Live-Scale Testing of Granular Materials Stabilized with Alkali-Activated Waste Glass and Carbide Lime
Abstract
:1. Introduction
2. Experimental Program
2.1. Materials
- BSHS (activated with a 3 molal sodium hydroxide solution);
- BH2O (hydrated with water).
2.2. Fabrication of the Laboratory Specimens
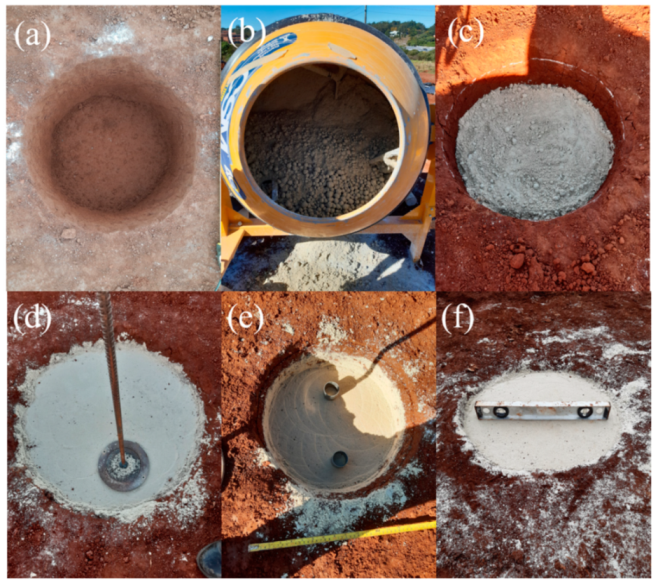
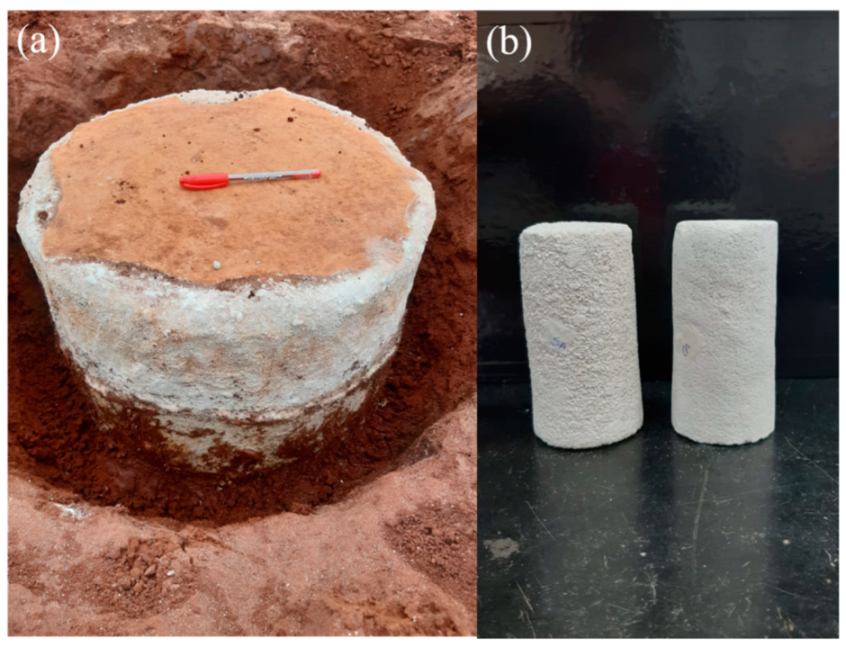
2.3. Recovery of the Field Specimens
2.4. Specimen Testing
2.5. Spread Footing Testing
3. Results and Discussion
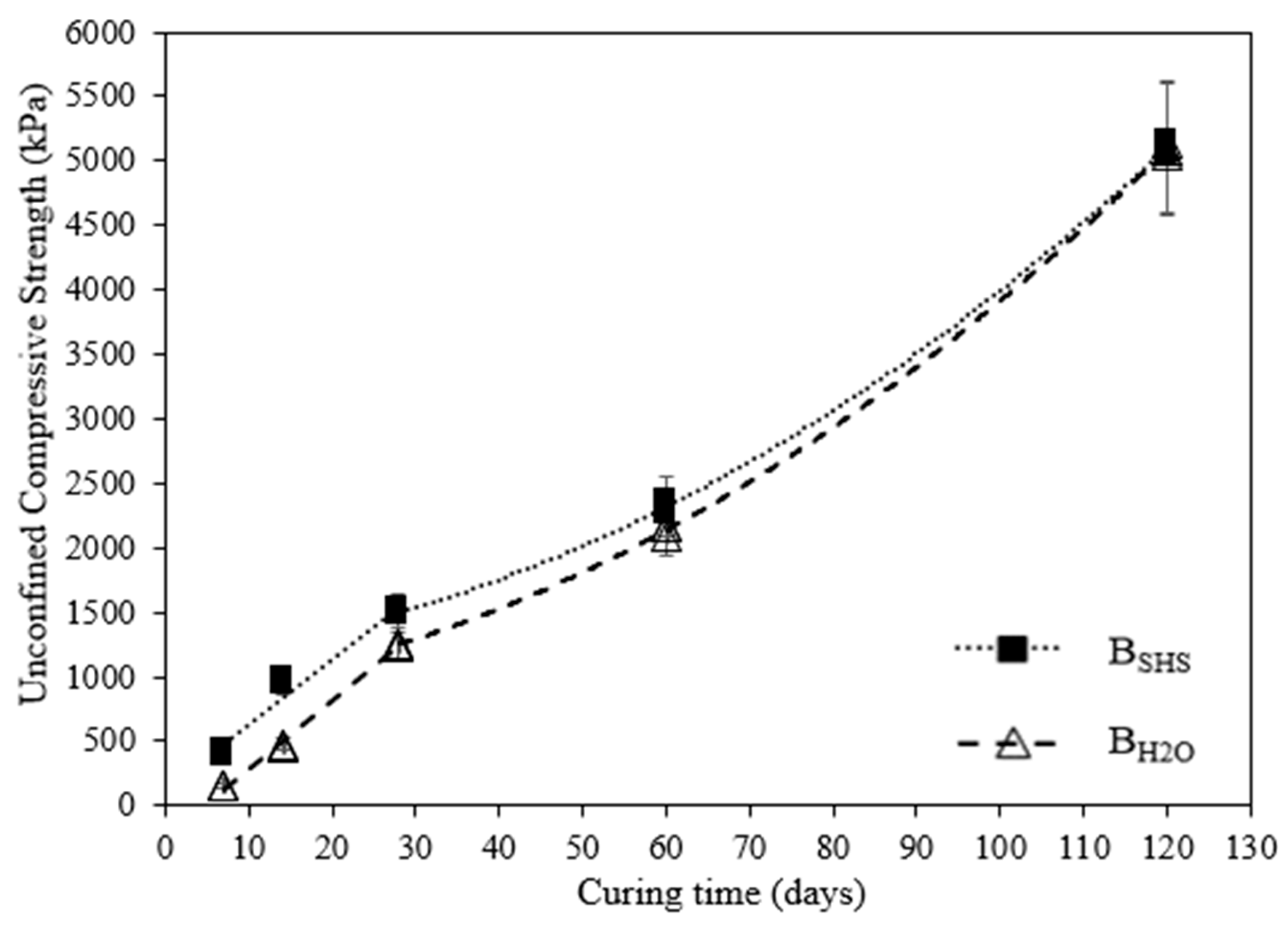
3.1. Evolution of UCS with Time
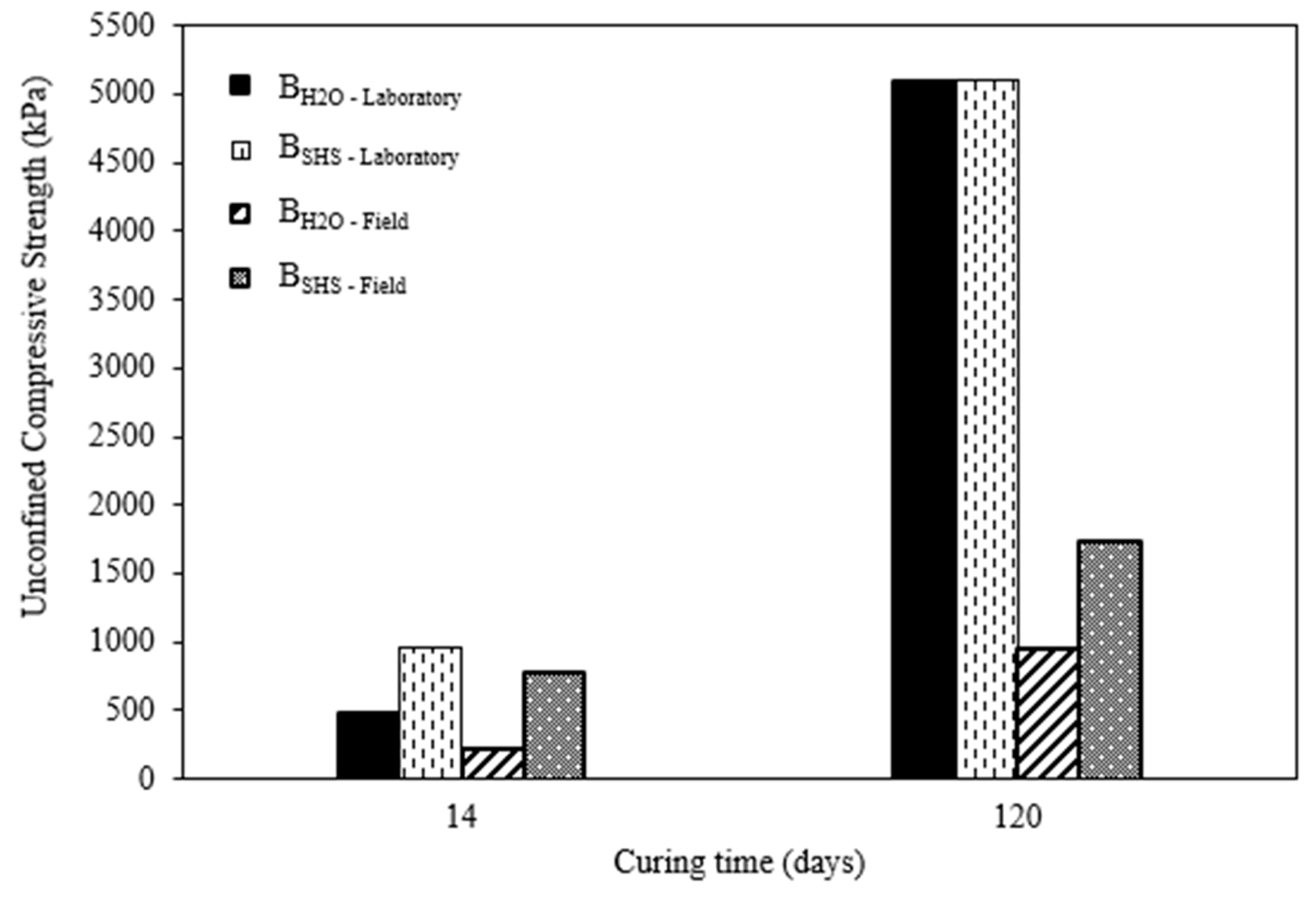
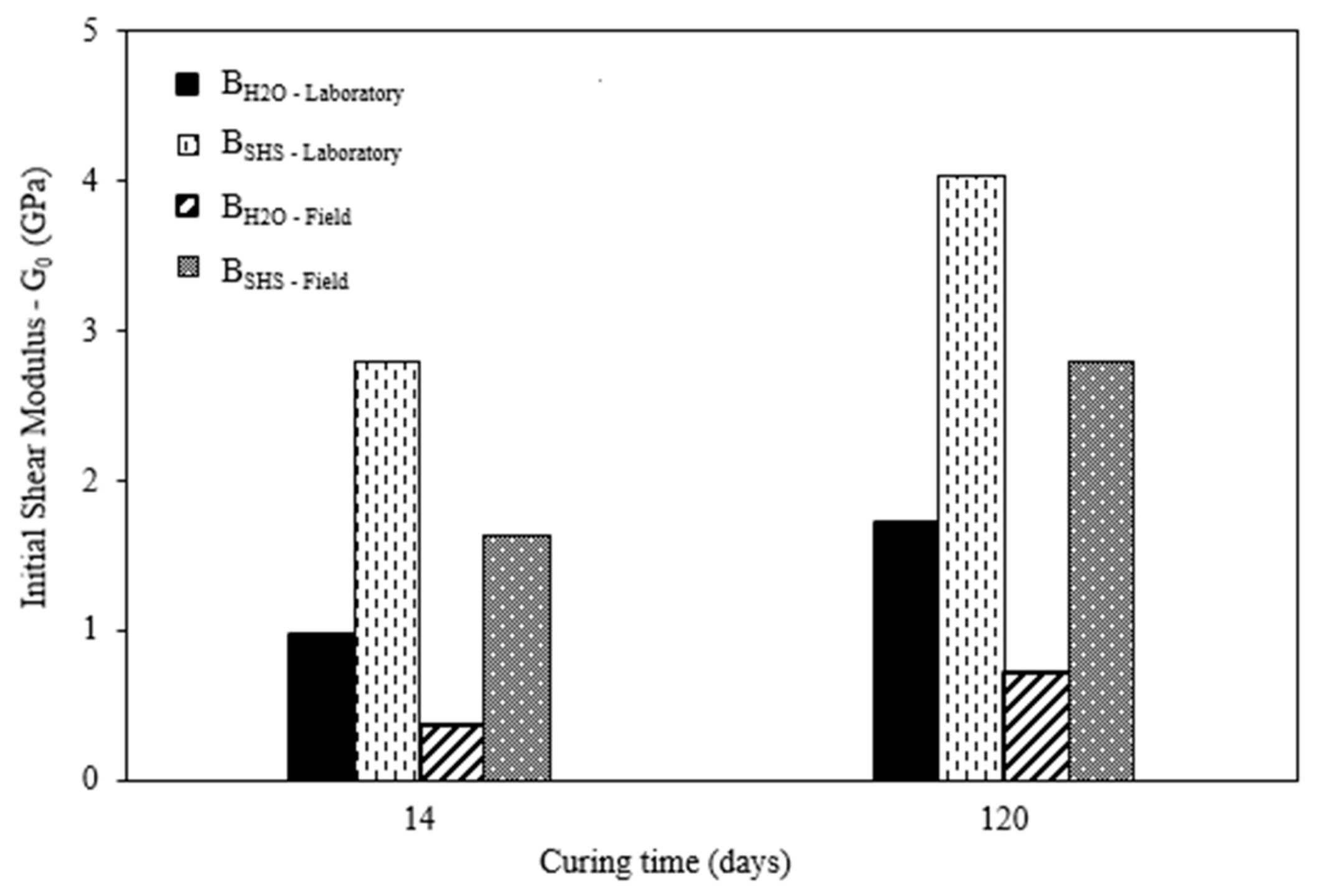
3.2. Field and Laboratory Results Comparison
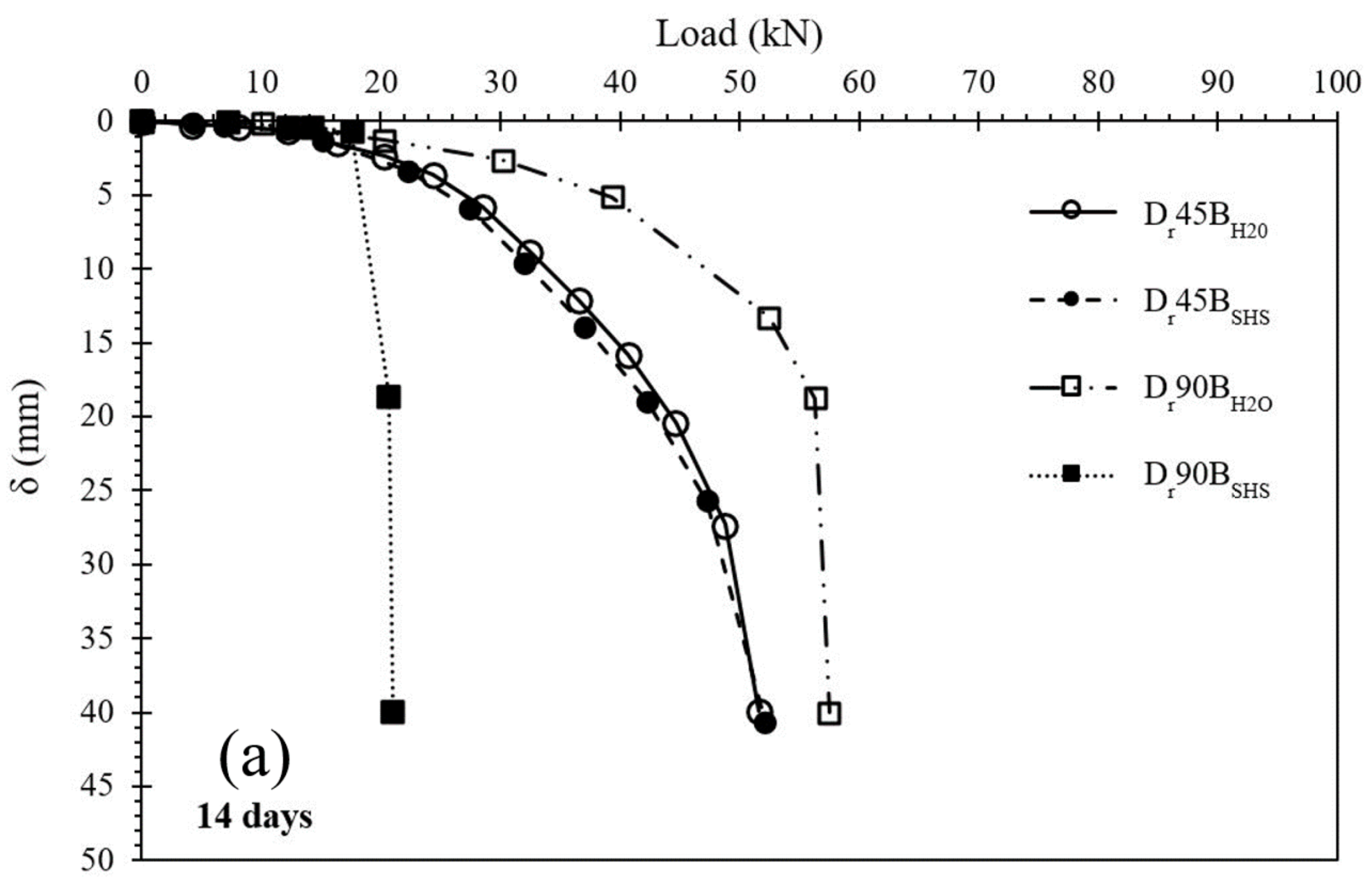
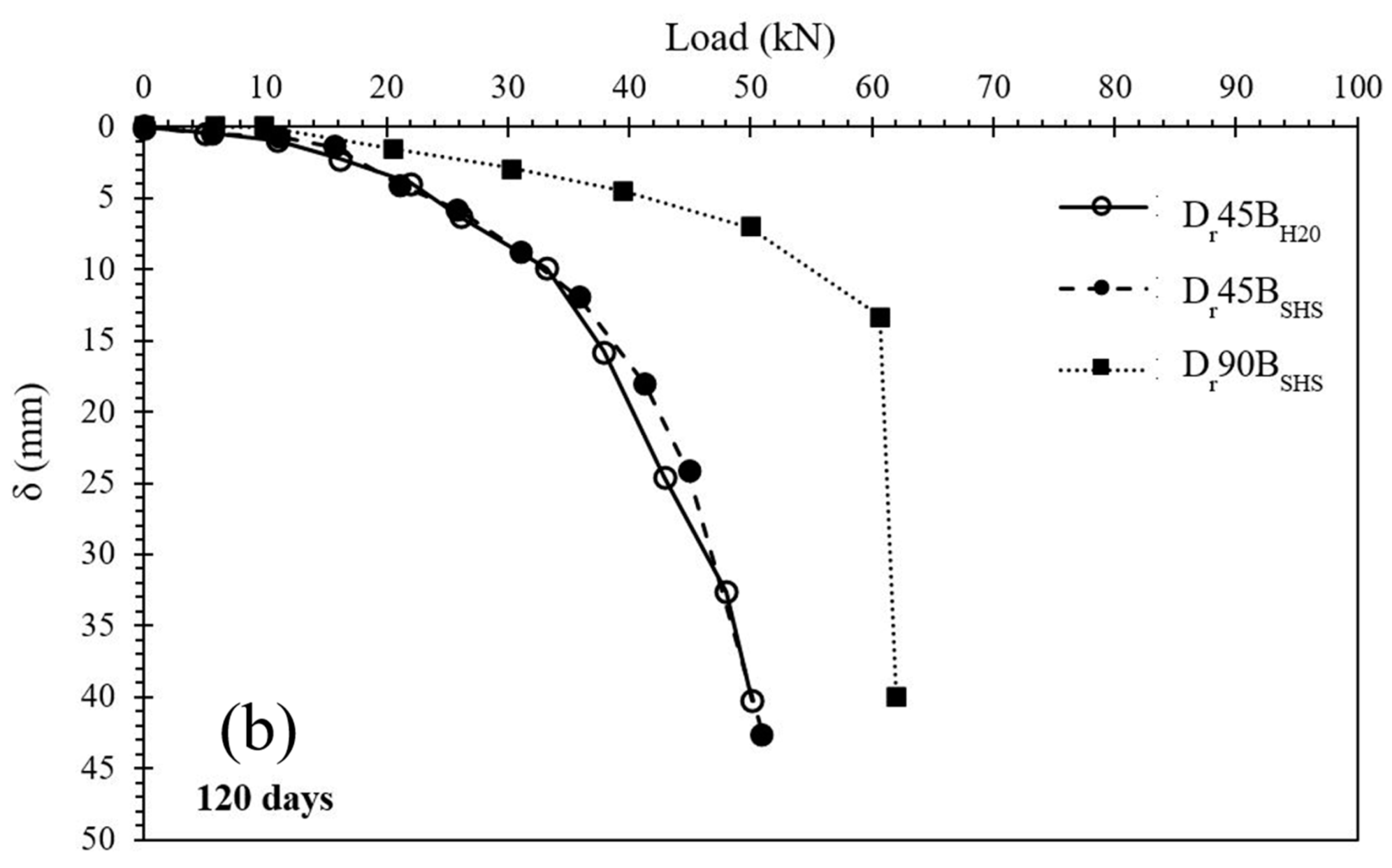
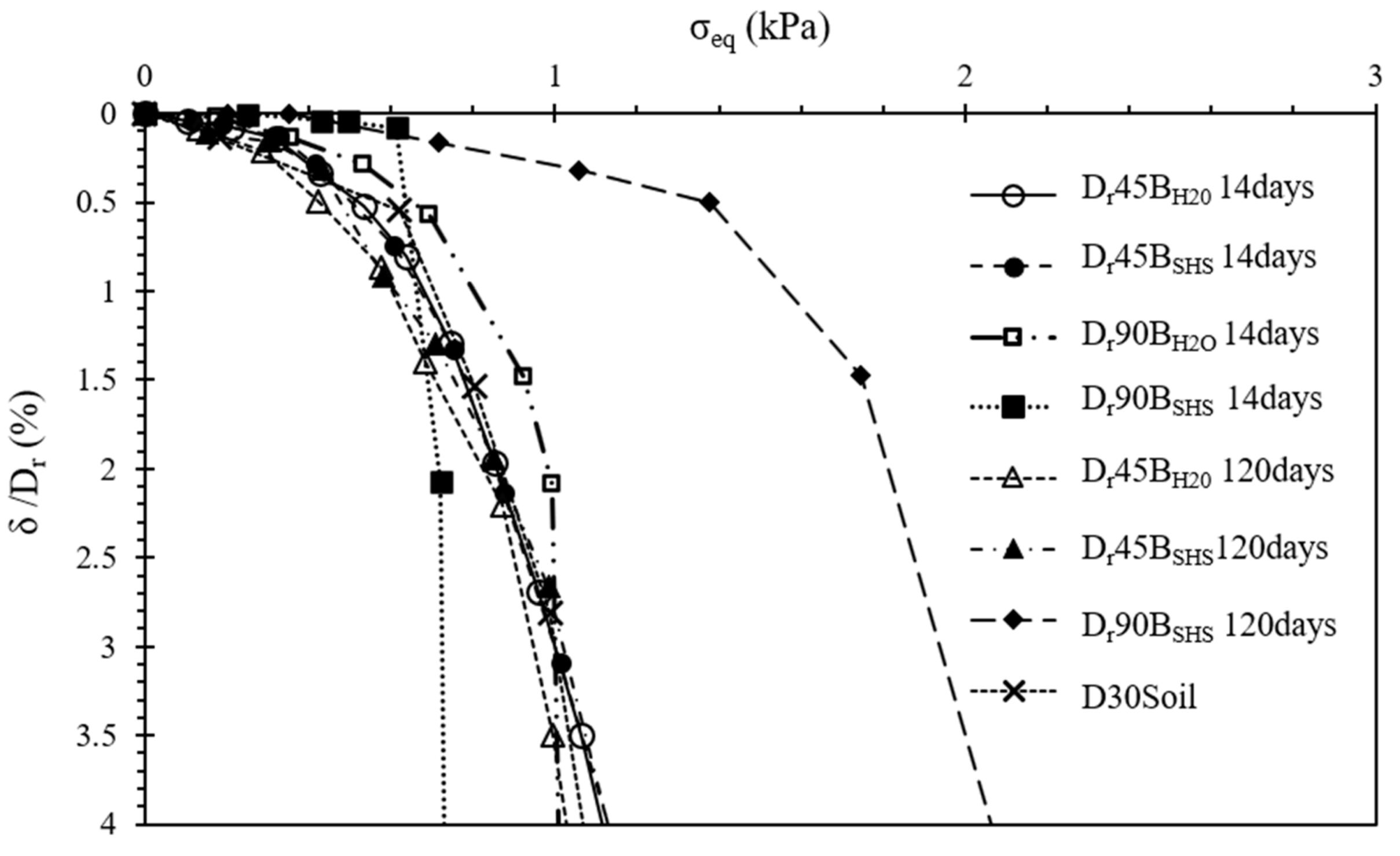
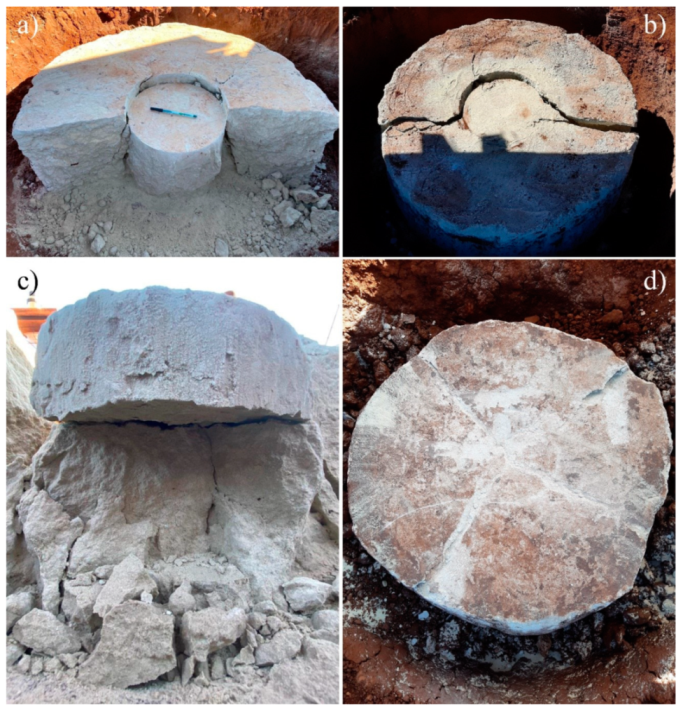
3.3. Spread Footing Testing
4. Conclusions
- (a)
- Regarding the laboratory mixtures, the material activated with a 3M sodium hydroxide solution presented a higher compressive strength (UCS) than the material hydrated with water, but only for short-term curing periods. The alkalinity of the activator enhances the initial dissolution of the original silica, resulting in a strength 2.6× higher than the water-based mixtures. With the increase in curing time, the latter was able to develop similar volumes of binder, thus obtaining similar UCS values. In the present research, the results for NaOH mixtures varied from 0.4 MPa to 5.0 MPa for laboratory tests and 0.2 MPa to 1.7 MPa for field results, depending on the curing period. Therefore, some of the results are contained in the minimum values required by soil-cement standards and can also be considered for projects with lower stresses;
- (b)
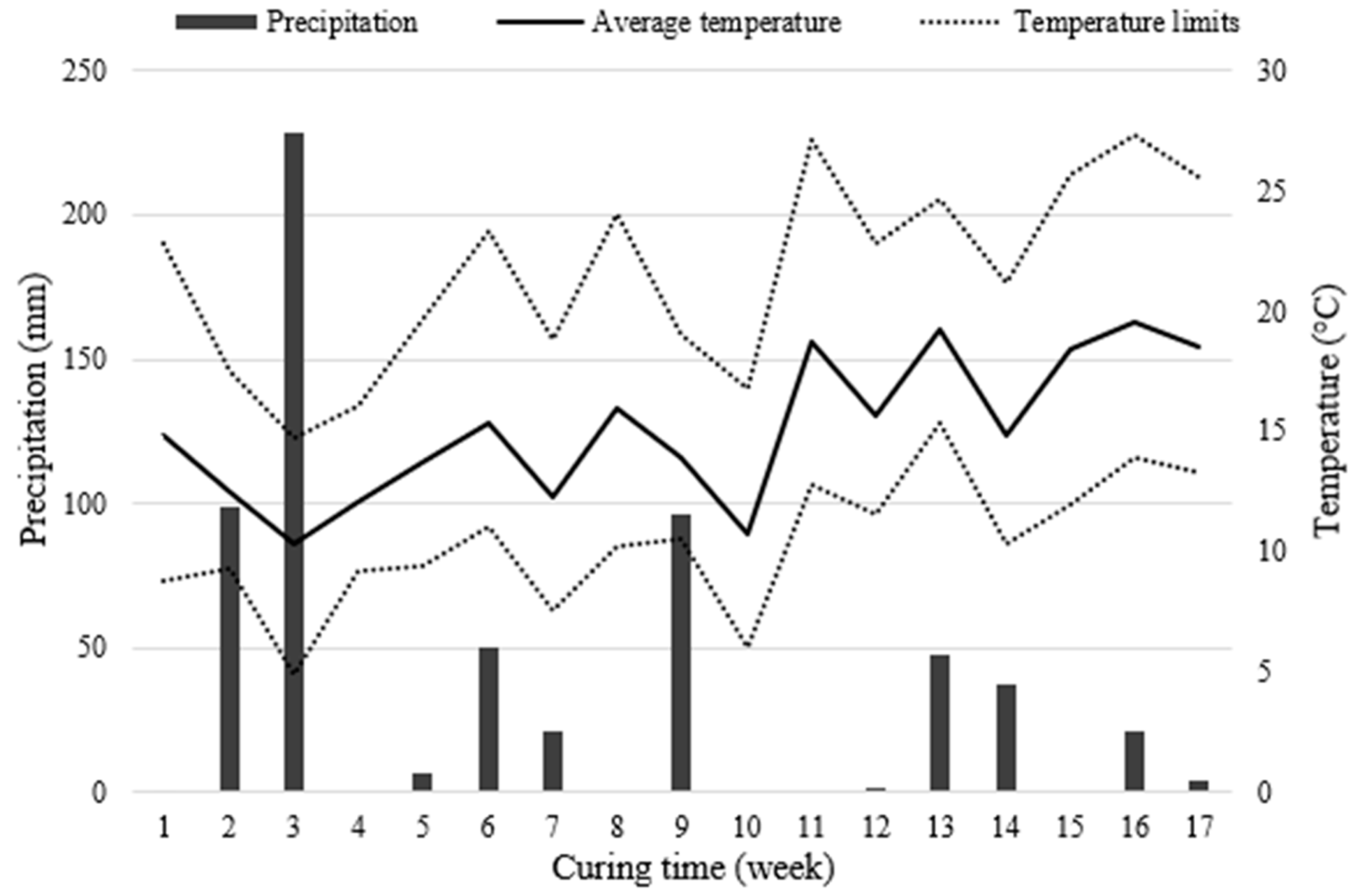
- Comparing the field and laboratory UCS data, it is clear that the former showed lower strength for both types of the mixture, which is attributed to the influence of the climatic conditions during curing, which was controlled in the laboratory, and to a molding precision of the specimens made in the laboratory with greater control regarding mixtures homogeneity, degree of compaction and control of shape and measurements (i.e., vertical sides and flat surfaces). As the curing period increased, the difference between field and laboratory became more significant, which was aggravated by the higher precipitation registered in the region during the last 7 weeks of the curing period, compared with the first 10 weeks;
- (c)
- The stiffness (G0) results obtained in the field were lower than those obtained in the laboratory for both types of mixture. With the increase in curing time, there was an increase in stiffness. However, the AAC-based mixtures showed higher initial stiffness values than the water-based mixtures in the laboratory and in the field. This was contrary to what was observed with the UCS, after 120 days, when the hydrated material showed higher compressive strength than the activated material;
- (d)
- The plate load tests showed that the stabilization of the surface layer significantly increased the load-bearing capacity compared with the natural soil. An increase in the diameter of the stabilized layer also provided a higher load-bearing capacity. Two different failure mechanisms were observed, depending on the dimensions of the reinforcement layers, with the 450 mm layer showing a puncture mechanism, while the 900 mm layer showed the appearance of cracks;
- (e)
- Data normalization suggests that, in the case of the 450 mm layers, the foundation (plate) and the stabilized layer behaved as a single element, supported on the lower stabilized base, meaning that the failure load is controlled by the capacity of the soil beneath it. For the 900 mm diameter, the failure occurred when the applied load exceeded its tensile stress.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Abdeldjouad, L.; Asadi, A.; Ball, R.; Nahazanan, H.; Huat, B.B. Application of alkali-activated palm oil fuel ash reinforced with glass fibers in soil stabilization. Soils Found. 2019, 59, 1552–1561. [Google Scholar] [CrossRef]
- ABNT NBR 9813. Solo—Determinação da Massa Específica Aparente In Situ, Com Emprego de Cilindro de Cravação. Available online: http://files.ilcoribeiro.webnode.com.br/200000080-6bf7f6cf24/NBR%209813.pdf (accessed on 13 March 2021).
- ABNT NBR 12253. Solo-Cimento—Dosagem Para Emprego Como Camada de Pacimento. Available online: https://www.target.com.br/produtos/normas-tecnicas/36229/nbr12253-solo-cimento-dosagem-para-emprego-como-camada-de-pavimento-procedimento (accessed on 13 March 2021).
- Alonso, S.; Palomo, A. Alkaline activation of metakaolin and calcium hydroxide mixtures: Influence of temperature, activator concentration and solids ratio. Mater. Lett. 2001, 47, 55–62. [Google Scholar] [CrossRef]
- ASTM. ASTM D7928. Standard Test Method for Particle-Size Distribution (Gradation) of Fine-Grained Soils Using the Sedi-Mentation (Hydrometer) Analysis; ASTM: West Conshohocken, FL, USA, 2021. [Google Scholar]
- ASTM. ASTM D698. Standard Test Methods for Laboratory Compaction Characteristics of Soil Using Standard Effort (12, 400 ft-lbf/ft3 (600 kN-m/m3)); ASTM: West Conshohocken, FL, USA, 2021. [Google Scholar]
- ASTM. ASTM C39/C39M. Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens; ASTM: West Conshohocken, FL, USA, 2021. [Google Scholar]
- ASTM. ASTM C511. Standard Specification for Mixing Rooms, Moist Cabinets, Moist Rooms, and Water Storage Tanks Used in the Testing of Hydraulic Cements and Concretes; ASTM: West Conshohocken, FL, USA, 2019. [Google Scholar]
- ASTM. ASTM D2487. Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System); ASTM: West Conshohocken, FL, USA, 2017. [Google Scholar]
- ASTM. ASTM D1194. Standard Test Method for Bearing Capacity of Soil for Static Load and Spread Footings; ASTM: West Conshohocken, FL, USA, 1994. [Google Scholar]
- Baldovino, J.J.; Izzo, R.L.; Rose, J.L.; Domingos, M.D. Strength, durability, and microstructure of geopolymers based on recycled-glass powder waste and dolomitic lime for soil stabilization. Constr. Build. Mater. 2021, 271, 121874. [Google Scholar] [CrossRef]
- Bicca Neto, V. Commitment Business for Recycling—Review; Coca Cola Company: Brasilia, Brazil, 2015; Available online: https://cempre.org.br/wp-content/uploads/2020/11/CEMPRE-Review2015.pdf (accessed on 20 March 2021).
- Bruschi, G.J.; dos Santos, C.P.; de Araújo, M.T.; Ferrazzo, S.T.; Marques, S.F.V.; Consoli, N.C. Green Stabilization of Bauxite Tailings: Mechanical Study on Alkali-Activated Materials. J. Mater. Civ. Eng. 2021, 33, 06021007. [Google Scholar] [CrossRef]
- Bruschi, G.J.; dos Santos, C.P.; Ferrazzo, S.T.; de Araújo, M.T.; Consoli, N.C. Parameters controlling loss of mass and stiffness degradation of ‘green’ stabilised tailings. Proc. Inst. Civ. Eng. Geotech. Eng. 2021, 164, 00119. [Google Scholar] [CrossRef]
- Caballero, R.D. Development of a Design Methodology for Circular Surface Foundations Laid on a Soil-Cement Layer; Federal University of Rio Grande do Sul: Porto Alegre, Spain, 2019. [Google Scholar]
- Carretta, M.D.S.; Cristelo, N.; Festugato, L.; Miguel, G.D.; Consoli, N.C. Experimental assessment of the small-strain response of residual soil under monotonic and cyclic loading. Proc. Inst. Civ. Eng. Geotech. Eng. 2021, 00073. [Google Scholar] [CrossRef]
- Consoli, N.C.; Carretta, M.D.S.; Leon, H.B.; Filho, H.C.S.; Tomasi, L.F. Strength and Stiffness of Ground Waste Glass–Carbide Lime Blends. J. Mater. Civ. Eng. 2019, 31, 06019010. [Google Scholar] [CrossRef]
- Consoli, N.C.; Casagrande, M.D.T.; Thomé, A.; Rosa, F.D.; Fahey, M. Effect of relative density on plate loading tests on fibre-reinforced sand. Géotechnique 2009, 59, 471–476. [Google Scholar] [CrossRef]
- Consoli, N.C.; Daassi-Gli, C.A.P.; Ruver, C.A.; Lotero, A.; Filho, H.C.S.; Moncaleano, C.J.; Lourenço, D.E. Lime–Ground Glass–Sodium Hydroxide as an Enhanced Sustainable Binder Stabilizing Silica Sand. J. Geotech. Geoenviron. Eng. 2021, 147, 06021011. [Google Scholar] [CrossRef]
- Consoli, N.C.; Moreira, E.B.; Festugato, L.; Caballero, R.D.; Foppa, D.; Ruver, C.A. Enhancing bearing capacity of shallow foundations through cement-stabilised sand layer over weakly bonded residual soil. Géotechnique 2021, 064. [Google Scholar] [CrossRef]
- Consoli, N.C.; Moreira, E.B.; Festugato, L.; Lopes, L.D.S.; Carretta, M.D.S.; Ceolin, A.O. Spread Footings on Green Stabilized Sand Layers over Weakly Bonded Residual Soil. J. Geotech. Geoenviron. Eng. 2020, 146, 06020022. [Google Scholar] [CrossRef]
- Consoli, N.C.; Rosa, A.D.; Saldanha, R.B. Variables Governing Strength of Compacted Soil–Fly Ash–Lime Mixtures. J. Mater. Civ. Eng. 2011, 23, 432–440. [Google Scholar] [CrossRef]
- Consoli, N.C.; Rosa, F.D.; Fonini, A. Plate Load Tests on Cemented Soil Layers Overlaying Weaker Soil. J. Geotech. Geoenviron. Eng. 2009, 135, 1846–1856. [Google Scholar] [CrossRef]
- Consoli, N.C.; Rossi, J.G.; Festugato, L.; Ruver, C.A.; Filho, H.C.S.; Foppa, D.; Carretta, M.D.S.; Leon, H.B. Circular-Plate Load Tests on Bounded Cemented Layers above Weak Cohesive-Frictional Soil. J. Geotech. Geoenviron. Eng. 2019, 145, 06019011. [Google Scholar] [CrossRef]
- Consoli, N.C.; Vendruscolo, M.A.; Prietto, P.D.M. Behavior of Plate Load Tests on Soil Layers Improved with Cement and Fiber. J. Geotech. Geoenviron. Eng. 2003, 129, 96–101. [Google Scholar] [CrossRef]
- Consoli, N.C.; Winter, D.; Leon, H.B.; Filho, H.C.S. Durability, Strength, and Stiffness of Green Stabilized Sand. J. Geotech. Geoenviron. Eng. 2018, 144, 04018057. [Google Scholar] [CrossRef]
- Coppola, L.; Bellezze, T.; Belli, A.; Bignozzi, M.C.; Bolzoni, F.; Brenna, A.; Cabrini, M.; Candamano, S.; Cappai, M.; Caputo, D.; et al. Binders alternative to Portland cement and waste management for sustainable construction—Part 2. J. Appl. Biomater. Funct. Mater. 2018, 16, 207–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrêa-Silva, M.; Miranda, T.; Rouainia, M.; Araújo, N.; Glendinning, S.; Cristelo, N. Geomechanical behaviour of a soft soil stabilised with alkali-activated blast-furnace slags. J. Clean. Prod. 2020, 267, 122017. [Google Scholar] [CrossRef]
- Cristelo, N.; Fernández-Jiménez, A.; Castro, F.; Fernandes, L.; Tavares, P. Sustainable alkaline activation of fly ash, aluminium anodising sludge and glass powder blends with a recycled alkaline cleaning solution. Constr. Build. Mater. 2019, 204, 609–620. [Google Scholar] [CrossRef]
- Cristelo, N.; Glendinning, S.; Fernandes, L.S.G.; Pinto, A.T. Effect of calcium content on soil stabilisation with alkaline activation. Constr. Build. Mater. 2012, 29, 167–174. [Google Scholar] [CrossRef]
- Daassi-Gli, C.A.P. Estabilização de um Solo Granular com Misturas de Pó de Vidro–Cal de Carbureto–Hidróxido de Sódio (NaOH); Federal University of Rio Grande do Sul: Porto Alegre, Spain, 2020. [Google Scholar]
- Duxson, P.; Jimenez, A.M.F.; Provis, J.; Lukey, G.C.; Palomo, Á.; Van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2006, 42, 2917–2933. [Google Scholar] [CrossRef]
- Escalante-Garcia, I.J. Overview of potential of urban waste glass as a cementitious material in alternative chemically activated binders. J. Chin. Ceram. Soc. 2015, 43, 1441–1448. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Composition and microstructure of alkali activated fly ash binder: Effect of the activator. Cem. Concr. Res. 2005, 35, 1984–1992. [Google Scholar] [CrossRef]
- Lee, S.; Chung, M.; Park, H.M.; Song, K.-I.; Chang, I. Xanthan Gum Biopolymer as Soil-Stabilization Binder for Road Construction Using Local Soil in Sri Lanka. J. Mater. Civ. Eng. 2019, 31, 06019012. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, C.; Zhang, Z.; Li, N. An overview on the reuse of waste glasses in alkali-activated materials. Resour. Conserv. Recycl. 2019, 144, 297–309. [Google Scholar] [CrossRef]
- Lotero, A.; Consoli, N.C.; Moncaleano, C.J.; Neto, A.T.; Koester, E. Mechanical Properties of Alkali-Activated Ground Waste Glass–Carbide Lime Blends for Geotechnical Uses. J. Mater. Civ. Eng. 2021, 33, 04021284. [Google Scholar] [CrossRef]
- Miranda, T.; Leitão, D.; Oliveira, J.; Corrêa-Silva, M.; Araújo, N.; Coelho, J.; Fernández-Jiménez, A.; Cristelo, N. Application of alkali-activated industrial wastes for the stabilisation of a full-scale (sub)base layer. J. Clean. Prod. 2020, 242, 118427. [Google Scholar] [CrossRef]
- Mohajerani, A.; Vajna, J.; Cheung, T.H.H.; Kurmus, H.; Arulrajah, A.; Horpibulsuk, S. Practical recycling applications of crushed waste glass in construction materials: A review. Constr. Build. Mater. 2017, 156, 443–467. [Google Scholar] [CrossRef]
- Pinto, A.T. Binding Systems Obtained by Alkaline Activation of Metacaulim; University of Minho: Braga, Portugal, 2004. [Google Scholar]
- Bilondi, M.P.; Toufigh, M.M.; Toufigh, V. Using calcium carbide residue as an alkaline activator for glass powder–clay geopolymer. Constr. Build. Mater. 2018, 183, 417–428. [Google Scholar] [CrossRef]
- Pourakbar, P.S.; Asadi, A.; Huat, B.B.K.; Cristelo, N.; Fasihnikoutalab, M.H. Application of Alkali-Activated Agro-Waste Reinforced with Wollastonite Fibers in Soil Stabilization. J. Mater. Civ. Eng. 2017, 29, 04016206. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Binder Chemistry—Blended Systems and Intermediate Ca Content; Springer: Singapore, 2014; pp. 125–144. [Google Scholar]
- Redden, R.; Neithalath, N. Microstructure, strength, and moisture stability of alkali activated glass powder-based binders. Cem. Concr. Compos. 2014, 45, 46–56. [Google Scholar] [CrossRef]
- Rios, S.; Cristelo, N.; Da Fonseca, A.V.; Ferreira, C. Structural Performance of Alkali-Activated Soil Ash versus Soil Cement. J. Mater. Civ. Eng. 2016, 28, 04015125. [Google Scholar] [CrossRef] [Green Version]
- Rivera, J.F.; Cuarán-Cuarán, Z.I.; Vanegas-Bonilla, N.; de Gutiérrez, R.M. Novel use of waste glass powder: Production of geopolymeric tiles. Adv. Powder Technol. 2018, 29, 3448–3454. [Google Scholar] [CrossRef]
- Rivera, J.F.; Orobio, A.; Cristelo, N.; de Gutiérrez, R.M. Fly ash-based geopolymer as A4 type soil stabiliser. Transp. Geotech. 2020, 25, 100409. [Google Scholar] [CrossRef]
- Saldanha, R.B.; Consoli, N.C. Accelerated Mix Design of Lime Stabilized Materials. J. Mater. Civ. Eng. 2016, 28, 06015012. [Google Scholar] [CrossRef]
- Saldanha, R.B.; Filho, H.C.S.; Mallmann, J.E.C.; Consoli, N.C.; Reddy, K. Physical–Mineralogical–Chemical Characterization of Carbide Lime: An Environment-Friendly Chemical Additive for Soil Stabilization. J. Mater. Civ. Eng. 2018, 30, 06018004. [Google Scholar] [CrossRef]
- Sales, L.F.P. Estudo do Comportamento de Fundações Superficiais Assentes em Solos Tratados; Fedral University of Rio Grande do Sul: Porto Alegre, Spain, 1998. [Google Scholar]
- Filho, H.C.S.; Saldanha, R.B.; Da Rocha, C.G.; Consoli, N.C. Sustainable Binders Stabilizing Dispersive Clay. J. Mater. Civ. Eng. 2021, 33, 06020026. [Google Scholar] [CrossRef]
- Filho, H.C.S.; Sacco, R.L.; Consoli, N.C. The effect of grain size of ground glass particles on the strength of green stabilized sand. Soils Rocks 2020, 43, 669–677. [Google Scholar] [CrossRef]
- Thomé, A.; Donato, M.; Consoli, N.C.; Graham, J. Circular footings on a cemented layer above weak foundation soil. Can. Geotech. J. 2005, 42, 1569–1584. [Google Scholar] [CrossRef]
- Torres-Carrasco, M.; Puertas, F. Alkaline Activation of Different Aluminosilicates as an alternative to Portland Cement: Alkali Activated Cements or Geopolymers. Rev. Ing. Constr. 2017, 32, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Yip, C.K.; Lukey, G.C.; Van Deventer, J.S.J. The coexistence of geopolymeric gel and calcium silicate hydrate at the early stage of alkaline activation. Cem. Concr. Res. 2005, 35, 1688–1697. [Google Scholar] [CrossRef]
- Zhang, Z.; Tao, M. Durability of Cement Stabilized Low Plasticity Soils. J. Geotech. Geoenviron. Eng. 2008, 134, 203–213. [Google Scholar] [CrossRef]
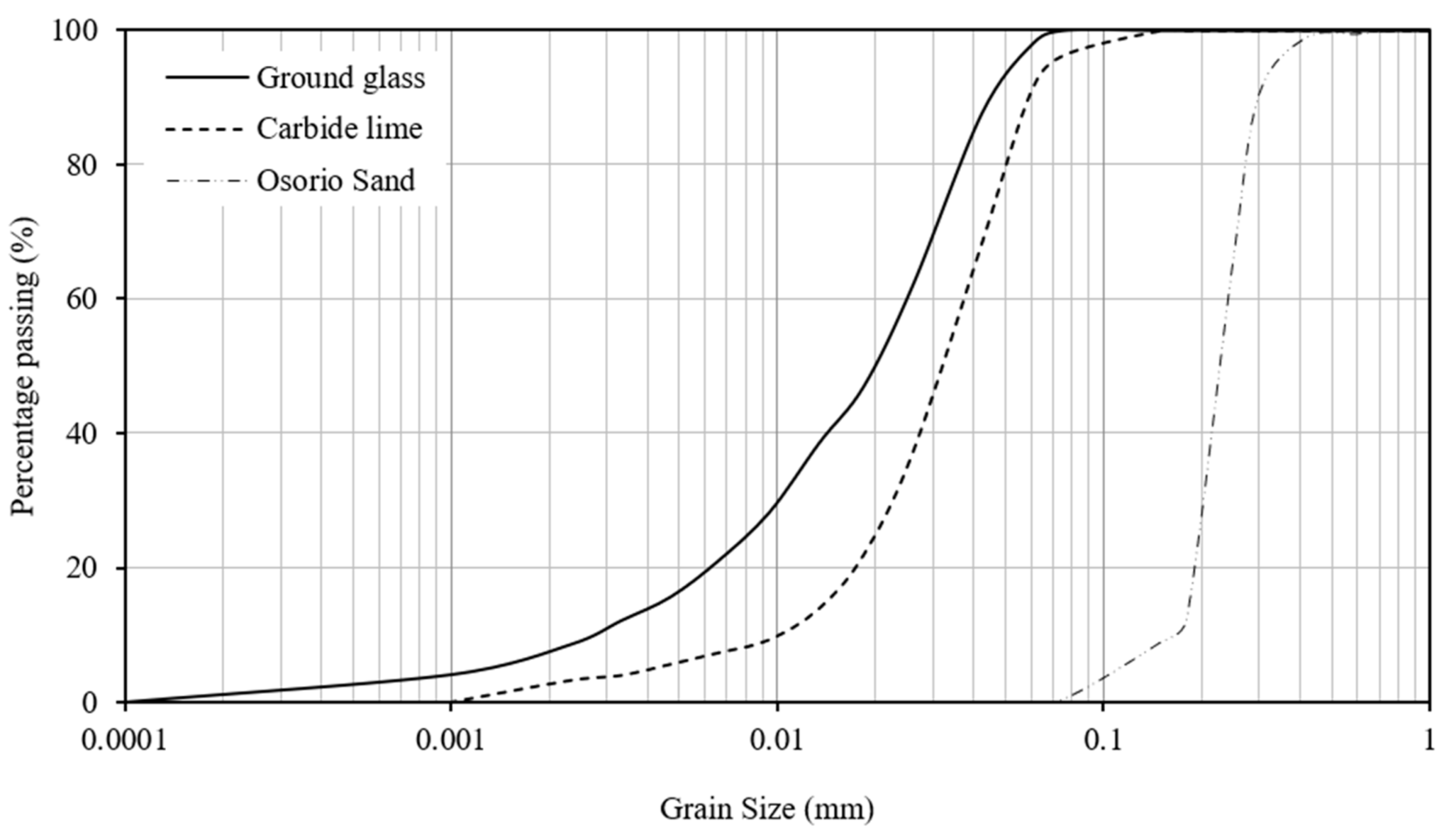

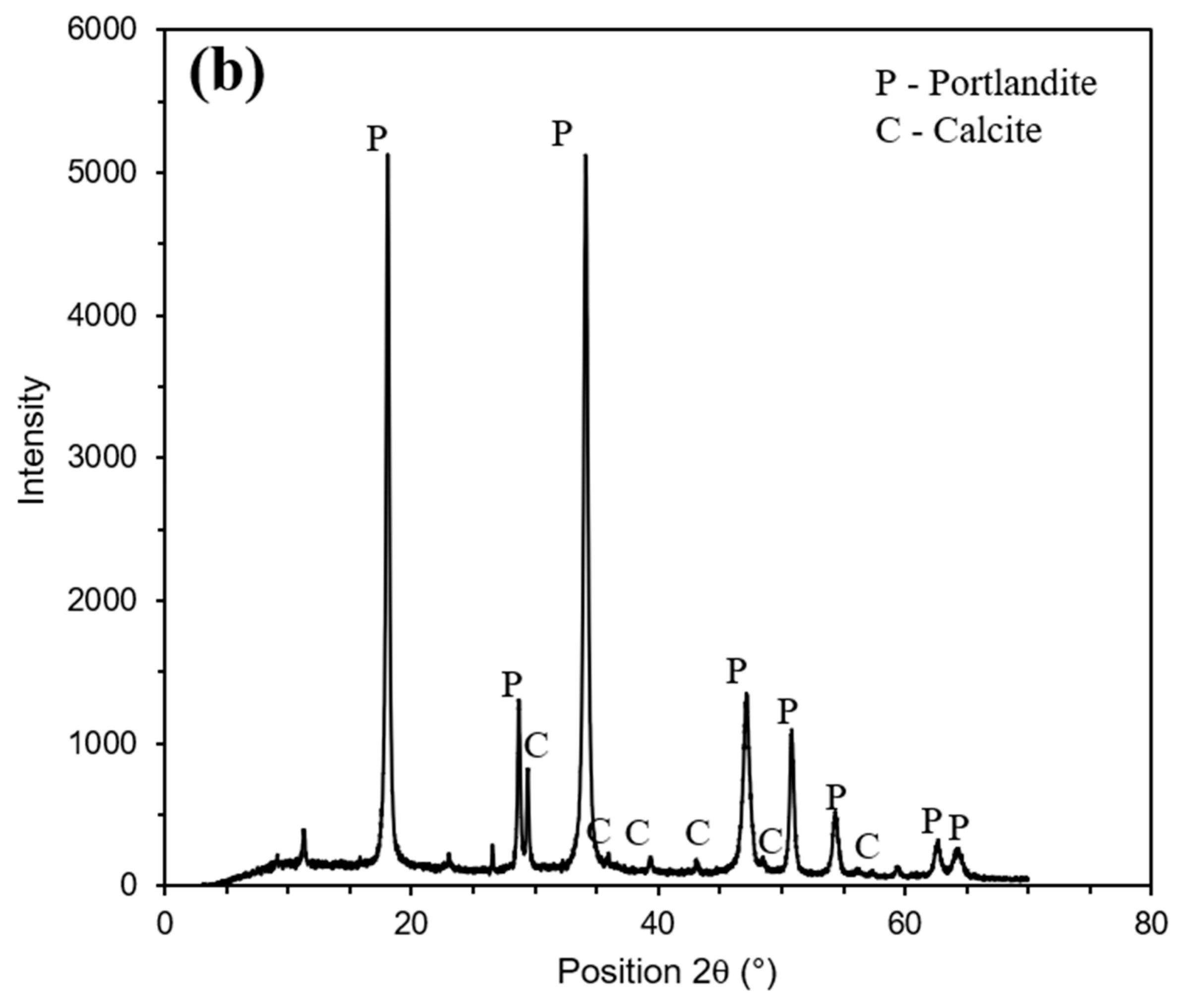




| Properties | Osorio Sand | GWG | CL |
|---|---|---|---|
| Specific gravity (g/cm3) | 2.65 | 2.47 | 2.19 |
| Medium sand (0.425 mm < diameter < 2.0 mm) (%) | 1.0 | - | - |
| Fine sand (0.075 mm < diameter < 0.425 mm) (%) | 98.8 | 30.6 | 2.0 |
| Silt (0.002 mm < diameter < 0.075 mm) (%) | 0.2 | 60.4 | 94.75 |
| Clay (diameter < 0.002 mm) (%) | 0 | 9.0 | 3.25 |
| Liquid limit | - | - | - |
| Plastic index | Non-plastic | Non-plastic | Non-plastic |
| Soil classification (ASTM D2487) | SP | ML | ML |
| Element | SiO2 | Al2O3 | Fe2O3 | CaO | Na2O | MgO | TiO2 | MnO | P2O5 | K2O |
|---|---|---|---|---|---|---|---|---|---|---|
| Ground Waste Glass | 69.02 | 4.34 | 0.91 | 9.72 | 10.89 | 4.2 | 0.05 | 0.01 | - | 0.25 |
| Carbide lime | 3.18 | 1.99 | 0.98 | 69.62 | - | 0.56 | 0.08 | 0.01 | 0.01 | - |
| Specimens | GWG (%) | CL (%) | ω (%) | Liquid Fase | γd (kN/m3) | Curing Period (Days) |
|---|---|---|---|---|---|---|
| BSHS | 30 | 7 | 11 | SHS (3 molal) | 16.0 | 7, 14, 28, 60 and 120 |
| BH2O | Water |
| Material | Dry Unit Weight (kN/m3) | Moisture Content (%) | ||
|---|---|---|---|---|
| 14d Curing | 120d Curing | 14d Curing | 120d Curing | |
| BH2O | 16.43 | 16.31 | 10.58 | 11.05 |
| BSHS | 16.41 | 16.12 | 10.58 | 10.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Secco, M.P.; Mesavilla, D.T.; Floss, M.F.; Cesar Consoli, N.; Miranda, T.; Cristelo, N. Live-Scale Testing of Granular Materials Stabilized with Alkali-Activated Waste Glass and Carbide Lime. Appl. Sci. 2021, 11, 11286. https://doi.org/10.3390/app112311286
Secco MP, Mesavilla DT, Floss MF, Cesar Consoli N, Miranda T, Cristelo N. Live-Scale Testing of Granular Materials Stabilized with Alkali-Activated Waste Glass and Carbide Lime. Applied Sciences. 2021; 11(23):11286. https://doi.org/10.3390/app112311286
Chicago/Turabian StyleSecco, Marina Paula, Débora Thaís Mesavilla, Márcio Felipe Floss, Nilo Cesar Consoli, Tiago Miranda, and Nuno Cristelo. 2021. "Live-Scale Testing of Granular Materials Stabilized with Alkali-Activated Waste Glass and Carbide Lime" Applied Sciences 11, no. 23: 11286. https://doi.org/10.3390/app112311286
APA StyleSecco, M. P., Mesavilla, D. T., Floss, M. F., Cesar Consoli, N., Miranda, T., & Cristelo, N. (2021). Live-Scale Testing of Granular Materials Stabilized with Alkali-Activated Waste Glass and Carbide Lime. Applied Sciences, 11(23), 11286. https://doi.org/10.3390/app112311286







