Microalgae: Potential for Bioeconomy in Food Systems
Abstract
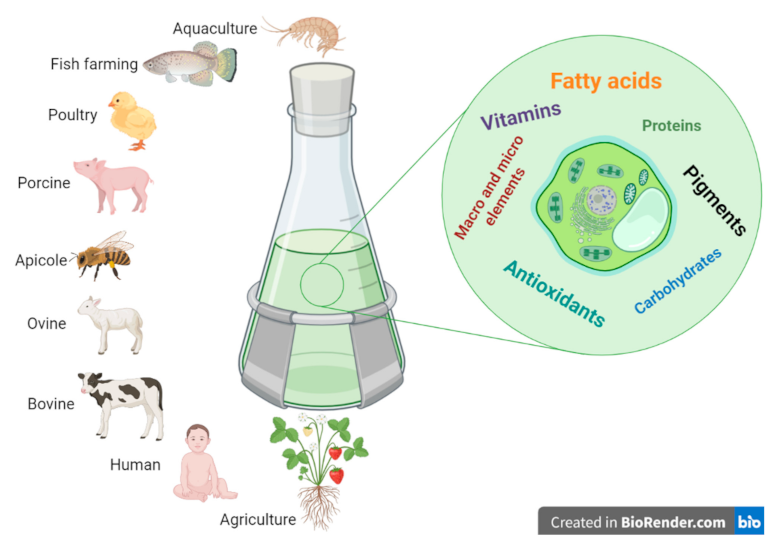
1. Introduction
2. Biodiversity
3. Nutritional Composition
3.1. Proteins
3.2. Carbohydrates
3.3. Lipids and Fatty Acids
3.4. Vitamins, Pigments, and Antioxidants
4. Animal Feed
5. Applications in Human Nutrition
6. Agriculture
7. Microalgae Production in Colombia
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IDEAM. Zonificación y Codificación de Unidades Hidrográficas e Hidrogeológicas de Colombia; Publicación Comité de Comunicaciones y Publicaciones del IDEAM: Bogotá, Colombia, 2013; pp. 1–47.
- Moreno, M.; Aguirre, R. Estado Del Arte de la Limnología de Lagos de Planos Inundables state of the art of limnology and flood plain lakes (Swamps) in Colombia. Rev. Gestión Ambient. 2009, 12, 85–105. [Google Scholar]
- Invemar. Los Ambientes Marinos y Costeros en Colombia: Año 2005; Serie de Publicaciones Periódicas; Instituto de Investigaciones Marionas y Costera: Santa Marta, Columbia, 2006; Volume 8, pp. 36–43. [Google Scholar]
- Noreña, P.A.; González Muñoz, A.; Mosquera-Rendón, J.; Botero, K.; Cristancho, M.A. Colombia, an unknown genetic diversity in the era of Big Data. BMC Genom. 2018, 19, 859. [Google Scholar] [CrossRef] [PubMed]
- Etter, A.; Andrade, Á.; Saavedra, K.; Amaya, P.; Arévalo, P. Estado de los Ecosistemas Colombianos: Una aplicación de la Metodología de la Lista Roja de Ecosistemas; Pontificia Universidad Javeriana y Conservación Internacional: Bogotá, Colombia, 2017; pp. 1–108. [Google Scholar]
- DANE. Incidencia de la Pobreza Monetaria. Indicadores Relevantes. Available online: https://sitios.dane.gov.co/indicadores-relevantes/ (accessed on 20 July 2021).
- Aguilar, A.; Twardowski, T.; Wohlgemuth, R. Bioeconomy for sustainable development. Biotechnol. J. 2019, 14, e1800638. [Google Scholar] [CrossRef] [PubMed]
- Issa, I.; Delbrück, S.; Hamm, U. Bioeconomy from experts’ perspectives—Results of a global expert survey. PLoS ONE 2019, 14, e0215917. [Google Scholar] [CrossRef] [PubMed]
- International Advisory Council on Global Bioeconomy. Global Bioeconomy Policy Report (IV): A Decade of Bioeconomy Policy Development around the World; International Advisory Council on Global Bioeconomy: Berlin, Germany, 2020; Volume 4, Available online: https://gbs2020.net/about/international-advisory-council/ (accessed on 22 July 2021).
- CONPES. CONPES-3934-Política de Crecimiento Verde. Dep. Nac. Planeac. 2018, 1, 1–44. [Google Scholar]
- Singh, J.; Saxena, R.C. An introduction to microalgae. In Handbook of Marine Microalgae; Elsevier: Amsterdam, The Netherlands, 2015; pp. 11–24. [Google Scholar]
- Gómez-Luna, L. Microalgas: Aspectos ecológicos y biotecnológicos. Rev. Cuba. Quim. 2007, 19, 3–20. [Google Scholar]
- Metting, F.B. Biodiversity and application of microalgae. J. Ind. Microbiol. Biotechnol. 1996, 17, 477–489. [Google Scholar] [CrossRef]
- Henry, E.C. Handbook of microalgal culture: Biotechnology and applied phycology. J. Phycol. 2004, 40, 1001–1002. [Google Scholar] [CrossRef]
- Núñez-Avellaneda, M. Microalgas acuáticas: La otra escala de la biodiversidad en la Amazonía colombiana. Inst. Amaz. Investig. Cient. SINCHI 2008, 1, 221. [Google Scholar]
- Norton, T.A.; Melkonian, M.; Andersen, R.A. Algal biodiversity. Phycologia 1996, 35, 308–326. [Google Scholar] [CrossRef]
- Kusmayadi, A.; Leong, Y.K.; Yen, H.W.; Huang, C.Y.; Chang, J.S. Microalgae as sustainable food and feed sources for animals and humans—Biotechnological and environmental aspects. Chemosphere 2021, 271, 129800. [Google Scholar] [CrossRef]
- Alvarez, A.L.; Weyers, S.L.; Goemann, H.M.; Peyton, B.M.; Gardner, R.D. Microalgae, soil and plants: A critical review of microalgae as renewable resources for agriculture. Algal Res. 2021, 54, 102200. [Google Scholar] [CrossRef]
- Sandesh Suresh, K.; Suresh, P.V.; Kudre, T.G. Prospective ecofuel feedstocks for sustainable production. In Advances in Eco-Fuels for a Sustainable Environment; Elsevier: London, UK, 2019; pp. 89–117. [Google Scholar]
- Broady, P.A. Diversity, distribution and dispersal of Antarctic terrestrial algae. Biodivers. Conserv. 1996, 5, 1307–1335. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Pandey, A.; Sukumaran, R.K.; Sahoo, D. Bioremediation by microalgae: Current and emerging trends for effluents treatments for value addition of waste streams. In Energy, Environment, and Sustainability, 1st ed.; Springer: Singapore, 2018; pp. 355–375. ISBN 978-981-10-7434-9. [Google Scholar]
- Heimann, K.; Huerlimann, R. Microalgal Classification: Major Classes and Genera of Commercial Microalgal Species. In Handbook of Marine Microalgae; Elsevier: London, UK, 2015; pp. 25–41. [Google Scholar]
- Pagels, F.; Salvaterra, D.; Amaro, H.M.; Guedes, A.C. Pigments from microalgae. In Handbook of Microalgae-Based Processes and Products; Elsevier: London, UK, 2020; pp. 465–492. [Google Scholar]
- Matsunaga, T.; Takeyama, H.; Miyashita, H.; Yokouchi, H. Marine microalgae. Adv. Biochem. Eng. Biotechnol. 2005, 96, 165–188. [Google Scholar] [PubMed]
- Arbeláez, M.N.; Mancera-Pineda, J.E.; Reguera, B. Structural variation of potentially toxic epiphytic dinoflagellates on thalassia testudinum from two coastal systems of Colombian Caribbean. Harmful Algae 2020, 92, 101738. [Google Scholar] [CrossRef]
- Morales-Parrado, J.; García-Alzate, C.A. Estructura Trófica de la Ictiofauna de los arroyos del Corral de San Luis, Cuenca del Bajo Magdalena, Colombia. Rev. Biol. Trop. 2016, 64, 715–732. [Google Scholar] [CrossRef]
- Jiménez, D.J.; Andreote, F.D.; Chaves, D.; Montaña, J.S.; Osorio-Forero, C.; Junca, H.; Zambrano, M.M.; Baena, S. Structural and functional insights from the metagenome of an acidic hot spring microbial planktonic community in the Colombian Andes. PLoS ONE 2012, 7, e52069. [Google Scholar] [CrossRef]
- Bohorquez, L.C.; Delgado-Serrano, L.; López, G.; Osorio-Forero, C.; Klepac-Ceraj, V.; Kolter, R.; Junca, H.; Baena, S.; Zambrano, M.M. In-depth characterization via complementing culture-independent approaches of the microbial community in an acidic hot spring of the Colombian Andes. Microb. Ecol. 2012, 63, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Mujtaba, G.; Memon, S.A.; Lee, K.; Rashid, N. Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renew. Sustain. Energy Rev. 2018, 92, 394–404. [Google Scholar] [CrossRef]
- Fernández, F.G.A.; Reis, A.; Wijffels, R.H.; Barbosa, M.; Verdelho, V.; Llamas, B. The role of microalgae in the bioeconomy. New Biotechnol. 2021, 61, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Yaakob, M.A.; Mohamed, R.M.S.R.; Al-Gheethi, A.; Aswathnarayana Gokare, R.; Ambati, R.R. Influence of nitrogen and phosphorus on microalgal growth, biomass, lipid, and fatty acid production: An overview. Cells 2021, 10, 393. [Google Scholar] [CrossRef] [PubMed]
- Christaki, E.; Florou-Paneri, P.; Bonos, E. Microalgae: A novel ingredient in nutrition. Int. J. Food Sci. Nutr. 2011, 62, 794–799. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.T.; Show, P.L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Chacón-Lee, T.L.; González-Mariño, G.E. Microalgae for ‘healthy’ foods-possibilities and challenges. Compr. Rev. Food Sci. Food Saf. 2010, 9, 655–675. [Google Scholar] [CrossRef]
- Delattre, C.; Pierre, G.; Laroche, C.; Michaud, P. Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotechnol. Adv. 2016, 34, 1159–1179. [Google Scholar] [CrossRef] [PubMed]
- Gaignard, C.; Laroche, C.; Pierre, G.; Dubessay, P.; Delattre, C.; Gardarin, C.; Gourvil, P.; Probert, I.; Dubuffet, A.; Michaud, P. Screening of marine microalgae: Investigation of new exopolysaccharide producers. Algal Res. 2019, 44, 101711. [Google Scholar] [CrossRef]
- Singh, S.; Kant, C.; Yadav, R.K.; Reddy, Y.P.; Abraham, G. Cyanobacterial Exopolysaccharides: Composition, Biosynthesis, and Biotechnological Applications in Cyanobacteria: From Basic Science to Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 347–358. [Google Scholar]
- Demura, M.; Ioki, M.; Kawachi, M.; Nakajima, N.; Watanabe, M.M. Desiccation tolerance of Botryococcus braunii (Trebouxiophyceae, Chlorophyta) and extreme temperature tolerance of dehydrated cells. J. Appl. Phycol. 2014, 26, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, F.; Liu, N.; Ge, F.; Xiao, H.; Yang, Y. Role of extracellular polymeric substances from Chlorella vulgaris in the removal of ammonium and orthophosphate under the stress of cadmium. Bioresour. Technol. 2015, 190, 299–306. [Google Scholar] [CrossRef]
- Pierre, G.; Delattre, C.; Dubessay, P.; Jubeau, S.; Vialleix, C.; Cadoret, J.P.; Probert, I.; Michaud, P. What is in store for EPS microalgae in the next decade? Molecules 2019, 24, 4296. [Google Scholar] [CrossRef]
- Kumar, D.; Kaštánek, P.; Adhikary, S.P. Exopolysaccharides from cyanobacteria and microalgae and their commercial application. Curr. Sci. 2018, 115, 234–241. [Google Scholar] [CrossRef]
- Zienkiewicz, K.; Du, Z.Y.; Ma, W.; Vollheyde, K.; Benning, C. Stress-induced neutral lipid biosynthesis in microalgae—Molecular, cellular and physiological insights. Biochim. Biophys. Acta 2016, 1861, 1269–1281. [Google Scholar] [CrossRef] [PubMed]
- Ananthi, V.; Raja, R.; Carvalho, I.S.; Brindhadevi, K.; Pugazhendhi, A.; Arun, A. A realistic scenario on microalgae based biodiesel production: Third generation biofuel. Fuel 2021, 284, 118965. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, K. Fatty acids of microalgae: Diversity and applications. Rev. Environ. Sci. Bio Technol. 2021, 20, 515–547. [Google Scholar] [CrossRef]
- Adarme-Vega, T.C.; Lim, D.K.Y.; Timmins, M.; Vernen, F.; Li, Y.; Schenk, P.M. Microalgal biofactories: A promising approach towards sustainable omega-3 fatty acid production. Microb. Cell Factories 2012, 11, 96. [Google Scholar] [CrossRef]
- Ramesh Kumar, B.; Deviram, G.; Mathimani, T.; Duc, P.A.; Pugazhendhi, A. Microalgae as rich source of polyunsaturated fatty acids. Biocatal. Agric. Biotechnol. 2019, 17, 583–588. [Google Scholar] [CrossRef]
- Freitas, H.R. Chlorella vulgaris as a source of essential fatty acids and micronutrients: A brief commentary. Open Plant. Sci. J. 2017, 10, 92–99. [Google Scholar] [CrossRef][Green Version]
- Silva, S.C.; Ferreira, I.C.F.R.; Dias, M.M.; Barreiro, M.F. Microalgae-derived pigments: A 10-year bibliometric review and industry and market trend analysis. Molecules 2020, 25, 3406. [Google Scholar] [CrossRef]
- Mobin, S.M.A.; Chowdhury, H.; Alam, F. Commercially important bioproducts from microalgae and their current applications-A review. Energy Procedia 2019, 160, 752–760. [Google Scholar] [CrossRef]
- Kaur, P. Microalgae as nutraceutical for achieving sustainable food solution in future. In Environmental and Microbial Biotechnology; Springer: Singapore, 2020; pp. 91–125. [Google Scholar]
- Camacho, F.; Macedo, A.; Malcata, F. Potential industrial applications and commercialization of microalgae in the functional food and feed industries: A short review. Mar. Drugs 2019, 17, 312. [Google Scholar] [CrossRef]
- Yaakob, Z.; Ali, E.; Zainal, A.; Mohamad, M.; Takriff, M.S. An overview: Biomolecules from microalgae for animal feed and aquaculture. J. Biol. Res. 2014, 21, 6. [Google Scholar] [CrossRef]
- Ricigliano, V.A. Microalgae as a promising and sustainable nutrition source for managed honey bees. Arch. Insect Biochem. Physiol. 2020, 104, e21658. [Google Scholar] [CrossRef]
- Gatrell, S.; Lum, K.; Kim, J.; Lei, X.G. Nonruminant nutrition symposium: Potential of defatted microalgae from the biofuel industry as an ingredient to replace corn and soybean meal in swine and poultry diets. J. Anim. Sci. 2014, 92, 1306–1314. [Google Scholar] [CrossRef]
- Zhou, W.; Hu, B.; Li, Y.; Min, M.; Mohr, M.; Du, Z.; Chen, P.; Ruan, R. Mass cultivation of microalgae on animal wastewater: A sequential two-stage cultivation process for energy crop and Omega-3-rich animal feed production. Appl. Biochem. Biotechnol. 2012, 168, 348–363. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Grünewald, C.; Ignacio Gayo-Peláez, J.; Ndovela, V.; Wood, E.; Vijay Kapoore, R.; Anne Llewellyn, C. Towards a circular economy: A novel microalgal two-step growth approach to treat excess nutrients from digestate and to produce biomass for animal feed. Bioresour. Technol. 2021, 320, 124349. [Google Scholar] [CrossRef]
- Lee, A.V.; You, L.; Oh, S.Y.; Li, Z.; Fisher-Heffernan, R.E.; Regnault, T.R.H.; de Lange, C.F.M.; Huber, L.; Karrow, N.A. Microalgae supplementation to late gestation sows and its effects on the health status of weaned piglets fed diets containing high or low-quality protein sources. Vet. Immunol. Immunopathol. 2019, 218, 109937. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Lee, A.V.; Oh, S.Y.; Fisher-Heffernan, R.E.; Edwards, M.; de Lange, K.; Karrow, N.A. Effect of lipopolysaccharide-induced immune stimulation and maternal fish oil and microalgae supplementation during late pregnancy on nursery pig hypothalamic–pituitary–adrenal function1. J. Anim. Sci. 2019, 97, 2940–2951. [Google Scholar] [CrossRef] [PubMed]
- de Tonnac, A.; Guillevic, M.; Mourot, J. Fatty acid composition of several muscles and adipose tissues of pigs fed n-3 PUFA rich diets. Meat Sci. 2018, 140, 1–8. [Google Scholar] [CrossRef]
- Moran, C.A.; Morlacchini, M.; Keegan, J.D.; Delles, R.; Fusconi, G. Effects of a DHA-rich unextracted microalgae as a dietary supplement on performance, carcass traits and meat fatty acid profile in growing-finishing pigs. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1026–1038. [Google Scholar] [CrossRef]
- Stokes, R.S.; Van Emon, M.L.; Loy, D.D.; Hansen, S.L. Assessment of algae meal as a ruminant feedstuff: Nutrient digestibility in sheep as a model species. J. Anim. Sci. 2015, 93, 5386–5394. [Google Scholar] [CrossRef]
- Holman, B.W.B.; Malau-Aduli, A.E.O. Spirulina as a livestock supplement and animal feed. J. Anim. Physiol. Anim. Nutr. 2013, 97, 615–623. [Google Scholar] [CrossRef]
- Till, B.E.; Huntington, J.A.; Posri, W.; Early, R.; Taylor-Pickard, J.; Sinclair, L.A. Influence of rate of inclusion of microalgae on the sensory characteristics and fatty acid composition of cheese and performance of dairy cows. J. Dairy Sci. 2019, 102, 10934–10946. [Google Scholar] [CrossRef] [PubMed]
- Till, B.E.; Huntington, J.A.; Kliem, K.E.; Taylor-Pickard, J.; Sinclair, L.A. Long term dietary supplementation with microalgae increases plasma docosahexaenoic acid in milk and plasma but does not affect plasma 13,14-dihydro-15-keto PGF 2α concentration in dairy cows. J. Dairy Res. 2020, 87, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Flaga, J.; Korytkowski, Ł.; Górka, P.; Kowalski, Z.M. The effect of docosahexaenoic acid-rich algae supplementation in milk replacer on performance and selected immune system functions in calves. J. Dairy Sci. 2019, 102, 8862–8873. [Google Scholar] [CrossRef] [PubMed]
- Lamminen, M.; Halmemies-Beauchet-Filleau, A.; Kokkonen, T.; Vanhatalo, A.; Jaakkola, S. The effect of partial substitution of rapeseed meal and faba beans by Spirulina platensis microalgae on milk production, nitrogen utilization, and amino acid metabolism of lactating dairy cows. J. Dairy Sci. 2019, 102, 7102–7117. [Google Scholar] [CrossRef]
- Stokes, R.S.; Loy, D.D.; Hansen, S.L. Effects of increased inclusion of algae meal on finishing steer performance and carcass characteristics1. J. Anim. Sci. 2016, 94, 687–696. [Google Scholar] [CrossRef]
- Holman, B. Growth and body conformation responses of genetically divergent Australian sheep to Spirulina (Arthrospira platensis) supplementation. Am. J. Exp. Agric. 2012, 2, 160–173. [Google Scholar] [CrossRef]
- Fan, Y.; Ren, C.; Meng, F.; Deng, K.; Zhang, G.; Wang, F. Effects of algae supplementation in high-energy dietary on fatty acid composition and the expression of genes involved in lipid metabolism in Hu sheep managed under intensive finishing system. Meat Sci. 2019, 157, 107872. [Google Scholar] [CrossRef]
- Ciliberti, M.G.; Albenzio, M.; Francavilla, M.; Neglia, G.; Esposito, L.; Caroprese, M. Extracts from microalga Chlorella sorokiniana exert an anti-proliferative effect and modulate cytokines in sheep peripheral blood mononuclear cells. Animals 2019, 9, 45. [Google Scholar] [CrossRef]
- Caroprese, M.; Albenzio, M.; Ciliberti, M.G.; Francavilla, M.; Sevi, A. A mixture of phytosterols from Dunaliella tertiolecta affects proliferation of peripheral blood mononuclear cells and cytokine production in sheep. Vet. Immunol. Immunopathol. 2012, 150, 27–35. [Google Scholar] [CrossRef]
- Tsiplakou, E.; Abdullah, M.A.M.; Mavrommatis, A.; Chatzikonstantinou, M.; Skliros, D.; Sotirakoglou, K.; Flemetakis, E.; Labrou, N.E.; Zervas, G. The effect of dietary Chlorella vulgaris inclusion on goat’s milk chemical composition, fatty acids profile and enzymes activities related to oxidation. J. Anim. Physiol. Anim. Nutr. (Berlin) 2018, 102, 142–151. [Google Scholar] [CrossRef]
- El-Bahr, S.; Shousha, S.; Shehab, A.; Khattab, W.; Ahmed-Farid, O.; Sabike, I.; El-Garhy, O.; Albokhadaim, I.; Albosadah, K. Effect of dietary microalgae on growth performance, profiles of amino and fatty acids, antioxidant status, and meat quality of broiler chickens. Animals 2020, 10, 761. [Google Scholar] [CrossRef] [PubMed]
- Tolba, S.A.; Sun, T.; Magnuson, A.D.; Liu, G.C.; Abdel-Razik, W.M.; El-Gamal, M.F.; Lei, X.G. Supplemental docosahexaenoic-acid-enriched microalgae affected fatty acid and metabolic profiles and related gene expression in several tissues of broiler chicks. J. Agric. Food Chem. 2019, 67, 6497–6507. [Google Scholar] [CrossRef]
- Tavernari, F.C.; Roza, L.F.; Surek, D.; Sordi, C.; Silva, M.L.B.D.; Albino, L.F.T.; Migliorini, M.J.; Paiano, D.; Boiago, M.M. Apparent metabolisable energy and amino acid digestibility of microalgae Spirulina platensis as an ingredient in broiler chicken diets. Br. Poult. Sci. 2018, 59, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.A.; Parker, N.B.; Löhr, C.V.; Cherian, G. Docosahexaenoic acid (22:6 n-3)-rich microalgae along with methionine supplementation in broiler chickens: Effects on production performance, breast muscle quality attributes, lipid profile, and incidence of white striping and myopathy. Poult. Sci. 2021, 100, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Ginzberg, A.; Cohen, M.; Sod-Moriah, U.A.; Shany, S.; Rosenshtrauch, A.; Arad, S. Chickens fed with biomass of the red microalga Porphyridium sp. have reduced blood cholesterol level and modified fatty acid composition in egg yolk. J. Appl. Phycol. 2000, 12, 325–330. [Google Scholar] [CrossRef]
- Feng, J.; Long, S.; Zhang, H.J.; Wu, S.G.; Qi, G.H.; Wang, J. Comparative effects of dietary microalgae oil and fish oil on fatty acid composition and sensory quality of table eggs. Poult. Sci. 2020, 99, 1734–1743. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Sheiha, A.M.; Taha, A.E.; Swelum, A.A.; Alarifi, S.; Alkahtani, S.; Ali, D.; AlBasher, G.; Almeer, R.; Falodah, F.; et al. Impacts of enriching growing rabbit diets with Chlorella vulgaris microalgae on growth, blood variables, carcass traits, immunological and antioxidant indices. Animals 2019, 9, 788. [Google Scholar] [CrossRef]
- Ribeiro, D.M.; Bandarrinha, J.; Nanni, P.; Alves, S.P.; Martins, C.F.; Bessa, R.J.B.; Falcão-E-Cunha, L.; Almeida, A.M. The effect of Nannochloropsis oceanica feed inclusion on rabbit muscle proteome. J. Proteom. 2020, 222, 103783. [Google Scholar] [CrossRef]
- Sumesh, K.; Roshan, D. Fish Farming Market by Environment (Marine Water, Fresh Water, and Brackish Water), and Fish Type (Pompano, Snappers, Groupers, Salmon, Milkfish, Tuna, Tilapia, Catfish, Sea Bass, & Others): Global Opportunity Analysis and Industry Forecast 2021–2027. Available online: https://www.giiresearch.com/report/amr980757-fish-farming-market-by-environment-marine-water.html (accessed on 27 July 2021).
- Sarker, P.K.; Kapuscinski, A.R.; McKuin, B.; Fitzgerald, D.S.; Nash, H.M.; Greenwood, C. Microalgae-blend tilapia feed eliminates fishmeal and fish oil, improves growth, and is cost viable. Sci. Rep. 2020, 10, 19328. [Google Scholar] [CrossRef]
- Hernández-Camacho, J.; Sánchez Páez, H. La diversidad biológica de Iberoamerica. In Biomas Terrestres de Colombia, 1st ed.; Instituto de Ecología: Ciudad de México, México, 1992; Volume 1, pp. 153–174. [Google Scholar]
- Kiron, V.; Phromkunthong, W.; Huntley, M.; Archibald, I.; De Scheemaker, G. Marine microalgae from biorefinery as a potential feed protein source for Atlantic salmon, common carp and whiteleg shrimp. Aquac. Nutr. 2012, 18, 1–11. [Google Scholar] [CrossRef]
- Sheikhzadeh, N.; Tayefi-Nasrabadi, H.; Oushani, A.K.; Enferadi, M.H. Effects of Haematococcus pluvialis supplementation on antioxidant system and metabolism in rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2012, 38, 413–419. [Google Scholar] [CrossRef]
- Stoneham, T.R.; Kuhn, D.D.; Taylor, D.P.; Neilson, A.P.; Smith, S.A.; Gatlin, D.M.; Chu, H.S.S.; O’Keefe, S.F. Production of omega-3 enriched tilapia through the dietary use of algae meal or fish oil: Improved nutrient value of fillet and offal. PLoS ONE 2018, 13, e0194241. [Google Scholar] [CrossRef]
- Svirčev, Z.; Lalić, D.; Bojadžija Savić, G.; Tokodi, N.; Drobac Backović, D.; Chen, L.; Meriluoto, J.; Codd, G.A. Global geographical and historical overview of cyanotoxin distribution and cyanobacterial poisonings. Arch. Toxicol. 2019, 93, 2429–2481. [Google Scholar] [CrossRef] [PubMed]
- Reguera, B.; Blanco, J. Dinophysis toxins: Distribution, fate in shellfish and impacts. Toxins 2019, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- Gross, R.; Schoeneberger, H.; Gross, U. The nutritional quality of Scenedesmus acutus in a semi-industrial plant in Peru. J. Environ. Pathol. Toxicol. Oncol. 1986, 6, 47–57. [Google Scholar] [PubMed]
- Lupatini, A.L.; Colla, L.M.; Canan, C.; Colla, E. Potential application of microalga Spirulina platensis as a protein source. J. Sci. Food Agric. 2017, 97, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Galarza, V.O. Carbohydrates and proteins in microalgaes: Potential functional foods. Braz. J. Food Technol. 2019, 22, 2019043. [Google Scholar] [CrossRef]
- Panahi, Y.; Darvishi, B.; Jowzi, N.; Beiraghdar, F.; Sahebkar, A. Chlorella vulgaris: A multifunctional dietary supplement with diverse medicinal properties. Curr. Pharm. Des. 2016, 22, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-producing green microalga Haematococcus pluvialis: From single cell to high value commercial products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef]
- Hu, C.C.; Lin, J.T.; Lu, F.J.; Chou, F.P.; Yang, D.J. Determination of carotenoids in Dunaliella salina cultivated in Taiwan and antioxidant capacity of the algal carotenoid extract. Food Chem. 2008, 109, 439–446. [Google Scholar] [CrossRef]
- Sikiru, A.B.; Arangasamy, A.; Alemede, I.C.; Guvvala, P.R.; Egena, S.S.A.; Ippala, J.R.; Bhatta, R. Chlorella vulgaris supplementation effects on performances, oxidative stress and antioxidant genes expression in liver and ovaries of New Zealand White rabbits. Heliyon 2019, 5, e02470. [Google Scholar] [CrossRef]
- Madhumathi, M. Antioxidant status of Penaeus monodon fed with Dunaliella salina supplemented diet and resistance against WSSV. Int. J. Eng. Sci. Technol. 2011, 3, 1–14. [Google Scholar]
- Sanhueza, J.; Nieto, S.; Valenzuela, A. Ácido docosahexaenoico (DHA), desarrollo cerebral, memoria y aprendizaje: La importancia de la suplementación Perinatal. Rev. Chil. Nutr. 2004, 31, 84–92. [Google Scholar] [CrossRef]
- Qiu, C.; He, Y.; Huang, Z.; Qiu, W.; Huang, J.; Wang, M.; Chen, B. Biosafety evaluation of Nannochloropsis oculata and Schizochytrium sp. oils as novel human milk fat substitutes. Food Funct. 2021, 12, 2972–2984. [Google Scholar] [CrossRef]
- Zhang, L.S.; Chu, M.Y.; Zong, M.H.; Yang, J.G.; Lou, W.Y. Facile and green production of human milk fat substitute through Rhodococcus opacus fermentation. J. Agric. Food Chem. 2020, 68, 9368–9376. [Google Scholar] [CrossRef] [PubMed]
- Boukid, F.; Rosell, C.M.; Rosene, S.; Bover-Cid, S.; Castellari, M. Non-animal proteins as cutting-edge ingredients to reformulate animal-free foodstuffs: Present status and future perspectives. Crit. Rev. Food Sci. Nutr. 2021, 27, 1–31. [Google Scholar] [CrossRef]
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017, 8, 2672–2685. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, T.; Fernández-Sevilla, J.M.; González-López, C.; Acién-Fernández, F.G. Spirulina for the food and functional food industries. Food Res. Int. 2020, 137, 109356. [Google Scholar] [CrossRef]
- Uribe-Wandurraga, Z.N.; Igual, M.; García-Segovia, P.; Martínez-Monzó, J. In vitro bioaccessibility of minerals from microalgae-enriched cookies. Food Funct. 2020, 11, 2186–2194. [Google Scholar] [CrossRef]
- Javier, H.-B.; Vanegas, R.E. Potencial Biotecnológico de Microalgas en Zonas Aridas, 1st ed.; Servicio Nacional de Aprendizaje: Bogotá, Colombia, 2018. [Google Scholar]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for high-value products towards human health and nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef] [PubMed]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.F. Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef]
- Schreiber, C.; Schiedung, H.; Harrison, L.; Briese, C.; Ackermann, B.; Kant, J.; Schrey, S.D.; Hofmann, D.; Singh, D.; Ebenhöh, O.; et al. Evaluating potential of green alga Chlorella vulgaris to accumulate phosphorus and to fertilize nutrient-poor soil substrates for crop plants. J. Appl. Phycol. 2018, 30, 2827–2836. [Google Scholar] [CrossRef]
- Karki, M.; Gibard, C.; Bhowmik, S.; Krishnamurthy, R. Nitrogenous derivatives of phosphorus and the origins of life: Plausible prebiotic phosphorylating agents in water. Life 2017, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Toner, J.D.; Catling, D.C. A carbonate-rich lake solution to the phosphate problem of the origin of life. Proc. Natl. Acad. Sci. USA 2020, 117, 883–888. [Google Scholar] [CrossRef]
- Pasek, M.A. Rethinking early Earth phosphorus geochemistry. Proc. Natl. Acad. Sci. USA 2008, 105, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Mau, L.; Kant, J.; Walker, R.; Kuchendorf, C.M.; Schrey, S.D.; Roessner, U.; Watt, M. Wheat can access phosphorus from algal biomass as quickly and continuously as from mineral fertilizer. Front. Plant Sci. 2021, 12, 631314. [Google Scholar] [CrossRef]
- Kholssi, R.; Marks, E.A.N.; Miñón, J.; Montero, O.; Debdoubi, A.; Rad, C. Biofertilizing effect of Chlorella sorokiniana suspensions on wheat growth. J. Plant Growth Regul. 2019, 38, 644–649. [Google Scholar] [CrossRef]
- Araujo Vidal, D.R.; Hernández Benítez, R.H.; Vanegas Guerrero, J. Efecto de la Inoculación de Cianobacterias en Cultivos de Interés Comercial en Zonas Semiáridas de la Guajira—Colombia. Rev. Colomb. Investig. Agroindust. 2018, 5, 20–31. [Google Scholar] [CrossRef]
- Carrera, S.; Velasco, L.A.; Barreto-Hernández, A. Potential of benthic microalgae of the Caribbean sea as food in mariculture. Rev. Biol. Mar. Oceanogr. 2018, 53, 321–333. [Google Scholar] [CrossRef]
- Grzesik, M.; Romanowska-Duda, Z.; Kalaji, H.M. Effectiveness of cyanobacteria and green algae in enhancing the photosynthetic performance and growth of willow (Salix viminalis L.) plants under limited synthetic fertilizers application. Photosynthetica 2017, 55, 510–521. [Google Scholar] [CrossRef]
- Nayak, M.; Swain, D.K.; Sen, R. Strategic valorization of de-oiled microalgal biomass waste as biofertilizer for sustainable and improved agriculture of rice (Oryza sativa L.) crop. Sci. Total Environ. 2019, 682, 475–484. [Google Scholar] [CrossRef]
- Rocca, S.; Agostini, A.; Giuntoli, J.; Marelli, L. Biofuels from Algae: Technology Options, Energy Balance and GHG Emissions; JRC98760; Publications Office of the European Union: Luxembourg, 2015. [Google Scholar]
- Vacca Jimeno, V.A.; Angulo Mercado, E.R.; Puentes Ballesteros, D.M.; Torres Yépez, J.G.; Plaza Vega, M.E. Uso de la microalga Chlorella sp. viva en suspensión en la decoloración del agua residual de una empresa Textil/Using the microalgae Chlorella sp. live suspended in decoloration wastewater from a textile factory. Prospectiva 2017, 15, 93–99. [Google Scholar] [CrossRef]
- Romero-Morales, M.A.; Ortiz-Villota, M.T.; Meza-Rodríguez, L.D. La biorremediación con microalgas (Spirulina máxima, Spirulina platensis y Chlorella vulgaris) como alternativa para tratar la eutrofización de la laguna de Ubaque, Colombia. Rev. Investig. Desarro Innov. 2018, 9, 163–176. [Google Scholar]
- Gómez Santos, J.A.; Rodríguez González, L.G. Obtención de biomasa de microalgas en aguas residuales para la producción de biocombustibles. Renov. Rev. Estud. Interdiscip. Cienc. Soc. Tecnol. Innovació 2019, 3, 21–36. [Google Scholar]
- Mora-Salguero, D.; Vives Florez, M.J.; Husserl Orjuela, J.; Fernández-Niño, M.; González Barrios, A.F. Evaluation of the phenol degradation capacity of microalgae-bacteria consortia from the bay of Cartagena, Colombia. TecnoLogicas 2019, 22, 149–158. [Google Scholar] [CrossRef]
- Torres, D.D.; Cáceres Sepúlveda, S.; Roa, A.L.; Suárez Gelvez, J.H.; Urbina Suárez, N.A. Utilización de microalgas de la división Chlorophyta en el tratamiento biológico de drenajes ácidos de Minas de carbón. Rev. Colomb. Biotechnol. 2017, 19, 95–104. [Google Scholar] [CrossRef]
- Muller-Feuga, A. Microalgae for aquaculture: The current global situation and future trends. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 1st ed.; Wiley: New York, NY, USA, 2013; pp. 615–627. [Google Scholar]
- Ramírez, B.D.G.; Valencia, J.U.S.; Arbelaez, A.F.A.; Herrera, J.M.; Rojano, B.A. Oxidative, sensory and fatty acid profile evaluation of a yogurt with docosahexaenoic acid (Dha) extracted from microalgae oil. Rev. Chil. Nutr. 2020, 47, 568–579. [Google Scholar]
- Colorado Gómez, M.A.; Tirado, D.A.M. Economía de recursos naturales a partir de la producción de Spirulina (Arthrospira maxima) en fotobiorreactores, La Guajira, Colombia. Reto 2017, 5, 50–59. [Google Scholar]
- Oviedo-Montiel, H.D.; Herrera-Cruz, E.E.; Hoya-Florez, J.K.; Prieto-Guevara, M.J.; Estrada-Posada, A.L.; Yepes-Blandón, J.A. Crecimiento poblacional de Macrothrix spinosa alimentada con Chlorella sp. Orinoquia 2019, 23, 79–86. [Google Scholar] [CrossRef]
- Torres-Valencia, G.A.; Imués-Figueroa, M.A.; Sanguino-Ortiz, W.R.; Chapman, F.A. Aislamiento de una cepa de rotiferos de agua dulce con potencial como alimento vivo en acuicultura. Rev. Investig. Pecu. 2018, 5, 25–32. [Google Scholar]
- García Salazar, C.A.; Pérez Cardona, Y.A.; Ríos Osorio, L.A.; Múnera Porras, L.M. Efecto de un Consorcio de cianobacterias sobre la obtención de biomasa vegetal de la gulupa (Passiflora edulis f. edulis sims) bajo condiciones de campo en el municipio de Marinilla—Antioquia”. Hechos Microbiológicos 2020, 11, 12–21. [Google Scholar] [CrossRef]
- Ardila, Á.A.M.; López, M.Y.; Vásquez, C.M.E.; González, D.Á.D.; Barajas, S.A.F. Obtaining lipids and carbohydrates from microalgae via design of selective culture media. TecnoLógicas 2017, 20, 83. [Google Scholar]
- Giraldo Calderón, N.D.; Díaz Bayona, K.C.; Atehortúa Garcés, L. Immobilization of the green microalga Botryococcus braunii in polyester wadding: Effect on biomass, fatty acids, and exopolysaccharide production. Biocatal. Agric. Biotechnol. 2018, 14, 80–87. [Google Scholar] [CrossRef]
- Leal Medina, G.I.; Abril Bonett, J.E.; Martínez Gélvez, S.J.; Muñoz Peñaloza, Y.A.; Peñaranda Lizarazo, E.M.; Urbina Suárez, N.A. Producción de Ácidos Grasos Poliinsaturados a partir de Biomasa Microalgal en un Cultivo Heterotrófico. Rev. Ion 2017, 30, 91–103. [Google Scholar] [CrossRef]
- Guarin-Villegas, E.; Remolina-Páez, L.; Bermúdez-Castro, J.; Mogollón-Londoño, S.; Contreras-Ropero, J.; García-Martínez, J. Effect of de Carbon/Nitrogen ratio on the production of microalgae-based carotenoids. Ing. Compet. 2020, 22, 8686. [Google Scholar]
- Niño Castillo, C.M.; Rodríguez Rivera, F.C.; Díaz, L.E.; Lancheros Díaz, A.G. Evaluación de las condiciones de crecimiento celular para la producción de astaxantina a patir de la microalga Haematococcus pluvialis. Nova 2017, 15, 19. [Google Scholar] [CrossRef]
- Miranda, A.M.; Ossa, E.A.; Vargas, G.J.; Sáez, A.A. Efecto de las Bajas Concentraciones de Nitratos y Fosfatos sobre la Acumulación de Astaxantina en Haematococcus pluvialis UTEX 2505. Inf. Tecnol. 2019, 30, 23–32. [Google Scholar] [CrossRef]
| Application | Microalga | Product | Conclusion | Reference |
|---|---|---|---|---|
| Human food | Crypthecodinium cohnii | Yogurt with microalgae oil | Addition of microalgae oil increases DHA in the diet. | [124] |
| Arthrospira maxima | Biomass | Mass production of dry biomass (1 g/L) was accomplished by outdoor scale-up. | [125] | |
| Animal feed | Scenedesmus sp. | Rotifer food | Increased population density was observed after feeding with Scenedesmus sp. | [126] |
| Chlorella sp. | Zooplankton food (Macrothrix spinosa) | M. spinosa fed with Chlorella sp. showed better performance. | [127] | |
| Cylindrotheca closterium, Entomoneis alata, Plagiotropis lepidoptera, Komvophoron crassum, Synechococcus sp., Tetraselmis chuii | Food in marine aquaculture | Benthic microalgae showed potential for use as feed in marine aquaculture. | [113] | |
| Agriculture | Gloeocapsa sp., Oscillatoria amphibia | Biofertilizer for rice, corn, and bean crops | Gloeocapsa sp. increased growth in rice plants by 15.0%. | [112] |
| Consortium of Microcystis aeruginosa, Synechococcus rubescens, Cyanobium gracile | Biofertilizer for gulupa crop | The results were similar to those of a traditional organic treatment. | [128] | |
| Other uses | Chlorella vulgaris | Lipid and carbohydrate production | Increased carbohydrates and lipids levels after addition of acetate, carbonate, and phosphate. | [129] |
| Botryococcus braunii | Fatty acids and exopolysaccharides | Galactose was the main component of exopolysaccharides (71.73%). | [130] | |
| Chlorella sp., Scenedesmus sp. | Polyunsaturated fatty acids | Oleic acid was the major fatty acid present (28.75%). | [131] | |
| Scenedesmus sp. | Carotenoids | C:N ratio affected biomass and carotenoid production. | [132] | |
| Haematococcus pluvialis | Astaxanthin | The highest astaxanthin production was obtained in RM growth medium (8.3 μg/mL). | [133] | |
| H. pluvialis | Astaxanthin | Higher concentrations of astaxanthin were obtained when limiting nitrogen and phosphorus. | [134] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado Sierra, E.; Serrano, M.C.; Manares, A.; Guerra, A.; Aranguren Díaz, Y. Microalgae: Potential for Bioeconomy in Food Systems. Appl. Sci. 2021, 11, 11316. https://doi.org/10.3390/app112311316
Machado Sierra E, Serrano MC, Manares A, Guerra A, Aranguren Díaz Y. Microalgae: Potential for Bioeconomy in Food Systems. Applied Sciences. 2021; 11(23):11316. https://doi.org/10.3390/app112311316
Chicago/Turabian StyleMachado Sierra, Elwi, María C. Serrano, Anderson Manares, Abraham Guerra, and Yani Aranguren Díaz. 2021. "Microalgae: Potential for Bioeconomy in Food Systems" Applied Sciences 11, no. 23: 11316. https://doi.org/10.3390/app112311316
APA StyleMachado Sierra, E., Serrano, M. C., Manares, A., Guerra, A., & Aranguren Díaz, Y. (2021). Microalgae: Potential for Bioeconomy in Food Systems. Applied Sciences, 11(23), 11316. https://doi.org/10.3390/app112311316







