Two-Phase Non-Newtonian Pulsatile Blood Flow Simulations in a Rigid and Flexible Patient-Specific Left Coronary Artery (LCA) Exhibiting Multi-Stenosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Acquisition and Processing
2.2. Material Properties
2.3. Development of 3D Models for Simulation (Computational Fluid Dynamics (CFD) and Fluid–Solid Interaction (FSI))
2.4. Computational Fluid Dynamics (CFD) Model
2.5. Multiphase Blood Flow Model
2.6. Continuity Equation for Multiphase Blood Flow
2.7. Momentum Equation
2.8. Slip and Drift Velocity
2.9. Constitutive Model
3. Artery Deformation Modelling
3.1. Fluid–Structure Interaction (FSI) Modelling
3.2. Wall Shear Stress (WSS)
4. Mesh Independent Study
5. Results and Discussion
5.1. Wall Pressure
5.2. Wall Shear Stress (WSS)
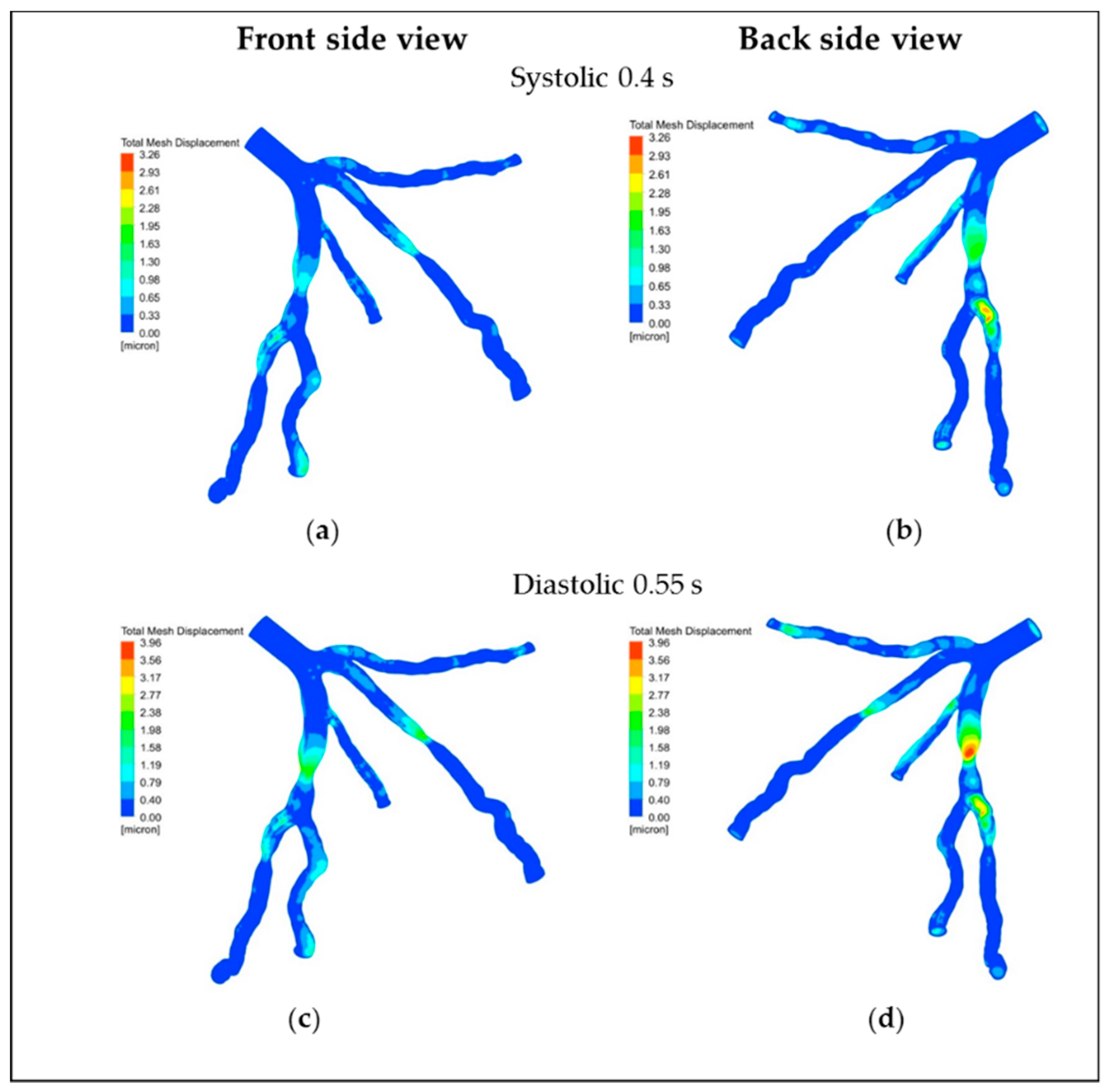
5.3. Displacement
5.4. Velocity Streamlines
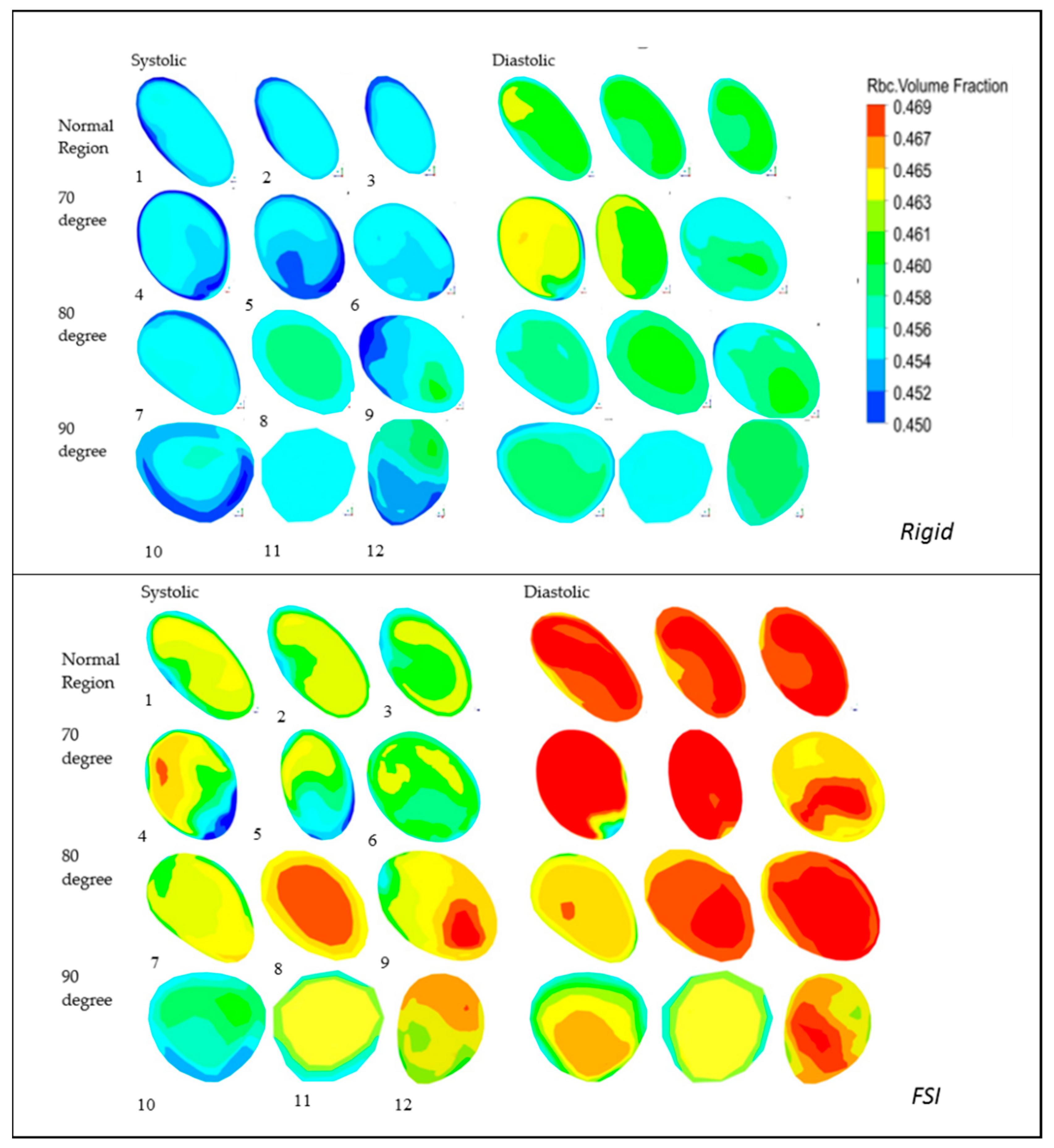
5.5. RBC Volume Fraction (VF)
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R. Heart disease and stroke statistics—2018 update: A report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Robertson, A.-K.L.; Söderberg-Nauclér, C. Inflammation and atherosclerosis. Annu. Rev. Pathol. Mech. Dis. 2006, 1, 297–329. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- McLaren, J.E.; Michael, D.R.; Ashlin, T.G.; Ramji, D.P. Cytokines, macrophage lipid metabolism and foam cells: Implications for cardiovascular disease therapy. Prog. Lipid Res. 2011, 50, 331–347. [Google Scholar] [CrossRef]
- Gerszten, R.E.; Tager, A.M. The monocyte in atherosclerosis—Should I stay or should I go now? N. Engl. J. Med. 2012, 366, 1734–1736. [Google Scholar] [CrossRef] [PubMed]
- Bongo, J.B.; Peng, D.Q. The neuroimmune guidance cue netrin-1: A new therapeutic target in cardiovascular disease. J. Cardiol. 2014, 63, 95–98. [Google Scholar] [CrossRef] [Green Version]
- Vlachopoulos, C.; O′Rourke, M.; Nichols, W.W. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Polanczyk, A.; Podgorski, M.; Wozniak, T.; Stefanczyk, L.; Strzelecki, M. Computational fluid dynamics as an engineering tool for the reconstruction of hemodynamics after carotid artery stenosis operation: A case study. Medicina 2018, 54, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopernik, M.; Tokarczyk, P. Development of multi-phase models of blood flow for medium-sized vessels with stenosis. Acta Bioeng. Biomech. 2019, 21, 63–70. [Google Scholar] [PubMed]
- Mallik, B.B.; Nanda, S.; Das, B.; Saha, D.; Das, D.S.; Paul, K. A non-newtonian fluid model for blood flow using power-law through an atherosclerotic arterial segment having slip velocity. Int. J. Pharm. Chem. Biol. Sci. 2013, 3, 752–760. [Google Scholar]
- Jung, J.; Lyczkowski, R.W.; Panchal, C.B.; Hassanein, A. Multiphase hemodynamic simulation of pulsatile flow in a coronary artery. J. Biomech. 2006, 39, 2064–2073. [Google Scholar] [CrossRef] [PubMed]
- Melka, B.; Nowak, M.; Gracka, M.; Adamczyk, W.; Golda, A.; Nowak, A.; Białecki, R.; Rojczyk, M.; Ostrowski, Z. CFD modeling of blood flow in rigid and elastic vessels-multifluid & multiscale approach. In Proceedings of the XXIII Zjazd Termodynamików, Gliwice, Poland, 10 September 2017. [Google Scholar]
- Joisar, K.; Bhoraniya, R.; Harichandan, A. Numerical analysis of two-phase blood flow in idealized artery with blockage. In Innovative Design, Analysis and Development Practices in Aerospace and Automotive Engineering (I-DAD 2018); Springer: Berlin/Heidelberg, Germany, 2019; pp. 259–267. [Google Scholar]
- Buradi, A.; Mahalingam, A. Effect of stenosis severity on wall shear stress based hemodynamic descriptors using multiphase mixture theory. J. Appl. Fluid Mech. 2018, 11, 1497–1509. [Google Scholar] [CrossRef]
- Wu, W.-T.; Aubry, N.; Antaki, J.F.; Massoudi, M. Simulation of blood flow in a sudden expansion channel and a coronary artery. J. Comput. Appl. Math. 2020, 376, 112856. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lyczkowski, R.W.; Gidaspow, D. Pulsatile flow in a coronary artery using multiphase kinetic theory. J. Biomech. 2009, 42, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Bit, A.; Chattopadhay, H. Acute aneurysm is more critical than acute stenoses in blood vessels: A numerical investigation using stress markers. BioNanoScience 2018, 8, 329–336. [Google Scholar] [CrossRef]
- Wu, X.; von Birgelen, C.; Li, Z.; Zhang, S.; Huang, J.; Liang, F.; Li, Y.; Wijns, W.; Tu, S. Assessment of superficial coronary vessel wall deformation and stress: Validation of in silico models and human coronary arteries in vivo. Int. J. Cardiovasc. Imaging 2018, 34, 849–861. [Google Scholar] [CrossRef]
- Malek, A.M.; Alper, S.L.; Izumo, S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 1999, 282, 2035–2042. [Google Scholar] [CrossRef]
- Athani, A.; Ghazali, N.; Badruddin, I.A.; Kamangar, S.; Anqi, A.E.; Algahtani, A. Investigation of two-way fluid-structure interaction of blood flow in a patient-specific left coronary artery. Bio-Med. Mater. Eng. 2021, 1–18. [Google Scholar] [CrossRef]
- Cameron, J.N.; Mehta, O.H.; Michail, M.; Chan, J.; Nicholls, S.J.; Bennett, M.R.; Brown, A.J. Exploring the relationship between biomechanical stresses and coronary atherosclerosis. Atherosclerosis 2020, 302, 43–51. [Google Scholar] [CrossRef]
- Jahangiri, M.; Saghafian, M.; Sadeghi, M.R. Numerical simulation of hemodynamic parameters of turbulent and pulsatile blood flow in flexible artery with single and double stenoses. J. Mech. Sci. Technol. 2015, 29, 3549–3560. [Google Scholar] [CrossRef]
- Ta, H.T.; Truong, N.P.; Whittaker, A.K.; Davis, T.P.; Peter, K. The effects of particle size, shape, density and flow characteristics on particle margination to vascular walls in cardiovascular diseases. Expert Opin. Drug Deliv. 2018, 15, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Eslami, P.; Tran, J.; Jin, Z.; Karady, J.; Sotoodeh, R.; Lu, M.T.; Hoffmann, U.; Marsden, A. Effect of wall elasticity on hemodynamics and wall shear stress in patient-specific simulations in the coronary arteries. J. Biomech. Eng. 2020, 142. [Google Scholar] [CrossRef] [PubMed]
- Gholipour, A.; Ghayesh, M.H.; Zander, A.C.; Psaltis, P.J. In Vivo based biomechanics of right and left coronary arteries. Int. J. Eng. Sci. 2020, 154, 103281. [Google Scholar] [CrossRef]
- Malvè, M.; García, A.; Ohayon, J.; Martínez, M. Unsteady blood flow and mass transfer of a human left coronary artery bifurcation: FSI vs. CFD. Int. Commun. Heat Mass Transf. 2012, 39, 745–751. [Google Scholar] [CrossRef]
- Chaichana, T.; Sun, Z.; Jewkes, J. Computation of hemodynamics in the left coronary artery with variable angulations. J. Biomech. 2011, 44, 1869–1878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamangar, S.; Badruddin, I.A.; Ahamad, N.A.; Govindaraju, K.; Nik-Ghazali, N.; Ahmed, N.; Badarudin, A.; Khan, T. The influence of geometrical shapes of stenosis on the blood flow in stenosed artery. Sains Malays. 2017, 46, 1923–1933. [Google Scholar] [CrossRef] [Green Version]
- Sakellarios, A.I.; Tsompou, P.; Kigka, V.; Siogkas, P.; Kyriakidis, S.; Tachos, N.; Karanasiou, G.; Scholte, A.; Clemente, A.; Neglia, D. Non-invasive prediction of site-specific coronary atherosclerotic plaque progression using lipidomics, blood flow, and LDL transport modeling. Appl. Sci. 2021, 11, 1976. [Google Scholar] [CrossRef]
- Berne, R.M.; Levy, M.N. Cardiovascular Physiology, Etc; The C.V.Mosby Company: Maryland Heights, MO, USA, 1967. [Google Scholar]
- Versteeg, H.K.; Malalasekera, W. An Introduction to Computational Fluid Dynamics: The Finite Volume Method; Pearson Education: London, UK, 2007. [Google Scholar]
- Massoudi, M.; Kim, J.; Antaki, J.F. Modeling and numerical simulation of blood flow using the theory of interacting continua. Int. J. Non-Linear Mech. 2012, 47, 506–520. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.-T.; Aubry, N.; Antaki, J.F.; Massoudi, M. Flow of blood in micro-channels: Recent results based on mixture theory. Int. J. Adv. Eng. Sci. Appl. Math. 2017, 9, 40–50. [Google Scholar] [CrossRef]
- Nowak, M.; Melka, B.; Rojczyk, M.; Gracka, M.; Nowak, A.J.; Golda, A.; Adamczyk, W.P.; Isaac, B.; Białecki, R.A.; Ostrowski, Z. The protocol for using elastic wall model in modeling blood flow within human artery. Eur. J. Mech.-B/Fluids 2019, 77, 273–280. [Google Scholar] [CrossRef]
- Manninen, M.; Taivassalo, V.; Kallio, S. On the Mixture Model for Multiphase Flow; Technical Research Centre of Finland: Otaniemi, Finland, 1996. [Google Scholar]
- Gidaspow, D. Multiphase Flow and Fluidization: Continuum and Kinetic Theory Descriptions; Academic Press: Cambridge, MA, USA, 1994. [Google Scholar]
- Hron, J.; Turek, S. A monolithic FEM/multigrid solver for an ALE formulation of fluid-structure interaction with applications in biomechanics. In Fluid-Structure Interaction; Springer: Berlin/Heidelberg, Germany, 2006; pp. 146–170. [Google Scholar]
- Rugonyi, S.; Bathe, K.-J. On finite element analysis of fluid flows fully coupled with structural interactions. CMES-Comput. Modeling Eng. Sci. 2001, 2, 195–212. [Google Scholar]
- Seo, T. Hemodynamic characteristics in the human carotid artery model induced by blood-arterial wall interactions. Int. J. Biomed. Biol. Eng. 2013, 7, 215–220. [Google Scholar]
- Lopes, D.; Puga, H.; Teixeira, J.; Teixeira, S. Fluid–Structure Interaction study of carotid blood flow: Comparison between viscosity models. Eur. J. Mech.-B/Fluids 2020, 83, 226–234. [Google Scholar] [CrossRef]
- Kamangar, S.; Badruddin, I.A.; Govindaraju, K.; Nik-Ghazali, N.; Badarudin, A.; Viswanathan, G.N.; Ahmed, N.S.; Khan, T.Y. Patient-specific 3D hemodynamics modelling of left coronary artery under hyperemic conditions. Med. Biol. Eng. Comput. 2017, 55, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, J.R.; Wolberg, A.S. Red blood cells in thrombosis. Blood J. Am. Soc. Hematol. 2017, 130, 1795–1799. [Google Scholar] [CrossRef] [PubMed]
- Berne, R.; Levy, M.N. Cardiovascular Physiology; CV Mosby Company: Saint Louis, MO, USA; Maryland Heights, MO, USA, 1967. [Google Scholar]
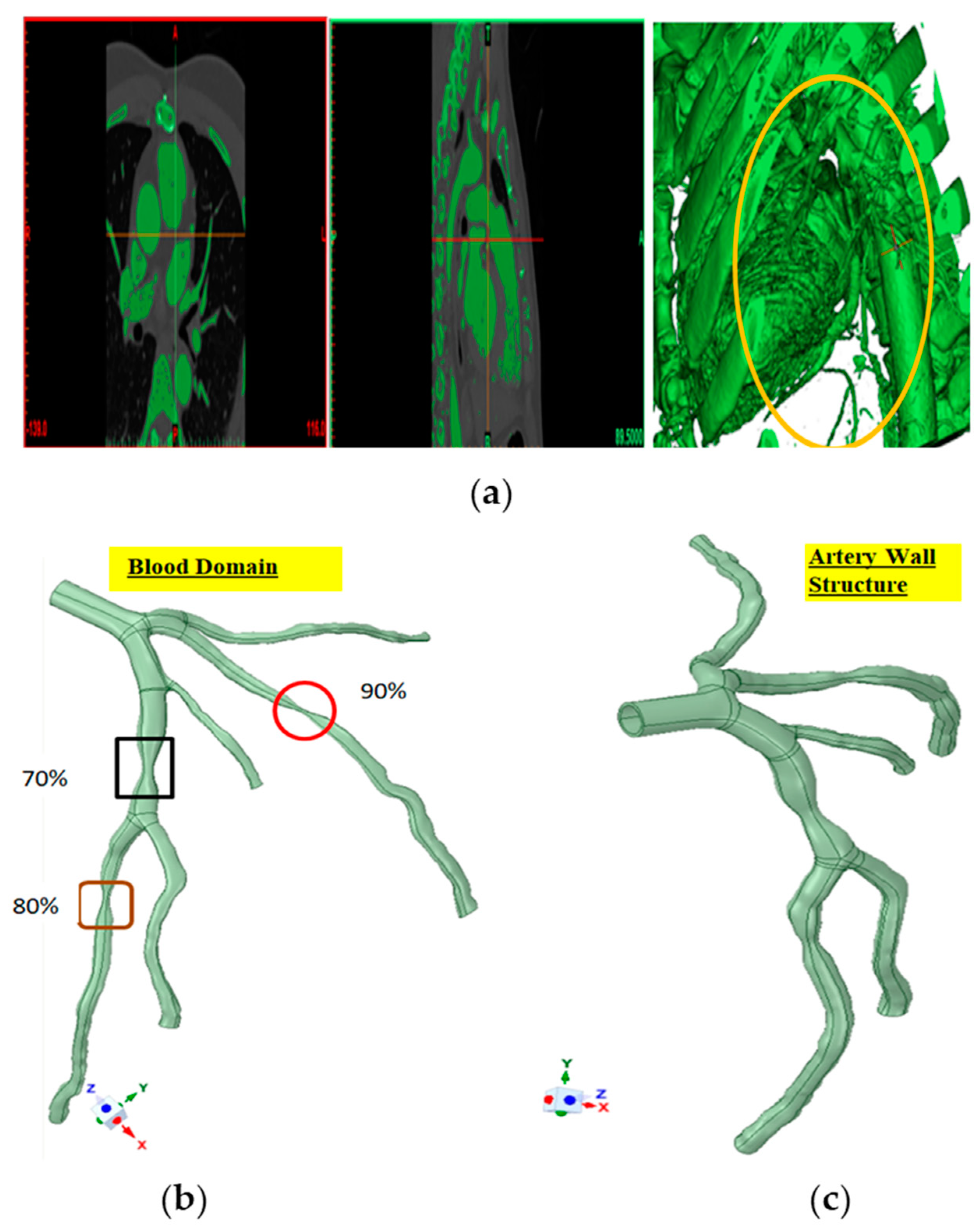
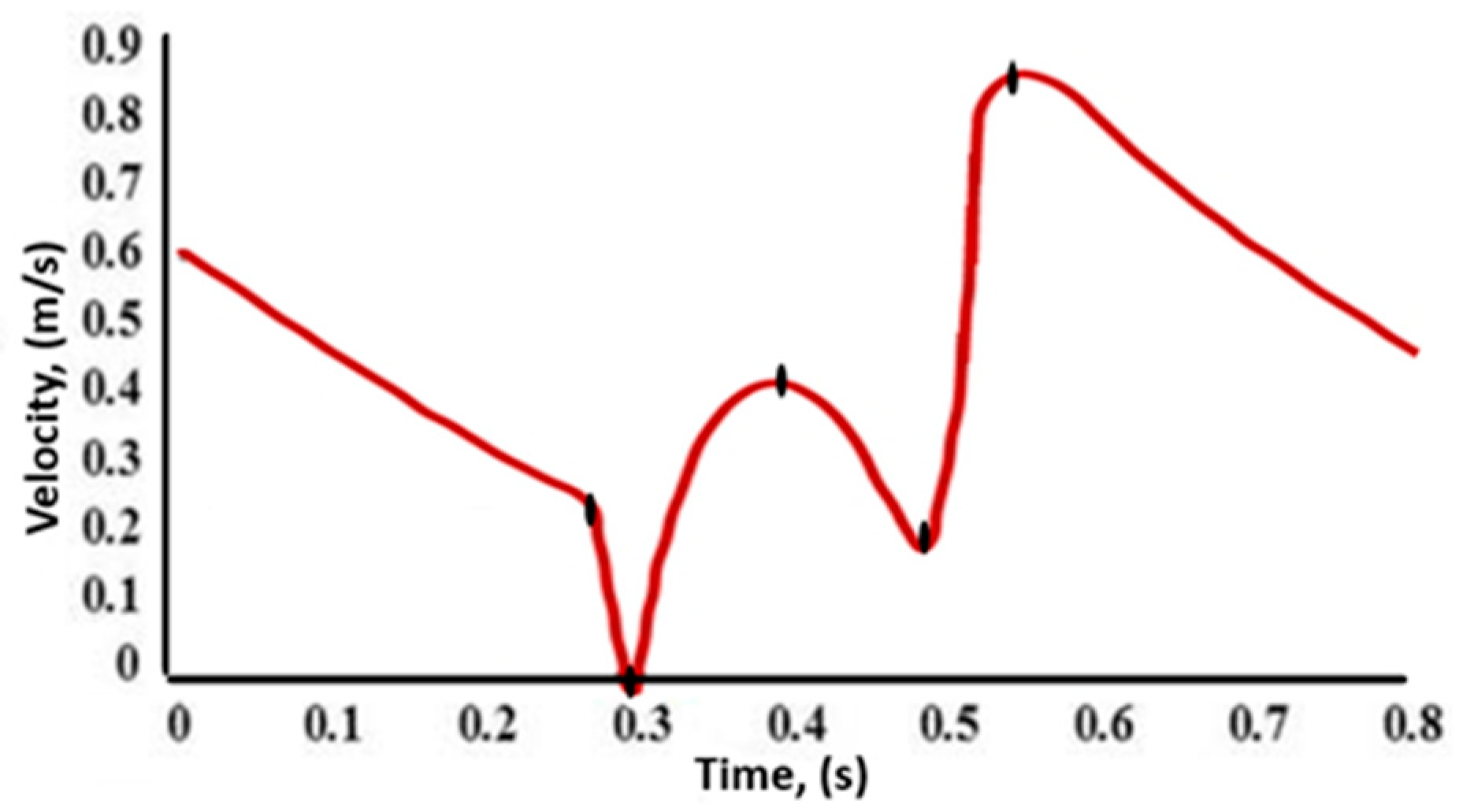

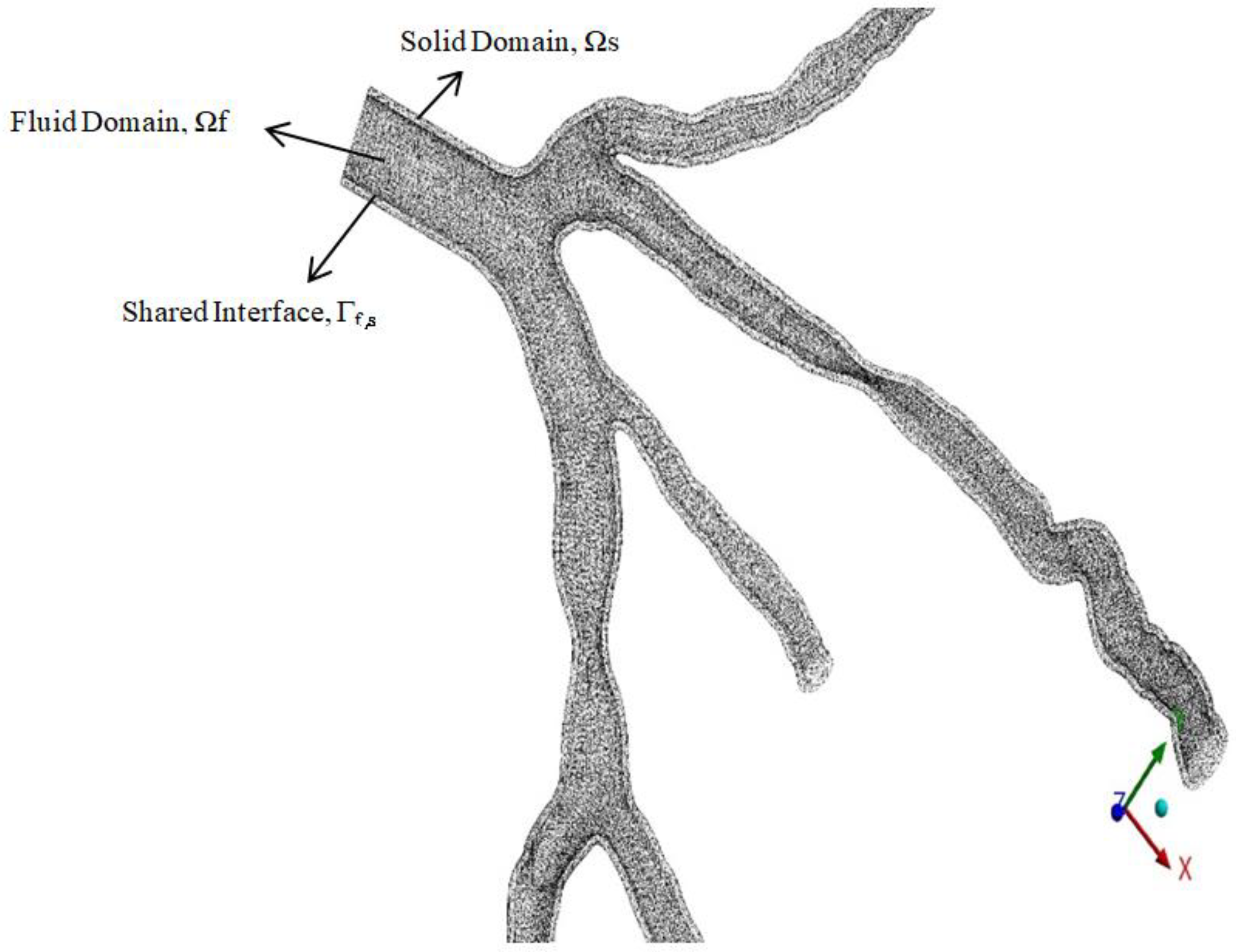


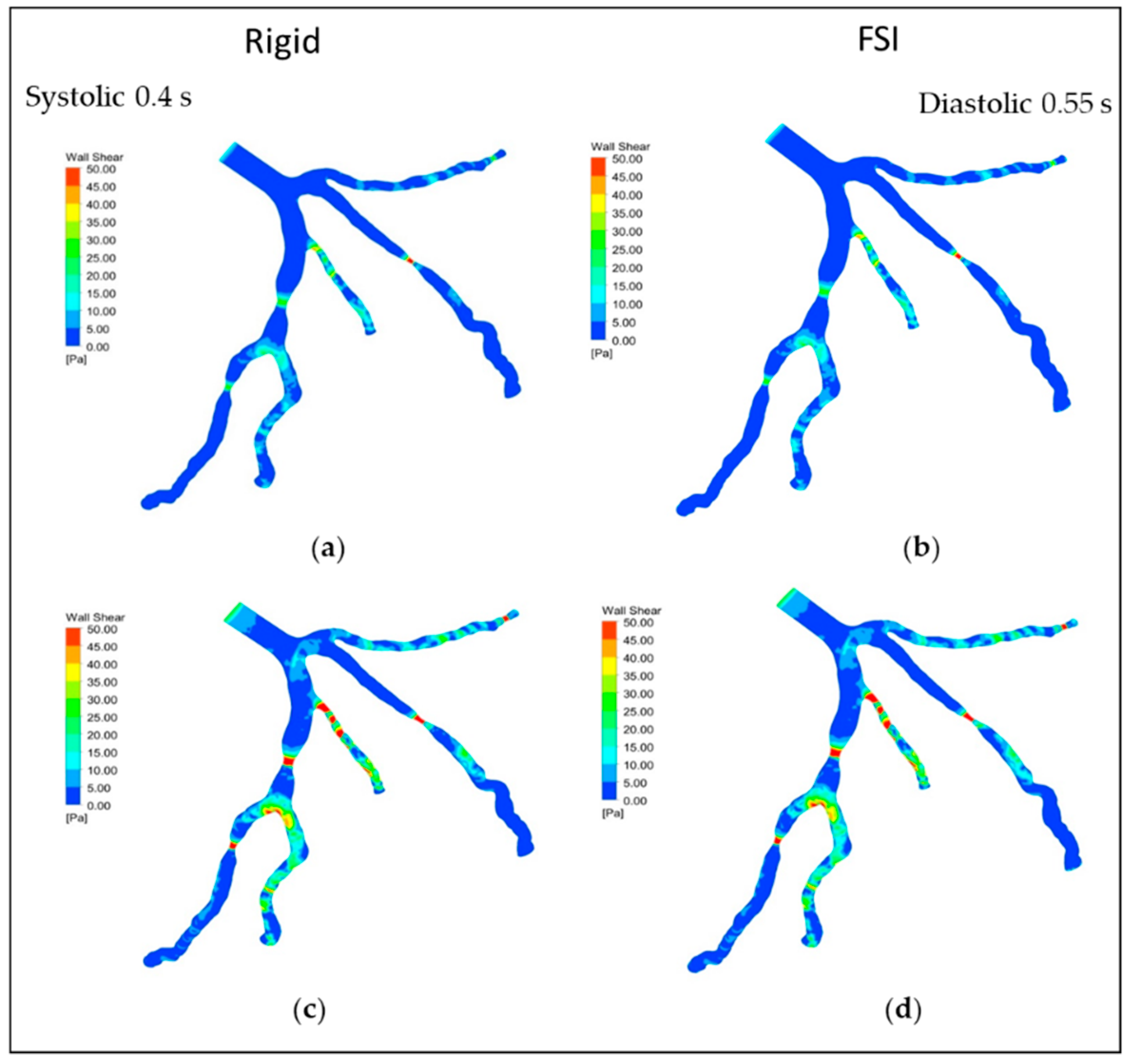

| Parameters | Value |
|---|---|
| Primary Phase | |
| Density of Plasma (p) | 1003 kg/m3 |
| Viscosity | 0.0013 kg/m-s |
| Secondary Phase | |
| RBC (Red Blood Cells) Density (RBC) | 1096 kg/m3 |
| Granular diameter of RBC | 8 µm |
| RBC volume fraction | 0.45 |
| Coefficient of Restitution, (e) | 0.99999 |
| Coefficient of Wall restitution (ew) | 0.9999 |
| Coefficient of Specularity (φ) | 0.60 |
| packing limit of RBC, (εs)max | 0.70 |
| Viscosity model | Carreau model |
| Time Constant (λ) [s], | 3.313 |
| Index of Power-Law (n) | 0.3568 |
| Zero shear viscosity (µ0) | 0.056 (kg/m-s) |
| Infinite shear viscosity (µ͚) | 0.00345 (kg/m-s) |
| Structural Properties | |
| Artery Wall Density(ρs) | 1300 kg/m3 |
| Wall thickness | 0.5 mm |
| Young’s Modulus | 1.08 (MPa) |
| Poisson’s ration(υ) | 0.49 |
| Bulk Modulus | 1.8 × 107 (Pa) |
| Shear Modulus | 3.6242 × 105 (Pa) |
| Parameters | Dimensions |
|---|---|
| Length of Left Main (LM) | 9.91 mm |
| Length of left circumflex (LCx) | 66.27 mm |
| Length of Left anterior descending (LAD) | 82.29 mm |
| Vessel wall thickness | 0.5 mm |
| Diameter of LM along Inlet | 3.186 mm |
| Diameter of LAD along Outlet | 2.168 mm |
| Diameter of LCx along Outlet | 2.823 mm |
| Area of inlet (LM) | 7.9715 mm2 |
| Angulation between LCx and LAD | 78.48° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Athani, A.; Ghazali, N.N.N.; Badruddin, I.A.; Usmani, A.Y.; Kamangar, S.; Anqi, A.E.; Ahammad, N.A. Two-Phase Non-Newtonian Pulsatile Blood Flow Simulations in a Rigid and Flexible Patient-Specific Left Coronary Artery (LCA) Exhibiting Multi-Stenosis. Appl. Sci. 2021, 11, 11361. https://doi.org/10.3390/app112311361
Athani A, Ghazali NNN, Badruddin IA, Usmani AY, Kamangar S, Anqi AE, Ahammad NA. Two-Phase Non-Newtonian Pulsatile Blood Flow Simulations in a Rigid and Flexible Patient-Specific Left Coronary Artery (LCA) Exhibiting Multi-Stenosis. Applied Sciences. 2021; 11(23):11361. https://doi.org/10.3390/app112311361
Chicago/Turabian StyleAthani, Abdulgaphur, Nik Nazri Nik Ghazali, Irfan Anjum Badruddin, Abdullah Y. Usmani, Sarfaraz Kamangar, Ali E. Anqi, and Nandalur Ameer Ahammad. 2021. "Two-Phase Non-Newtonian Pulsatile Blood Flow Simulations in a Rigid and Flexible Patient-Specific Left Coronary Artery (LCA) Exhibiting Multi-Stenosis" Applied Sciences 11, no. 23: 11361. https://doi.org/10.3390/app112311361







