Accounting for Interelement Interferences in Atomic Emission Spectroscopy: A Nonlinear Theory
Abstract
:1. Introduction
- the state of matter and the type of substances to be analyzed (gases, liquids, and solids);
- the atomization method (flame atomization; electrothermal atomization in graphite furnaces; laser ablation);
- the method of optical excitation (excitation of atoms and ions in different types of plasma; selective excitation by light sources);
- the type of system for recording light emission produced by the excited atoms and (or) ions of the substance being analyzed (photomultiplier tubes; photodiodes; charge-coupled devices).
- (1)
- changes in the concentration of the principal component at different concentrations, the impurities being determined when working with relative intensities (see above);
- (2)
- superposition of the spectral line of the analyte and that of another element on the focal surface of the spectrometer;
- (3)
- changes in the physicochemical properties (atomic structure, crystal structure, melting point, evaporation temperature, etc.) of the substance when the concentration of the influencing element changes, causing the rate at which the atoms of the substance enter the optical excitation zone to change;
- (4)
- changes in the parameters of the optical excitation system due to the presence of atoms of influencing elements in the excitation zone (for example, changes in the temperature of plasma electrons when using arc discharge or other discharge methods).
- mathematical methods for calculating corrections;
- methods that adjust the procedure of measuring the analytical signal;
- methods optimizing the process of measuring the analytical signal;
- spectrum transformation methods (Fourier transform, the differentiation of spectra, etc.).
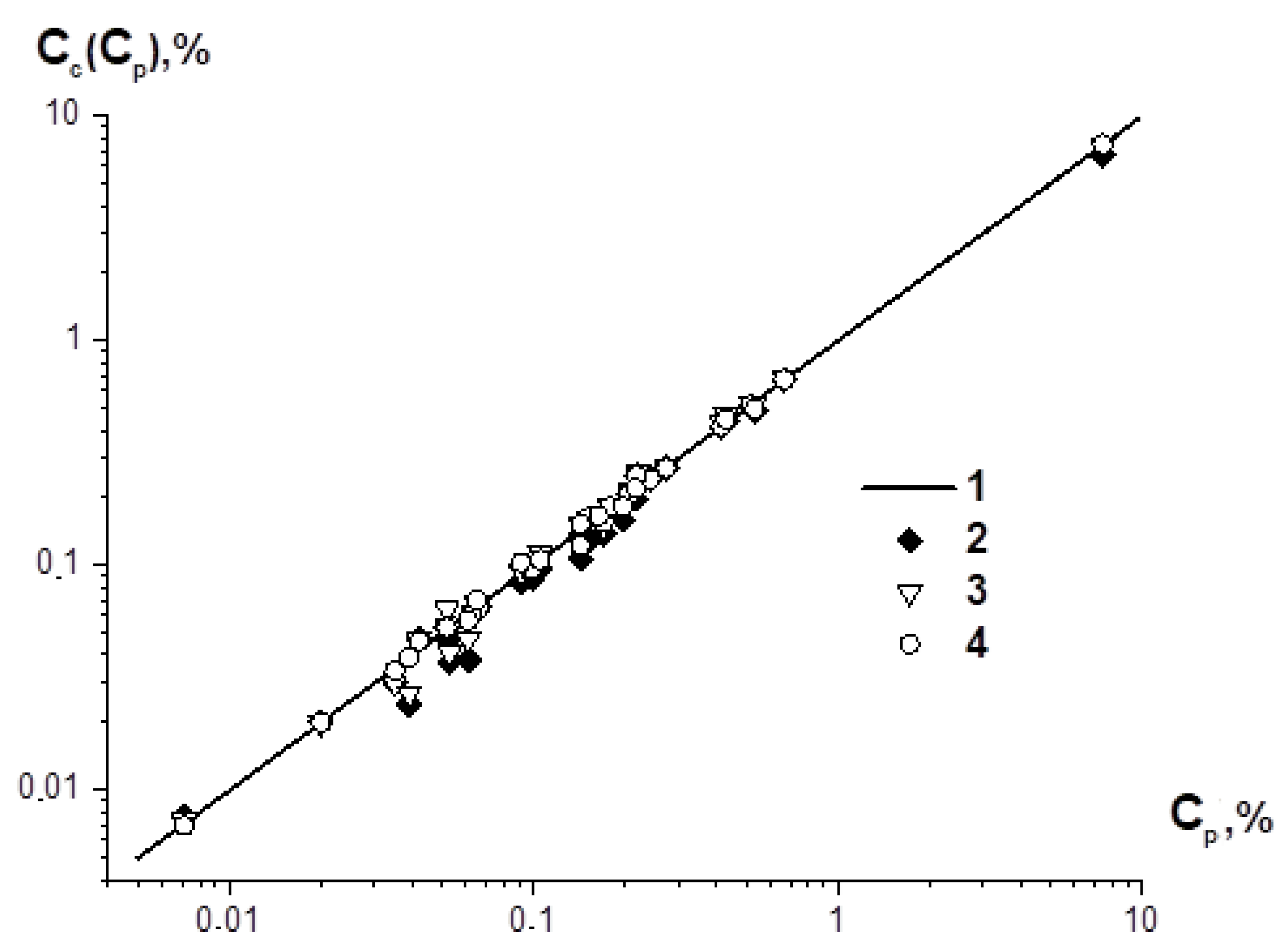
2. Materials and Methods
3. Discussion
4. Conclusions
- Third-element effects are additive. This assumption is justified by the fact that we considered a case of samples with small amounts of impurities, which makes it possible to neglect so-called effects superimposed on other effects.
- The main causes of third-element effects on the results of determining impurity content in the sample are: (1) changes in the conditions for the emission of impurity atoms from the sample to the plasma zone and the dependence of the plasma parameters (in particular, the excitation temperature) on the concentrations of the third elements in the sample; (2) changes in the registered signal of the analyte due to spectral line interferences and the dependence of the background plasma radiation on the concentrations of the third elements in the sample. This makes it possible to represent the relationship between the measured impurity concentration and the intensities of the analytical lines in the form of (4).
- As a rule, the concentrations of impurities are small, which makes it possible to use expansions in series of the type (10).
- When there are third-element effects, the relationship between the concentration of the analyte and the intensity of its analytical line can be represented as a polynomial of some degree that coincides with the degree of the corresponding polynomial in the absence of third-element effects. When they are present, the coefficients of this polynomial depend on the intensities of the analytical lines of the influencing elements and, at , are equal to the corresponding coefficients in the absence of third-element effects.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix B
References
- Hollas, J.M. Modern Spectroscopy; John Wiley & Sons: Chichester, UK, 2004. [Google Scholar]
- Marcus, R.K.; Broekaert, J.A.C. Glow Discharge Plasmas in Analytical Spectroscopy; John Wiley & Sons: New York, NY, USA, 2003. [Google Scholar]
- Bogaerts, A. Plasma diagnostics and numerical simulations: Insight into the heart of analytical glow discharges. J. Anal. At. Spectrom. 2007, 22, 13–40. [Google Scholar] [CrossRef]
- Payling, R.; Jones, D.G.; Bengtson, A. Glow Discharge Optical Emission Spectrometry; John Wiley & Sons: Chichester, UK, 1997. [Google Scholar]
- Joosten, H.G.; Golloch, A.; Flock, J.; Killewald, S. Atomic Emission Spectrometry. AES—Spark, Arc, Laser Excitation; De Gruyter: New York, NY USA; Berlin, Germany, 2020. [Google Scholar] [CrossRef]
- Mandelstam, S.L. Introduction to Spectral Analysis; Gostekhizdat: Moscow, Russia, 1946. (In Russian) [Google Scholar]
- Russo, R.E.; Mao, X.; Liu, H.; Gonzalez, J.; Mao, S.S. Laser ablation in analytical chemistry—A review. Talanta 2002, 57, 425–451. [Google Scholar] [CrossRef]
- Buravlev, Y.M.; Neuimina, G.P.; Ustinova, V.I. Study of the influence of boron on the results of spectral analysis of structural steels. In Bulletin of Scientific and Technical Information; Ural Institute of Ferrous Metals: Sverdlovsk, Russia, 1959; pp. 120–125. (In Russian) [Google Scholar]
- Buravlev, Y.M.; Neuimina, G.P.; Perepelkina, M.A.; Maramygina, G.P. Study of the influence of structure on the results of spectral analysis. In Proceedings of the Materials of the 2nd Urals Meeting on Spectroscopy, Sverdlovsk, Russia, 14–16 May 1958; pp. 62–66. (In Russian). [Google Scholar]
- Petrov, V.V.; Borok, B.A.; Kravets, T.P.; Buravlev, Y.M.; Popov, A.A.; Vilesov, F.I.; Koritsky, V.G.; Nalimov, V.V.; Nedler, V.V.; Kislovsky, L.D.; et al. 10th All-Union Meeting on Spectroscopy, 4–14 July 1956, Lvov, Russia; Volume 2: Atomic Spectroscopy; Landsberg, G.S., Shaevich, A.B., Eds.; Publishing House of Lvov University: Lvov, Russia, 1958; 586p. (In Russian) [Google Scholar]
- Ulyanina, I.Y.; Zagulyaeva, S.V.; Vinograd, M.I.; Uralova, V.A.; Kokorin, G.A.; Pavperova, I.A.; Zoteev, V.S.; Konovalov, L.V.; Williams, O.S.; Kashparova, O.D.; et al. New Methods of Metal Tests; Timoshenko, N.N., Ed.; I. P. Bardin Central Research Institute for Ferrous Metallurgy: Moscow, Russia, 1962; Volume 24, 455p. (In Russian) [Google Scholar]
- Buravlev, Y.M. Physical Optics and Spectroscopy. In Methods of Suppressing the Effect of Sample Composition and Structure on the Results of Spectral Analysis of Alloys; Kiev House of Scientific and Technical Propaganda “Znanie” Society: Kiev, Russia, 1965; 39p. (In Russian) [Google Scholar]
- Korotkov, V.F.; Titovets, A.V. On the Influence of “Third” Elements in Steels during Work on the Vacuum Quantometer; I. P. Bardin Central Research Institute for Ferrous Metallurgy: Moscow, Russia, 1964; Volume 37, pp. 113–117. (In Russian) [Google Scholar]
- Buravlev, Y.M.; Buyanov, N.V.; Ustinova, V.I. About Influence of Phosphorus, Carbon and Silicon on Results of Spectral Analysis of Cast Irons and Steels; All-Union Research Institute of Standard Reference Materials and Spectral Standards: Moscow, Russia, 1965; Volume 2, pp. 114–123. (In Russian) [Google Scholar]
- Prokofiev, V.K. Spectral Analysis of Elements which are Difficult to Excite. Izv. AS USSR Phys. Ser. 1945, 9, 691–699. (In Russian) [Google Scholar]
- Buravlev, Y.M.; Rudnevskii, N.K.; Grikit, I.A. Spectral Analysis of Metals and Alloys (Processes on Electrodes); Tekhnika: Kiev, Russia, 1976; p. 195. (In Russian) [Google Scholar]
- Vovk, V.N. Some features of the spectral analysis of metals and alloys in controlled atmospheres. In Atomic Spectroscopy and Spectral Analysis; Naukova Dumka: Kiev, Russia, 1974; pp. 159–164. (In Russian) [Google Scholar]
- Eroshenko, L.E.; Dem’yanchuk, A.S. Features of the arcs and high-frequency sparks in air and argon used in spectral analysis of metals. J. Appl. Spectrosc. 1983, 39, 746–750. [Google Scholar] [CrossRef]
- Borzov, V.P.; Sventitsky, N.S. Spectral Analysis of Aluminum Alloys and Determination of Carbon in Steels; Leningrad House of Scientific and Technical Propaganda “Znanie” Society: Leningrad, Russia, 1950; p. 14. (In Russian) [Google Scholar]
- Sorokina, N.N.; Golubeva, V.M. Spectral Analysis of Microadditions of Rare Earth and Alkali Earth Metals in Steel and Alloys; I. P. Bardin Central Research Institute for Ferrous Metallurgy: Moscow, Russia, 1964; Volume 37, pp. 108–113. (In Russian) [Google Scholar]
- Rezchikov, V.G.; Kostrikov, I.N.; Legkov, A.V. Atomic-emission determination of carbon in metals using reactions in low-temperature plasma. J. Appl. Spectrosc. 1988, 48, 255–258. [Google Scholar] [CrossRef]
- Burakov, V.S.; Yankovsky, A.A. Practical Guide to Spectral Analysis; Publishing House of the BSSR Academy of Sciences: Minsk, Russia, 1960; p. 232. (In Russian) [Google Scholar]
- Leshchev, G.V.; Makarova, S.A. Spectral Analysis of Steel Using Low-Voltage Spark; Leningrad House of Scientific and Technical Propaganda “Znanie” Society: Leningrad, Russia, 1954; p. 7. (In Russian) [Google Scholar]
- Gudkova, K.V.; Taganov, K.I. On the Prospects of Creating a Unified Method for Suppression of Influencing Factors in Spectral Analysis of Metals and Alloys. In Proceedings of the Fourth Ural Conference on Spectroscopy, Sverdlovsk, Russia, 15–17 May 1963; Metallurgy: Moscow, Russia, 1965; pp. 60–63, 263. (In Russian). [Google Scholar]
- Buravlev, Y.M. Influence of Composition and Sample Size on the Results of Spectral Analysis of Alloys; Tekhnika: Kiev, Russia, 1970; p. 212. (In Russian) [Google Scholar]
- Buravlev, Y.M. Influence of Structure on the Results of Spectral Analysis of Alloys; Metallurgizdat: Moscow, Russia, 1963; p. 151. (In Russian) [Google Scholar]
- Belkevich, Y.P. Experience of Spectral Analysis of Copper-Based Alloys; Sudpromgiz: Leningrad, Russia, 1955; p. 40. (In Russian) [Google Scholar]
- Maiorov, I.; Filimonov, L.N.; Yarkin, B. Prospects of establishing a high-temperature source of great accuracy and stability for the spectral analysis of small series of sample solutions. J. Appl. Spectrosc. 1975, 22, 431–444. [Google Scholar] [CrossRef]
- Prokofiev, V.K. Photographic Methods of Quantitative Spectral Analysis of Metals and Alloys; State Publishing House of Technical and Theoretical Literature: Moscow, Russia; Leningrad, Russia, 1951. (In Russian) [Google Scholar]
- Sukhenko, K.A. Photoelectric Methods of Spectral Analysis; Oborongiz: Moscow, Russia, 1961; p. 96. (In Russian) [Google Scholar]
- Török, T.; Kovács, M.; Cséti, S. Untergrundkorrektur und Auswertung mit einem projizierbaren Graphikon für die Spektralanalyse. Fresenius’ Zeitschrift für analytische Chemie 1959, 165, 241–247. [Google Scholar] [CrossRef]
- Suranov, A.Y.; Shpomer, A.V.; Yakunin, A.G. Spectral analysis of metals with an automated spectrum-recording system on the basis of a linear charge-coupling photodiode detector. J. Appl. Spectrosc. 1985, 43, 955–960. [Google Scholar] [CrossRef]
- Shayevich, A.B. Methods for Estimating the Accuracy of Spectral Analysis; Metallurgizdat: Sverdlovsk, Russia, 1959; p. 57. (In Russian) [Google Scholar]
- Rotman, L.E.; Vorobeichik, V.M. Reference Book on Emission Spectral Analysis; Mashinostroenie: Moscow, Russia, 1982; p. 347. (In Russian) [Google Scholar]
- Borbat, A.M. New Developments in Emission Spectral Analysis; Kiev House of Scientific and Technical Propaganda “Znanie” Society: Kiev, Russia, 1972; p. 15. (In Russian) [Google Scholar]
- Kaplan, V.Y.; Filimonov, L.H.; Mayorov, I.A. Metrology of Analytical Production Control in Ferrous Metallurgy; Metallurgy: Moscow, Russia, 1989; p. 200. (In Russian) [Google Scholar]
- Pliner, Y.L.; Svechnikova, E.A.; Ogurtsov, V.M. Quality Management of Chemical Analysis in Metallurgy; Metallurgy: Moscow, Russia, 1979; p. 208. (In Russian) [Google Scholar]
- Kazennova, M.N.; Gusev, V.V. Spectral Analysis as a Method of Quality Control of the Output Products; Leningrad House of Scientific and Technical Propaganda “Znanie” Society: Leningrad, Russia, 1981; p. 32. (In Russian) [Google Scholar]
- Shayevich, A.B.; Shubina, S.B. Industrial Methods of Spectral Analysis; Metallurgy: Moscow, Russia, 1965; p. 224. (In Russian) [Google Scholar]
- Orlov, A.G. Calculation Methods in Quantitative Spectral Analysis; Nedra: Leningrad, Russia, 1977; p. 108. (In Russian) [Google Scholar]
- Pliner, Y.L.; Stepin, V.V.; Ustinova, V.I. Standard Samples of Metallurgical Materials; Metallurgy: Moscow, Russia, 1976; p. 296. (In Russian) [Google Scholar]
- Török, T.; Cséti, S. Einwirkung des Magnesiums als dritter Legierungsbestandteil auf die Bestimmung von Zink in Aluminium. Microchimica Acta 1958, 46, 538–544. [Google Scholar] [CrossRef]
- Khodonov, M.T. Mathematical Support for Correction of Inter-Element Effects in Atomic Emission Spectral Analysis with Induction Plasma. Ph.D. Thesis, Research and Design Institute of Rare Metals Industry Giredmet, Moscow, Russia, 1994; p. 78. (In Russian). [Google Scholar]
- Moskvin, L.N. Problems of Modern Analytical Chemistry; Leningrad State University: Leningrad, Russia, 1989; Volume 6, pp. 26–38, 176. (In Russian) [Google Scholar]
- Karpov, Y.A.; Savostin, A.P.; Salnikov, V.D. Analytical Control in Metallurgical Production; Akademkniga: Moscow, Russia, 2006; p. 352. (In Russian) [Google Scholar]
- Gorsky, E.V.; Livshits, A.M.; Peleznev, A.V. Accounting of the influence of “third” elements in the analysis of aluminum alloys on the emission spectrometer PAPUAS-4. Ind. Lab. Diagn. Mater. 2006, 3, 11–15. [Google Scholar]
- Gorsky, E.V.; Livshits, A.M. Consideration of Inter-Element Effects in Analysis of High-Alloyed Steels on an Emission Spectrometer (PAPUAS-4). Ind. Lab. Diagn. Mater. 2017, 83, 26–30. (In Russian) [Google Scholar]
- Gorsky, E.V.; Livshits, A.M. Analysis of metal alloys on low-resolution emission spectrometers. Correction of inter-element effects. Analytics 2018, 2, 58–62. [Google Scholar] [CrossRef] [Green Version]
- Gorsky, E.V. Construction of Small-Size Equipment for the Analysis of Metal Alloys on the Basis of Emission Spectral Analysis. Ph.D. Thesis, Institute of Spectroscopy RAS, Moscow, Russia, 2007; p. 113. (In Russian). [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popova, A.N.; Sukhomlinov, V.S.; Mustafaev, A.S. Accounting for Interelement Interferences in Atomic Emission Spectroscopy: A Nonlinear Theory. Appl. Sci. 2021, 11, 11237. https://doi.org/10.3390/app112311237
Popova AN, Sukhomlinov VS, Mustafaev AS. Accounting for Interelement Interferences in Atomic Emission Spectroscopy: A Nonlinear Theory. Applied Sciences. 2021; 11(23):11237. https://doi.org/10.3390/app112311237
Chicago/Turabian StylePopova, Anna N., Vladimir S. Sukhomlinov, and Aleksandr S. Mustafaev. 2021. "Accounting for Interelement Interferences in Atomic Emission Spectroscopy: A Nonlinear Theory" Applied Sciences 11, no. 23: 11237. https://doi.org/10.3390/app112311237
APA StylePopova, A. N., Sukhomlinov, V. S., & Mustafaev, A. S. (2021). Accounting for Interelement Interferences in Atomic Emission Spectroscopy: A Nonlinear Theory. Applied Sciences, 11(23), 11237. https://doi.org/10.3390/app112311237





