & Wu, Z. (2021). A Durable PVDF/PFOTES-SiO2 Superhydrophobic Coating on AZ31B Mg Alloy with Enhanced Abrasion Resistance Performance and Anti-Corrosion Properties. Applied Sciences, 11(23), 11172. https://doi.org/10.3390/app112311172







