Abstract
This study investigates the effects of zoledronic acid (ZA) and compressive force on osteoblast functions, to elucidate the pathogenesis of medication-related osteonecrosis of the jaw (MRONJ). MC3T3-E1 cells were exposed to ZA (1, 10 and 100 µM) to evaluate the effects of ZA on cell proliferation. Furthermore, to investigate the influence of ZA with or without compressive force on osteoblast differentiation, real-time polymerase chain reaction and Alizarin Red S staining were performed. ZA concentrations > 10 μM were highly cytotoxic to MC3T3-E1 cells. Combining 1-μM ZA with compressive force influenced expression levels of osteoblast-related genes and matrix mineralization. The inhibitory effects of ZA on cell proliferation and the combination of ZA and compressive force on osteoblast differentiation may contribute to the pathogenesis of MRONJ.
1. Introduction
Bisphosphonates (BPs) have seen wide use in the treatment of various bone diseases [,]. Zoledronic acid (ZA) is considered one of the most intensive and effective BPs, and is widely used in clinical practice for the treatment of various bone diseases.
Despite the many beneficial effects of BPs, bisphosphonate-related osteonecrosis of the jaw (BRONJ) is a problem, and the number of identified BRONJ cases appears to be increasing rapidly. BRONJ is often accompanied by pain and leads to reduced quality of life in patients []. Recently, the term “medication-related osteonecrosis of the jaw” (MRONJ) has been used to include cases of osteonecrosis of the jaw showing associations with other antiresorptive and antiangiogenic therapies [].
Diverse hypotheses regarding the pathogenesis of MRONJ have been advocated, such as over-suppression of both bone resorption by osteoclasts and bone turnover, antiangiogenic activity, and toxicity against the oral epithelium []. Several reports have demonstrated that diabetes mellitus, severe periodontitis and traumatic tooth extraction represent risk factors for the progression of MRONJ [,,]. Furthermore, compressive force as seen in traumatic occlusion, bruxism and bite force from ill-fitting dentures may also contribute to the pathogenesis of MRONJ. However, the effects of compressive force and ZA on osteoblast proliferation and differentiation remain uncertain. In the present study, we investigated the effects of ZA and compressive force on osteoblast functions, to clarify the possible pathogenesis of MRONJ.
2. Materials and Methods
2.1. Cell Culture
MC3T3-E1 cells (mouse calvaria-derived osteoblast) were obtained from the European Collection of Authenticated Cell Cultures (ECACC, Salisbury, UK). Cells were cultured in a standard medium comprising α-minimum essential medium (Sigma-Aldrich, M4655, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (Gibco®; Thermo Fisher Scientific, 12662029, Waltham, MA, USA) and antibiotic reagent (Sigma-Aldrich, P0781, St. Louis, MO, USA), and incubated at 37 °C in a humidified atmosphere of 5% CO2/95% air.
2.2. Cell Proliferation Assay and Morphological Analysis
MC3T3-E1 cells were seeded in 6-well plates at 2.0 × 104 cells/well. After incubation for 24 h, the cells were treated with ZA (Sigma-Aldrich, St. Louis, MO, USA) at concentrations of 1, 10 or 100 µM. Cell proliferation of cells cultured for 1, 3 or 7 days was determined using an MTS assay (CellTiter 96 AQ One Solution Cell Proliferation Assay Kit; Promega, G3580, Madison, WI, USA) in accordance with the manufacturer’s protocol. The absorbance of each well was then observed at 490 nm using a spectral scanning multimode reader (Varioskan Flash 2.4; Thermo Science, Waltham, MA, USA). Cell morphologies were observed under an inverted microscope (ZEISS Primovert; ZEISS, Oberkochen, Germany).
2.3. Osteoblast Differentiation, ZA Treatment and Compressive Force Application
MC3T3-E1 cells were seeded in 6-well plates at 3.0 × 105 cells/well. After cultures reached subconfluence, cells were provided with an osteogenic medium (standard medium supplemented with Osteoblast-Inducer Reagent; Takara Bio, MK430, Kusatsu, Shiga, Japan). Cells were incubated with 1 µM of ZA. The medium was replaced every 3 days. Furthermore, some cells incubated with ZA were subjected to 4.0 g/cm2 of continuously compressive force, using a compression method based on that described previously []. In short, a glass cylinder and lead ball were placed over the cell layer in the well of the 6-well plate. The weight of the lead ball placed on the glass cylinder determined the compressive force.
Cells without addition of ZA and without compressive force were used as controls.
2.4. cDNA Preparation and Real-Time Polymerase Chain Reaction (PCR)
Expression levels of osteoblast marker genes after 7 days of culture were measured using real-time PCR. Using Easy DNA Extraction kit version 2 (Kaneka, Minato City, Tokyo, Japan), cDNA was prepared from cultured cells according to the manufacturer’s protocol. Real-time PCR was performed using a CFX96 PCR detection system (Bio Rad, Hercules, CA, USA) with TB Green® Premix Ex Taq II (Takara Bio, RR820, Kusatsu, Shiga, Japan). Osteoblast-related genes including alkaline phosphatase (ALP), runt-related transcription factor 2 (Runx2), type I collagen (Col I), vascular endothelial growth factor (VEGF)-A and VEGF receptor 1 (VEGFR-1) were amplified using forward and reverse primers. The primers used were: 5′- GCAGTATGAATTGAATCGGAACAAC-3′, 5′- ATGGCCTGGTCCATCTCCAC-3′(ALP); 5′- TGCAAGCAGTATTTACAACAGAGG-3′, 5′- GGCTCACGTCGCTCATCTT-3′ (Runx2); 5′- TCAGTGCAATTGTGTTGCTGAAAG-3′, 5′- GATACCAAACTGGGCGTGCTG-3′(Col I); 5′- ACATTGGCTCACTTCCAGAAACAC-3′, 5′- TGGTTGGAACCGGCATCTTTA-3′ (VEGF-A); 5′- TCAGGTAGGGCTGGCCAAAG-3′, 5′- CCCAATTGCTGGATATCTGGATG-3′ (VEGFR-1); and 5′- TGTGTCCGTCGTGGATCTGA-3′, 5′- TTGCTGTTGAAGTCGCAGGAG-3′ (GAPDH).
Relative expression ratios of markers were calculated based on the comparative threshold cycle (ddCt) method. The levels of mRNA expression were calculated and normalized to the level of GAPDH mRNA expression.
2.5. Matrix Mineralization
Matrix mineralization after 14 and 21 days of cultures was identified by Alizarin Red S (ARS) staining. Cultures were fixed in 10% PFA and treated with ARS (Iwai Chemicals, Chuo City, Tokyo, Japan). ARS-stained cultures were eluted with 2% formic acid. ARS concentration was then determined by measuring absorbance at 450 nm.
2.6. Statistical Analysis
All data are presented as mean ± standard deviation (n = 5–7). Analysis was performed by Steel-Dwass testing. The software js-STAR_XR was used for calculations, and values of p < 0.05 were considered statistically significant.
3. Results
3.1. Cell Proliferation and Cell Morphology
Cell proliferation was evaluated by MTS assay following incubation with various concentrations of ZA. MTS assay showed that 10 and 100 μM of ZA significantly reduced cell proliferation at days 1–7. Cell proliferation was also reduced under the addition of ZA at 1 µM for 7 days, but cell proliferation was maintained at 94.3 ± 1.9% of that without ZA (Figure 1).
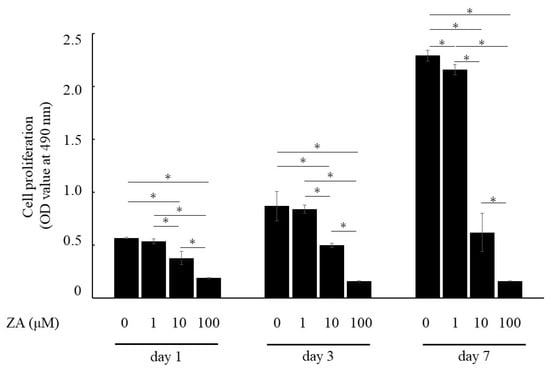
Figure 1.
Influence of ZA on proliferation of MC3T3-E1 cells. Results are expressed as mean ± SD. * p < 0.05 compared with control (0 μM of ZA) of each period.
MC3T3-E1 cells without ZA and with 1 μM of ZA cultured for 1 or 3 days exhibited fibroblast-like morphology, characteristic of immature osteoblasts. Furthermore, cells showed a cobblestone, cuboidal appearance on day 7. On the other hand, MC3T3-E1 cells with 10 μM of ZA exhibited unusual morphologies, lost cell extensions and globular appearance, and were floating and detached on day 7. MC3T3-E1 cells with 100 μM of ZA exhibited floating, detached and dead cells on days 1–7 (Figure 2). These results indicated that ZA concentrations > 10 μM were highly cytotoxic to MC3T3-E1 cells.

Figure 2.
Influence of ZA on cell morphology of MC3T3-E1 cells. The highest concentration (100 μM) of ZA shows toxic effects against cells on days 1–7. In addition, 10 μM of ZA induces unusual morphologies on day 7.
3.2. Real-Time PCR Analysis of Osteoblast-Related Genes
To analyze the influences of ZA with or without compressive force on osteoblast differentiation, cDNA was fabricated and real-time PCR was performed to measure the gene expressions of osteoblast marker genes. Adding 1 μM of ZA significantly decreased expressions of ALP, Runx2, Col I and VEGFR-1 when compared with controls (Figure 3). Furthermore, the combination of 1 μM of ZA and compressive force appeared to influence expression levels of osteoblast marker genes.
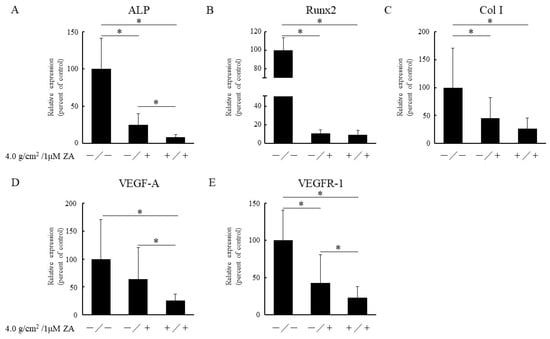
Figure 3.
Quantification of expressions of osteoblast-related genes (A: ALP; B: Runx2; C: Col I; D: VEGF-A; and E: VEGFR-1) using real-time PCR. Results are expressed as mean ± SD. * p < 0.05.
3.3. Matrix Mineralization
Matrix mineralization was demonstrated by ARS staining, which is specific for calcium-binding sites. The images of matrix mineralization are shown in Figure 4A.
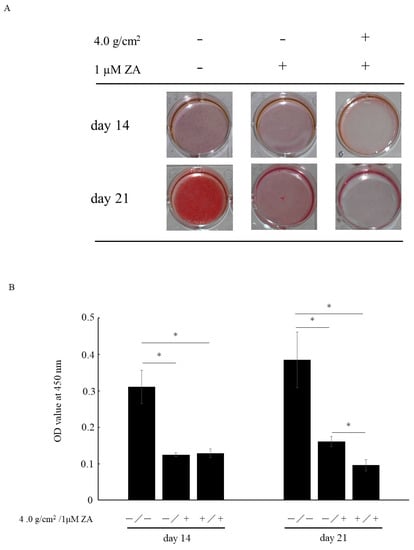
Figure 4.
Matrix mineralization is assessed by Alizarin Red S (ARS) staining (A). After ARS staining, dye is extracted and quantified (B). Data represent mean ± SD. * p < 0.05.
After 14 days of culture, matrix mineralization was found to be inhibited by the addition of 1 μM of ZA, with or without compressive force. After 21 days of culture, matrix mineralization was inhibited by the addition of 1 μM of ZA. Furthermore, matrix mineralization was suppressed by the combination of 1 μM of ZA and compressive force when compared with both control and 1 μM of ZA without compressive force (Figure 4B).
4. Discussion
This study explored the impacts of compressive force and ZA on osteoblast proliferation and differentiation. BPs are robustly related to osteonecrosis in the jaw, but the pathogenesis of MRONJ remains unclear. Recent studies have suggested that BPs might directly interfere with osteoblasts. As reported by Pozzi et al., high doses of ZA inhibit both osteoclast and osteoblast functions and bone remodeling in vivo []. A previous in vitro study also identified inhibitory effects of ZA on cell viability, cell proliferation, and osteoblast differentiation of mesenchymal stem cell []. Furthermore, other reports have mentioned that higher doses of ZA decrease osteoblast cell viability in a dose-dependent manner [,]. In the present study, higher micromolar dose (10 or 100 µM) of ZA decreased cell proliferation on days 1–7. Higher concentrations of ZA exert direct cytotoxic effects on cells, and a lower micromolar dose (1 µM) of ZA also affects cell proliferation with prolonged exposure.
Changes in expression levels of osteoblast markers are typical events during osteoblast differentiation. ALP and Runx2 are closely related to osteoblast differentiation and matrix mineralization []. Col I expression is one of the most widely appreciated early markers for osteoblast differentiation []. Furthermore, osteoblast differentiation and bone formation are closely related to blood vessel formation, and VEGF play a definitive role in bone repair, since angiogenesis and osteogenesis are highly coupled []. Both intramembranous and endochondral ossification depend on the VEGF signaling pathway, and blocking VEGF receptors decreases blood vessel formation and bone regeneration []. In addition, VEGF-A mRNA expression is increased during terminal differentiation with other mRNA expressions, such as bone sialoprotein and osteocalcin [,]. We obtained evidence of the anti-osteoblast differentiation properties of 1 µM of ZA according to the results of real-time PCR. Furthermore, the combination of 1 µM of ZA and compressive force robustly inhibited osteoblast differentiation (Figure 3).
Matrix mineralization is one of the bone formation events initiated by osteoblasts and is the key process in bone formation. In this study, we performed ARS staining to observe matrix mineralization in vitro. As reported by Maruotti et al., BPs including ZA may increase or decrease osteogenesis in a concentration-dependent manner []. Furthermore, previous reports have also mentioned that nanomolar doses of ZA induced no differences in matrix mineralization compared with the control, while micromolar levels of ZA produced decreases in mineralization []. The present study found that mineralized nodule formation was decreased with the addition of 1 μM of ZA, and the combination of 1 μM ZA with compressive force markedly decreased the matrix mineralization of cells; these results indicated that the combination of ZA and compressive force negatively affects osteoblast differentiation.
In the fields of dentistry and oral and maxillofacial surgery, mechanical stress (including compressive force) is closely related to the pathogenesis of various diseases, such as periodontal disease [,], peri-implant bone loss [] and temporomandibular joint disorder []. Alveolar bone experiences continuous mechanical stress such as occlusal forces from tooth contact. Furthermore, the oral mucosa and alveolar bone of edentulous patients also receive mechanical stress from the denture base. Yanagisawa et al. reported that a compressive force of 1.0 g/cm2 increased expression levels of osteogenesis-related transcript factors, mRNAs encoding Runx2, Osterix, Dlx5 and Msx2 []. They also revealed that a compressive force of 1.0 g/cm2 induced mineralized nodule formation, whereas 2.0 g/cm2 decreased expression of osteogenesis-related transcription factors. An in vitro study found that optimal compressive force induced mineralization, but excessive compressive force reduced osteoblast-related gene expression, protein expression and matrix mineralization []. Furthermore, alveolar bone resorption was induced by continuous mechanical pressure from the denture base in another in vivo study [].
To the best of our knowledge, this is the first report to describe the effects of compressive force and BPs together on osteoblast differentiation. According to our results, excessive mechanical stress from teeth and/or the denture base may contribute to the pathogenesis of MRONJ. However, our study had several limitations. One major limitation was that we could not identify certain factors which directly influence the pathogenesis of MRONJ. Therefore, further in vitro comprehensive studies investigating osteoblast- and/or osteoclast-related molecules concerned with differentiation or signaling using different cell lines and in vivo studies are necessary.
5. Conclusions
Based on the results of this in vitro study, high doses of ZA (10 or 100 μM) negatively affect the viability of MC3T3-E1 cells, due to cytotoxic effects from ZA. Even low-dose ZA (1 μM) induced down-regulation of osteoblast-related gene expression and matrix mineralization. Furthermore, the combination of 1 μM ZA with compressive force markedly decreased osteoblast-related gene expression and matrix mineralization. We hypothesize that the inhibitory effects of ZA on cell viability and the combination of ZA and compressive force on differentiation of osteoblasts may contribute to the pathogenesis of MRONJ.
Author Contributions
Conceptualization, K.Y. and H.T.; methodology, K.Y., S.I. and H.T.; data curation, K.Y. and S.I.; investigation, K.Y., S.I. and T.H.; writing original draft, K.Y., S.I., T.H., H.T. and M.F.; supervision, M.F. and M.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by KAKENHI Grants-in-Aid for Scientific Research (C) (numbers 17K11825 and 20K10089) from the Japan Society for the Promotion of Science (JSPS).
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Bone, H.G.; Hosking, D.; Devogelaer, J.P.; Tucci, J.R.; Emkey, R.D.; Tonino, R.P.; Rodriguez-Portales, J.A.; Downs, R.W.; Gupta, J.; Santora, A.C.; et al. Alendronate Phase III Osteoporosis Treatment Study Group. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N. Engl. J. Med. 2004, 350, 1189–1199. [Google Scholar] [CrossRef] [Green Version]
- Aapro, M.; Abrahamsson, P.A.; Body, J.J.; Coleman, R.E.; Colomer, R.; Costa, L.; Crinò, L.; Dirix, L.; Gnant, M.; Gralow, J.; et al. Guidance on the use of bisphosphonates in solid tumours: Recommendations of an international expert panel. Ann. Oncol. 2008, 19, 420–432. [Google Scholar] [CrossRef]
- Ohlrich, E.J.; Coates, D.E.; Cullinan, M.P.; Milne, T.J.; Zafar, S.; Zhao, Y.; Duncan, W.D.; Seymour, G.J. The bisphosphonate zoledronic acid regulates key angiogenesis-related genes in primary human gingival fibroblasts. Arch. Oral. Biol. 2016, 63, 7–14. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef]
- Gong, X.; Yu, W.; Zhao, H.; Su, J.; Sheng, Q. Skeletal Site-specific Effects of Zoledronate on in vivo Bone Remodeling and in vitro BMSCs Osteogenic Activity. Sci. Rep. 2017, 7, 36129. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, T.; Yamori, M.; Ishizaki, T.; Asai, K.; Goto, K.; Takahashi, K.; Nakayama, T.; Bessho, K. Increased incidence of osteonecrosis of the jaw after tooth extraction in patients treated with bisphosphonates: A cohort study. Int. J. Oral Maxillofac. Surg. 2012, 41, 1397–1403. [Google Scholar]
- Peer, A.; Khamaisi, M. Diabetes as a risk factor for medication-related osteonecrosis of the jaw. J. Dent. Res. 2015, 94, 252–260. [Google Scholar]
- Soutome, S.; Hayashida, S.; Funahara, M.; Sakamoto, Y.; Kojima, Y.; Yanamoto, S.; Umeda, M. Factors affecting development of medication-related osteonecrosis of the jaw in cancer patients receiving high-dose bisphosphonate or denosumab therapy: Is tooth extraction a risk factor? PLoS ONE 2018, 13, e0201343. [Google Scholar] [CrossRef] [Green Version]
- Ichimiya, H.; Takahashi, T.; Ariyoshi, W.; Takano, H.; Matayoshi, T.; Nishihara, T. Compressive mechanical stress promotes osteoclast formation through RANKL expression on synovial cells. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 103, 334–341. [Google Scholar]
- Pozzi, S.; Vallet, S.; Mukherjee, S.; Cirstea, D.; Vaghela, N.; Santo, L.; Rosen, E.; Ikeda, H.; Okawa, Y.; Kiziltepe, T.; et al. High-dose zoledronic acid impacts bone remodeling with effects on osteoblastic lineage and bone mechanical properties. Clin. Cancer Res. 2009, 15, 5829–5839. [Google Scholar] [CrossRef] [Green Version]
- Patntirapong, S.; Singhatanadgit, W.; Chanruangvanit, C.; Lavanrattanakul, K.; Satravaha, Y. Zoledronic acid suppresses mineralization through direct cytotoxicity and osteoblast differentiation inhibition. J. Oral Pathol. Med. 2012, 41, 713–720. [Google Scholar] [CrossRef]
- Huang, K.C.; Cheng, C.C.; Chuang, P.Y.; Yang, T.Y. The effects of zoledronate on the survival and function of human osteoblast-like cells. BMC Musculoskelet. Disord. 2015, 16, 355. [Google Scholar] [CrossRef] [Green Version]
- Pons-Fuster López, E.; Seoane Leston, J.; López Jornet, P. Epigallocatechin-3-gallate reduces damage to osteoblast-like cells treated with Zoledronic acid. Arch. Oral Biol. 2018, 94, 27–32. [Google Scholar] [CrossRef]
- Komori, T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res. 2010, 339, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Robling, A.G.; Castillo, A.B.; Turner, C.H. Biomechanical and molecular regulation of bone remodeling. Annu. Rev. Biomed. Eng. 2006, 8, 455–498. [Google Scholar] [CrossRef]
- Gerber, H.P.; Vu, T.H.; Ryan, A.M.; Kowalski, J.; Werb, Z.; Ferrara, N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 1999, 5, 623–628. [Google Scholar] [CrossRef]
- Jacobsen, K.A.; Al-Aql, Z.S.; Wan, C.; Fitch, J.L.; Stapleton, S.N.; Mason, Z.D.; Cole, R.M.; Gilbert, S.R.; Clemens, T.L.; Morgan, E.F.; et al. Bone formation during distraction osteogenesis is dependent on both VEGFR1 and VEGFR2 signaling. J. Bone Miner. Res. 2008, 23, 596–609. [Google Scholar] [CrossRef] [Green Version]
- Deckers, M.M.; Karperien, M.; van der Bent, C.; Yamashita, T.; Papapoulos, S.E.; Löwik, C.W. Expression of vascular endothelial growth factors and their receptors during osteoblast differentiation. Endocrinology 2000, 141, 1667–1674. [Google Scholar] [CrossRef]
- Schipani, E.; Maes, C.; Carmeliet, G.; Semenza, G.L. Regulation of osteogenesis-angiogenesis coupling by HIFs and VEGF. J. Bone. Miner. Res. 2009, 24, 1347–1353. [Google Scholar] [CrossRef]
- Maruotti, N.; Corrado, A.; Neve, A.; Cantatore, F.P. Bisphosphonates: Effects on osteoblast. Eur. J. Clin. Pharmacol. 2012, 68, 1013–1018. [Google Scholar] [CrossRef]
- Yang, X.; Lu, Y.; Li, Z.; Wang, Y.; Zhao, F.; Han, J. Low concentrations of zoledronic acid are better at regulating bone formation and repair. Intractable Rare Dis. Res. 2013, 2, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Svanberg, G.; Lindhe, J. Vascular reactions in the periodontal ligament incident to trauma from occlusion. J. Clin. Periodontol. 1974, 1, 58–69. [Google Scholar] [CrossRef]
- Branschofsky, M.; Beikler, T.; Schäfer, R.; Flemming, T.F.; Lang, H. Secondary trauma from occlusion and periodontitis. Quintessence Int. 2011, 42, 515–522. [Google Scholar]
- Naert, I.; Duyck, J.; Vandamme, K. Occlusal overload and bone/implant loss. Clin. Oral Implants Res. 2012, 23, 95–107. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Suzuki, N.; Mitsui, N.; Koyama, Y.; Otsuka, K.; Shimizu, N. Compressive force stimulates the expression of osteogenesis-related transcription factors in ROS 17/2.8 cells. Arch. Oral Biol. 2008, 53, 214–219. [Google Scholar] [CrossRef]
- Mitsui, N.; Suzuki, N.; Koyama, Y.; Yanagisawa, M.; Otsuka, K.; Shimizu, N.; Maeno, M. Effect of compressive force on the expression of MMPs, PAs, and their inhibitors in osteoblastic Saos-2 cells. Life Sci. 2006, 79, 575–583. [Google Scholar] [CrossRef]
- Mori, S.; Sato, T.; Hara, T.; Nakashima, K.; Minagi, S. Effect of continuous pressure on histopathological changes in denture-supporting tissues. J. Oral Rehabil. 1997, 24, 37–46. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).