Collagen Nanoparticles in Drug Delivery Systems and Tissue Engineering
Abstract
:1. Introduction
2. Methods of Collagen Nanoparticle Preparation
2.1. Emulsification/Solvent Extraction
2.2. Complex Coacervation/Polyelectrolyte Complexation
2.3. Phase Separation
2.4. Nano Spray Drying
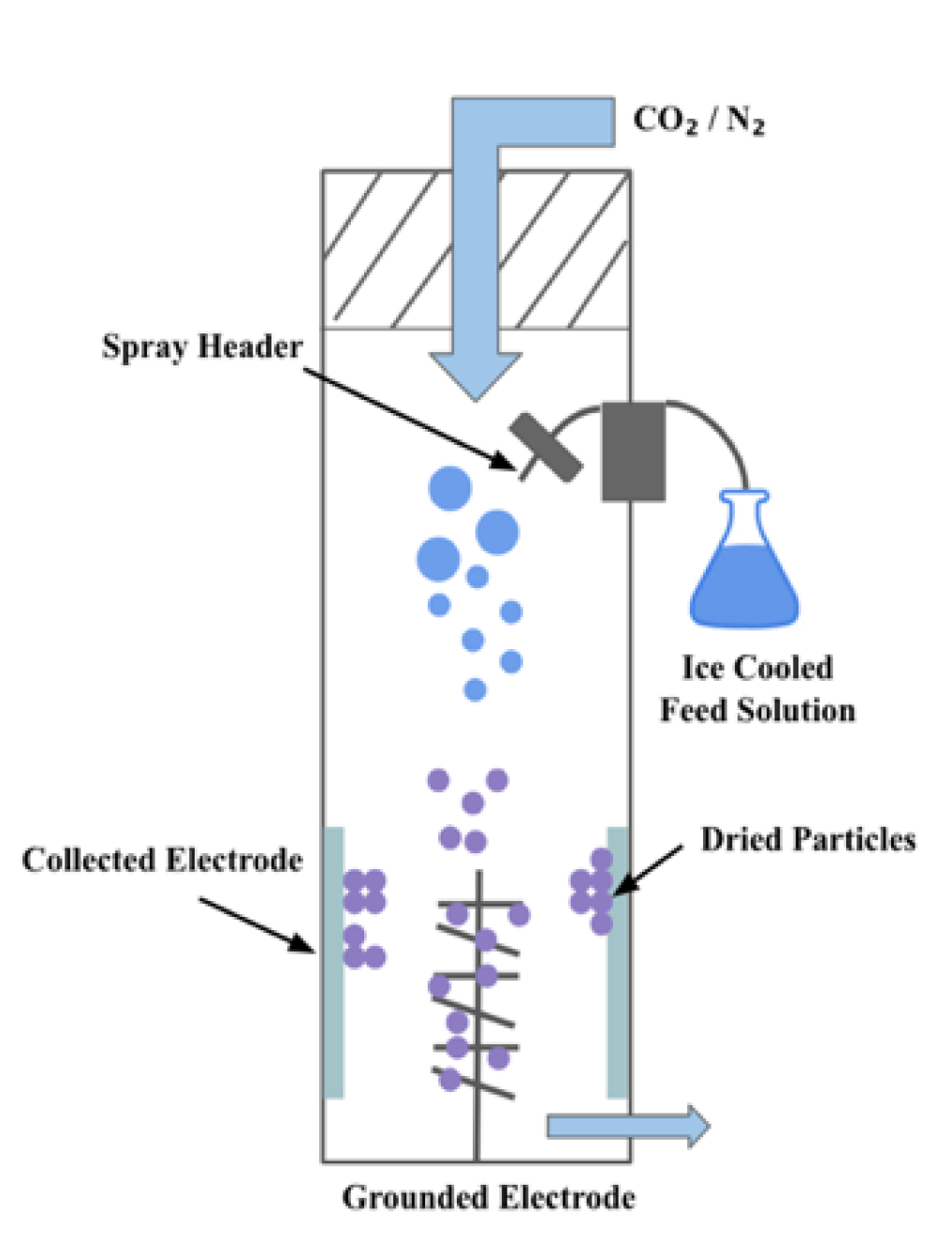
2.5. Electrospraying
2.6. Self Assembly
2.7. Desolvation
2.8. Milling
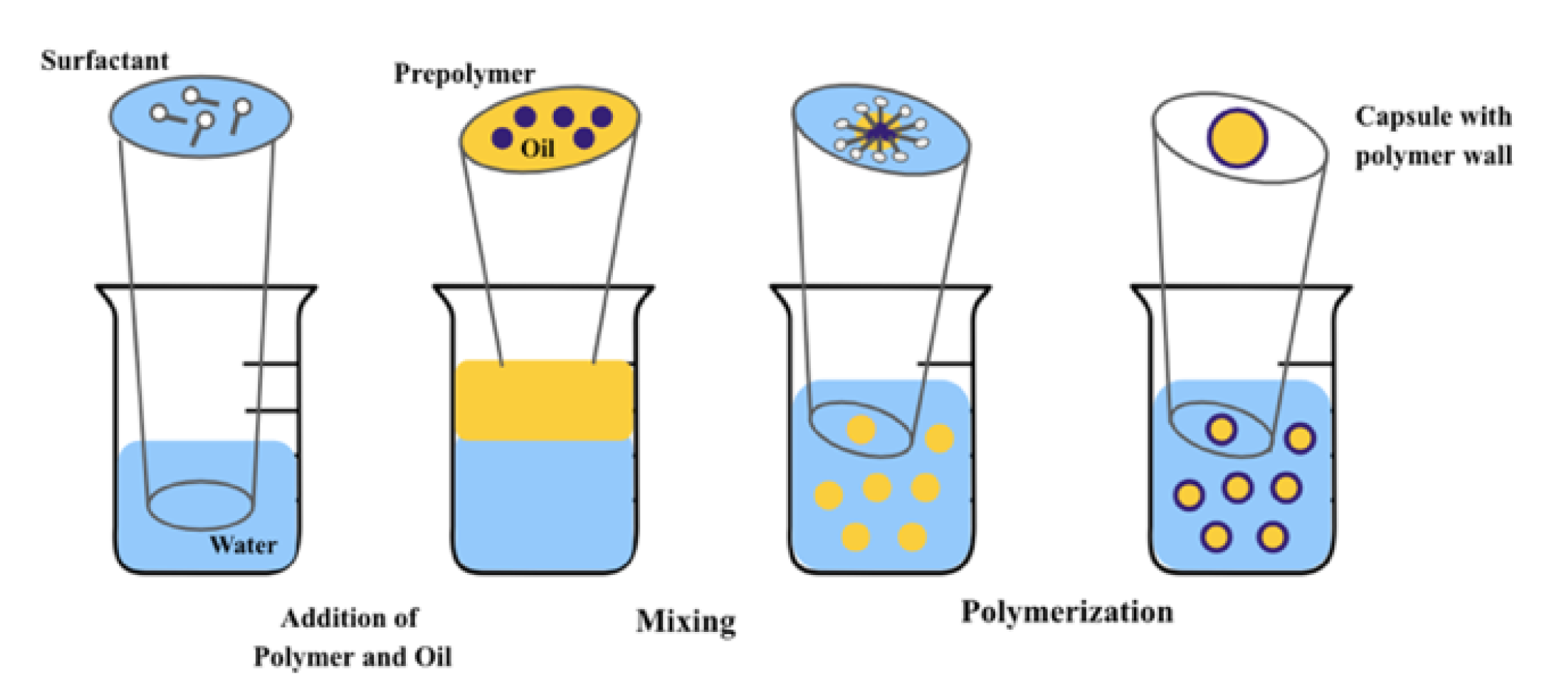
2.9. Interfacial Polymerization
2.10. Polymer Chain Collapse
3. Collagen-Based NPs in Biomedicine
3.1. Collagen Based NPs in DDS
3.2. Collagen Based NPs in TE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. In Drug Design, Development and Therapy; Dove Medical Press Ltd.: Macclesfield, UK, 2018; Volume 12, pp. 3117–3145. [Google Scholar]
- Patino, M.G.; Neiders, M.E.; Andreana, S.; Noble, B.; Cohen, R.E. Collagen as an Implantable Material in Medicine and Dentistry. Volume XXVIII. 2002. Available online: http://meridian.allenpress.com/joi/article-pdf/28/5/220/2033011/1548-1336 (accessed on 1 June 2021).
- Khan, R.; Khan, M.H. Use of collagen as a biomaterial: An update. J. Indian Soc. Periodontol. 2013, 17, 539–542. [Google Scholar] [CrossRef]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.v.; Peppas, N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uzel, S.G.; Buehler, M.J. Molecular structure, mechanical behavior and failure mechanism of the C-terminal cross-link domain in type I collagen. J. Mech. Behav. Biomed. Mater. 2011, 4, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Voicu, G.; Geanaliu, R.E.; Ghiţulică, C.D.; Ficai, A.; Grumezescu, A.M.; Bleotu, C. Synthesis, characterization and bioevaluation of irinotecan-collagen hybrid materials for biomedical applications as drug delivery systems in tumoral treatments. Open Chem. 2013, 11, 2134–2143. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Aditya, A.; Kim, B.; Koyani, R.D.; Oropeza, B.; Furth, M.; Kim, J.; Kim, N.P. Kinetics of collagen microneedle drug delivery system. J. Drug Deliv. Sci. Technol. 2019, 52, 618–623. [Google Scholar] [CrossRef]
- Collagen in Gene Delivery Applications|Sigma-Aldrich [Internet]. Available online: https://www.sigmaaldrich.com/technical-documents/articles/material-matters/collagen-in-gene-delivery-applications.html (accessed on 22 April 2021).
- Kumar, D. A review on collagen based drug delivery systems. Int. J. Pharm. Teach. Pract. 2013, 4, 811–820. [Google Scholar]
- Lee, J.H. Injectable hydrogels delivering therapeutic agents for disease treatment and tissue engineering. Biomater. Res. 2018, 22, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The role of collagen in cancer: From bench to bedside. J. Transl. Med. 2019, 17, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Shekhter, A.B.; Fayzullin, A.L.; Vukolova, M.N.; Rudenko, T.G.; Osipycheva, V.D.; Litvitsky, P.F. Medical Applications of Collagen and Collagen-Based Materials. Curr. Med. Chem. 2019, 26, 506–516. [Google Scholar] [CrossRef]
- Lazovic, G.; Colic, M.; Grubor, M.; Jovanovic, M. The application of collagen sheet in open wound healing. Ann. Burn. Fire Disasters 2005, 18, 151–156. [Google Scholar]
- Gouveia, R.M.; Connon, C.J. Collagen scaffolds for corneal regeneration. In Biomaterials and Regenerative Medicine in Ophthalmology, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 151–177. [Google Scholar]
- Chen, F.M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. In Progress in Polymer Science; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; Volume 53, pp. 86–168. [Google Scholar]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef] [Green Version]
- Panduranga, R.K. Recent developments of collagen-based materials for medical applications and drug delivery systems. J. Biomater. Sci. Polym. Ed. 1995, 7, 623–645. [Google Scholar] [CrossRef]
- Cheng, R.; Meng, F.; Deng, C.; Klok, H.-A.; Zhong, Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 2013, 34, 3647–3657. [Google Scholar] [CrossRef]
- Tang, Z.; He, C.; Tian, H.; Ding, J.; Hsiao, B.S.; Chu, B.; Chen, X. Polymeric nanostructured materials for biomedical applications. Prog. Polym. Sci. 2016, 60, 86–128. [Google Scholar] [CrossRef] [Green Version]
- Fathi-Achachelouei, M.; Knopf-Marques, H.; Da Silva, C.E.R.; Barthès, J.; Bat, E.; Tezcaner, A.; Vrana, N.E. Use of Nanoparticles in Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2019, 7, 113. [Google Scholar] [CrossRef] [Green Version]
- Perez, R.; Won, J.-E.; Knowles, J.C.; Kim, H.-W. Naturally and synthetic smart composite biomaterials for tissue regeneration. Adv. Drug Deliv. Rev. 2013, 65, 471–496. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef] [Green Version]
- Dang, Y.; Guan, J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Berthet, M.; Gauthier, Y.; Lacroix, C.; Verrier, B.; Monge, C. Nanoparticle-Based Dressing: The Future of Wound Treatment? Trends Biotechnol. 2017, 35, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Papi, M.; Palmieri, V.; Maulucci, G.; Arcovito, G.; Greco, E.; Quintiliani, G.; Fraziano, M.; De Spirito, M. Controlled self assembly of collagen nanoparticle. J. Nanoparticle Res. 2011, 13, 6141–6147. [Google Scholar] [CrossRef]
- Kandamachira, A.; Selvam, S.; Marimuthu, N.; Kalarical, S.J.; Nishter, N.F.; Kandamchira, A.; Sreeram, K.J.; Fathima, N.N. Collagen-nanoparticle Interactions: Type I Collagen Stabilization Using Functionalized Nanoparticles. Soft Mater. 2014, 13, 59–65. [Google Scholar] [CrossRef]
- Naskar, A.; Kim, K.-s. Recent Advances in Nanomaterial-Based Wound-Healing Therapeutics. Pharmaceutics 2020, 12, 499. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, E.I.; Udekwu, K.; Skog, M.; Pacioni, N.L.; Stamplecoskie, K.G.; González-Béjar, M.; Polisetti, N.; Wickham, A.; Richter-Dahlfors, A.; Griffith, M.; et al. The biocompatibility and antibacterial properties of collagen-stabilized, photochemically prepared silver nanoparticles. Biomaterials 2012, 33, 4947–4956. [Google Scholar] [CrossRef]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-Based Nanoparticles as Drug Delivery Systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef]
- Lo, S.; Fauzi, M.B. Current Update of Collagen Nanomaterials—Fabrication, Characterisation and Its Applications: A Review. Pharmaceutics 2021, 13, 316. [Google Scholar] [CrossRef] [PubMed]
- Hoa, L.T.M.; Chi, N.T.; Nguyen, L.H.; Chien, D.M. Preparation and characterisation of nanoparticles containing ketoprofen and acrylic polymers prepared by emulsion solvent evaporation method. J. Exp. Nanosci. 2011, 7, 189–197. [Google Scholar] [CrossRef]
- Mendoza-Muñoz, N.; Alcalá-Alcalá, S.; Quintanar-Guerrero, D. Preparation of Polymer Nanoparticles by the Emulsification-Solvent Evaporation Method: From Vanderhoff’s Pioneer Approach to Recent Adaptations. In Polymer Nanoparticles for Nanomedicines, 1st ed; Springer: Cham, Switzerland, 2016; pp. 87–121. [Google Scholar] [CrossRef]
- Wang, G.; Uludag, H. Recent developments in nanoparticle-based drug delivery and targeting systems with emphasis on protein-based nanoparticles. Expert Opin. Drug Deliv. 2008, 5, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Sharkawy, A.; Barreiro, M.F.; Rodrigues, A.E. New Pickering emulsions stabilized with chitosan/collagen peptides nanoparticles: Synthesis, characterization and tracking of the nanoparticles after skin application. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126327. [Google Scholar] [CrossRef]
- Singh, A.N.; Yethiraj, A. Liquid–Liquid Phase Separation as the Second Step of Complex Coacervation. J. Phys. Chem. B 2021, 125, 3023–3031. [Google Scholar] [CrossRef] [PubMed]
- Kariduraganavar, M.Y.; Heggannavar, G.B.; Amado, S.; Mitchell, G.R. Protein Nanocarriers for Targeted Drug Delivery for Cancer Therapy. In Nanocarriers for Drug Delivery; Elsevier: Amsterdam, Netherlands, 2018; pp. 173–204. [Google Scholar] [CrossRef]
- Suzuki, M.; Takayanagi, A.; Shimizu, N. Collagen-Based Non-Viral Gene Carriers. Mol. Ther. 2003, 7, S222. [Google Scholar]
- Feng, L.; Zhu, C.; Yuan, H.; Liu, L.; Lv, F.; Wang, S. Conjugated polymer nanoparticles: Preparation, properties, functionalization and biological applications. Chem. Soc. Rev. 2013, 42, 6620–6633. [Google Scholar] [CrossRef]
- Tuncel, D.; Demir, H.V. Conjugated polymer nanoparticles. Nanoscale 2010, 2, 484–494. [Google Scholar] [CrossRef]
- Yoon, J.; Kwag, J.; Shin, T.J.; Park, J.; Lee, Y.M.; Lee, Y.; Park, J.; Heo, J.; Joo, C.; Park, T.J.; et al. Nanoparticles of Conjugated Polymers Prepared from Phase-Separated Films of Phospholipids and Polymers for Biomedical Applications. Adv. Mater. 2014, 26, 4559–4564. [Google Scholar] [CrossRef]
- DeFrates, K.; Markiewicz, T.; Gallo, P.; Rack, A.; Weyhmiller, A.; Jarmusik, B.; Hu, X. Protein Polymer-Based Nanoparticles: Fabrication and Medical Applications. Int. J. Mol. Sci. 2018, 19, 1717. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.M.; Guimarães, K.L.; Cerize, N.N.; Tunussi, A.S.; Poço, J.G. Nano Spray Drying as an Innovative Technology for Encapsulating Hydrophilic Active Pharmaceutical Ingredients (API). J. Nanomed. Nanotechnol. 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Haggag, Y.A.; Faheem, A.M. Evaluation of nano spray drying as a method for drying and formulation of therapeutic peptides and proteins. Front. Pharmacol. 2015, 6, 140. [Google Scholar] [CrossRef] [Green Version]
- Gulfam, M.; Kim, J.-E.; Lee, J.M.; Ku, B.; Chung, B.H. Anticancer Drug-Loaded Gliadin Nanoparticles Induce Apoptosis in Breast Cancer Cells. Langmuir 2012, 28, 8216–8223. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Mackay, J.A.; Mcdaniel, J.R.; Chilkoti, A.; Clark, R.L. Fabrication of elastin-like polypeptide nanoparticles for drug delivery by electrospraying. Biomacromolecules 2009, 10, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Champion, J.A.; Katare, Y.K.; Mitragotri, S. Particle shape: A new design parameter for micro- and nanoscale drug delivery carriers. J. Control. Release 2007, 121, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bock, N.; Woodruff, M.A.; Hutmacher, D.W.; Dargaville, T.R. Electrospraying, a Reproducible Method for Production of Polymeric Microspheres for Biomedical Applications. Polymers 2011, 3, 131–149. [Google Scholar] [CrossRef] [Green Version]
- Nagarajan, U.; Kawakami, K.; Zhang, S.; Chandrasekaran, B.; Nair, B.U. Fabrication of Solid Collagen Nanoparticles Using Electrospray Deposition. Chem. Pharm. Bull. 2014, 62, 422–428. [Google Scholar] [CrossRef] [Green Version]
- Batrakova, E.V.; Bronich, T.K.; Vetro, J.A.; Kabanov, A.V. Polymer micelles as drug carriers. In Nanoparticulates as Drug Carriers; Imperial College Press: London, UK, 2006; pp. 57–93. [Google Scholar]
- Kempf, M.; Miyamura, Y.; Liu, P.-Y.; Chen, A.C.-H.; Nakamura, H.; Shimizu, H.; Tabata, Y.; Kimble, R.; McMillan, J.R. A denatured collagen microfiber scaffold seeded with human fibroblasts and keratinocytes for skin grafting. Biomaterials 2011, 32, 4782–4792. [Google Scholar] [CrossRef]
- Zhu, S.; Yuan, Q.; Yin, T.; You, J.; Gu, Z.; Xiong, S.; Hu, Y. Self-assembly of collagen-based biomaterials: Preparation, characterizations and biomedical applications. J. Mater. Chem. B 2018, 6, 2650–2676. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Coester, C.; Kreuter, J.; Langer, K. Desolvation process and surface characterisation of protein nanoparticles. Int. J. Pharm. 1999, 194, 91–102. [Google Scholar] [CrossRef]
- Mhetar, S.K.; Ashok Nerle, A.; Patil, R.L.; Pawar, R.A.; Patil, M.M.; Shinde, H.T. Cost Effective Ball Milling Machine for Producing Nanopowder. Int. Res. J. Eng. Technol. 2017, 4, 330–334. [Google Scholar]
- Kumar, M.; Xiong, X.; Wan, Z.; Sun, Y.; Tsang, D.C.; Gupta, J.; Gao, B.; Cao, X.; Tang, J.; Ok, Y.S. Ball milling as a mechanochemical technology for fabrication of novel biochar nanomaterials. Bioresour. Technol. 2020, 312, 123613. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Leung, H. Nano Scale Collagen Particles and Membranes. U.S. Patent 8,951,598, 7 May 2010. [Google Scholar]
- Morgan, P.W. Interfacial Polymerization. Encycl. Polym. Sci. Technol. 2011. [Google Scholar] [CrossRef]
- Song, Y.; Fan, J.-B.; Wang, S. Recent progress in interfacial polymerization. Mater. Chem. Front. 2017, 1, 1028–1040. [Google Scholar] [CrossRef]
- Prasher, A.; Loynd, C.M.; Tuten, B.T.; Frank, P.G.; Chao, D.; Berda, E.B. Efficient fabrication of polymer nanoparticles via sonogashira cross-linking of linear polymers in dilute solution. J. Polym. Sci. Part A Polym. Chem. 2015, 54, 209–217. [Google Scholar] [CrossRef]
- Kröger, A.P.P.; Hamelmann, N.M.; Juan, A.; Lindhoud, S.; Paulusse, J.M.J. Biocompatible Single-Chain Polymer Nanoparticles for Drug Delivery—A Dual Approach. ACS Appl. Mater. Interfaces 2018, 10, 30946–30951. [Google Scholar] [CrossRef] [Green Version]
- Hanlon, A.M.; Chen, R.; Rodriguez, K.; Willis, C.; Dickinson, J.G.; Cashman, M.; Berda, E.B. Scalable Synthesis of Single-Chain Nanoparticles under Mild Conditions. Macromolecules 2017, 50, 2996–3003. [Google Scholar] [CrossRef]
- Verso F lo Pomposo, J.A.; Colmenero, J.; Moreno, A.J. Multi-orthogonal folding of single polymer chains into soft nanoparticles. Soft Matter 2014, 10, 4813–4821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohcharoenkal, W.; Wang, L.; Chen, Y.C.; Rojanasakul, Y. Protein Nanoparticles as Drug Delivery Carriers for Cancer Therapy. BioMed Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitta, S.K.; Numata, K. Biopolymer-based nanoparticles for drug/gene delivery and tissue engineering. Int. J. Mol. Sci. 2013, 14, 1629–1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Posadas, I.; Monteagudo, S.; Ceña, V. Nanoparticles for brain-specific drug and genetic material delivery, imaging and diagnosis. Nanomedicine 2016, 11, 833–849. [Google Scholar] [CrossRef] [Green Version]
- Bender, A.R.; von Briesen, H.; Kreuter, J.; Duncan, I.B.; Rübsamen-Waigmann, H. Efficiency of nanoparticles as a carrier system for antiviral agents in human immunodeficiency virus-infected human monocytes/macrophages in vitro. Antimicrob. Agents Chemother. 1996, 40, 1467–1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathore, P.; Arora, I.; Rastogi, S.; Akhtar, M.; Singh, S.; Samim, M. Collagen Nanoparticle-Mediated Brain Silymarin Delivery: An Approach for Treating Cerebral Ischemia and Reperfusion-Induced Brain Injury. Front. Neurosci. 2020, 14, 538404. [Google Scholar] [CrossRef]
- Ucar, B.; Humpel, C. Collagen for brain repair: Therapeutic perspectives. Neural Regen. Res. 2018, 13, 595–598. [Google Scholar]
- Wohlfart, S.; Gelperina, S.; Kreuter, J. Transport of drugs across the blood-brain barrier by nanoparticles. J. Control. Release Off. J. Control. Release Soc. 2012, 161, 264–273. [Google Scholar] [CrossRef]
- Kurakhmaeva, K.B.; Djindjikhashvili, I.A.; Petrov, V.E.; Balabanyan, V.U.; Voronina, T.A.; Trofimov, S.S.; Kreuter, J.; Gelperina, S.; Begley, D.; Alyautdin, R.N. Brain targeting of nerve growth factor using poly(butyl cyanoacrylate) nanoparticles. J. Drug Target. 2009, 17, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Hoang, G.; Manivasagan, P.; Moorthy, M.S.; Phan, T.T.V.; Kim, H.H.; Nguyen, T.P.; Oh, J. Rapid microwave-assisted synthesis of gold loaded hydroxyapatite collagen nano-bio materials for drug delivery and tissue engineering application. Ceram. Int. 2018, 45, 2977–2988. [Google Scholar] [CrossRef]
- Deepa, C.; Suresh, G.; Devagi, P.; Kowsalya, A.; Kavitha, K.; Ramesh, B. Evaluation of the antibacterial activity of silver nanoparticles, doxycycline, collagen and their amalgamation—An in vitro study. Mater. Today Proc. 2021, 43, 3050–3053. [Google Scholar] [CrossRef]
- Le, V.M.; Lang, M.D.; Shi, W.B.; Liu, J.W. A collagen-based multicellular tumor spheroid model for evaluation of the efficiency of nanoparticle drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 540–544. [Google Scholar] [CrossRef]
- Yang, S.C.; Lu, L.F.; Cai, Y.; Zhu, J.B.; Liang, B.W.; Yang, C.Z. Body distribution in mice of intravenously injected camptothecin solid lipid nanoparticles and targeting effect on brain. J. Control. Release Off. J. Control. Release Soc. 1999, 59, 299–307. [Google Scholar] [CrossRef]
- Berthold, A.; Cremer, K.; Kreuter, J. Collagen microparticles: Carriers for glucocorticosteroids. Eur. J. Pharm. Biopharm. 1998, 45, 23–29. [Google Scholar] [CrossRef]
- Volkova, N.; Yukhta, M.; Pavlovich, O.; Goltsev, A. Application of Cryopreserved Fibroblast Culture with Au Nanoparticles to Treat Burns. Nanoscale Res. Lett. 2016, 11, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Vedhanayagam, M.; Nidhin, M.; Duraipandy, N.; Naresh, N.D.; Jaganathan, G.; Ranganathan, M.; Kiran, M.S.; Narayan, S.; Nair, B.U.; Sreeram, K.J. Role of nanoparticle size in self-assemble processes of collagen for tissue engineering application. Int. J. Biol. Macromol. 2017, 99, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Morshed, M.; Memic, A.; Hassan, S.; Webster, T.J.; Marei, H. Nanoparticles in tissue engineering: Applications, challenges and prospects. Int. J. Nanomed. 2018, 13, 5637–5655. [Google Scholar] [CrossRef] [Green Version]
- Patrascu, J.M.; Nedelcu, I.A.; Sonmez, M.; Ficai, D.; Ficai, A.; Vasile, B.S.; Ungureanu, C.; Albu, M.G.; Andor, B.; Andronescu, E.; et al. Composite Scaffolds Based on Silver Nanoparticles for Biomedical Applications. J. Nanomater. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Nidhin, M.; Vedhanayagam, M.; Sangeetha, S.; Kiran, M.S.; Nazeer, S.S.; Jayasree, R.S.; Sreeram, K.J.; Nair, B.U. Fluorescent nanonetworks: A novel bioalley for collagen scaffolds and Tissue Engineering. Sci. Rep. 2014, 4, srep05968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Z.; Teoh, S.-H. Surface modification of ultra thin poly (ε-caprolactone) films using acrylic acid and collagen. Biomaterials 2004, 25, 1991–2001. [Google Scholar] [CrossRef]
- Moon, D.G.; Christ, G.; Stitzel, J.D.; Atala, A.; Yoo, J.J. Cyclic Mechanical Preconditioning Improves Engineered Muscle Contraction. Tissue Eng. Part A 2008, 14, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Zhao, Y.; Yang, M.; Wang, S.F. Nano-collagen artificial bone for alveolar ridge preservation in the Kazakh from Xinjiang Tacheng Region. Chin. J. Tissue Eng. Res. 2015, 19, 1864–1871. [Google Scholar]
- Shen, L.; Wang, X.Y.; Chen, P.; Yu, T. Allogeneic adipose-derived stem cells combined with nano collagen-based bone for repair of ulna bone defects. Chin. J. Tissue Eng. Res. 2015, 19, 5162–5166. [Google Scholar]
- Quinlan, E.; Partap, S.; Azevedo, M.M.; Jell, G.; Stevens, M.M.; O’Brien, F.J. Hypoxia-mimicking bioactive glass/collagen glycosaminoglycan composite scaffolds to enhance angiogenesis and bone repair. Biomaterials 2015, 52, 358–366. [Google Scholar] [CrossRef]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L. Influence of freezing rate on pore structure in freeze-dried collagen-GAG scaffolds. Biomaterials 2004, 25, 1077–1086. [Google Scholar] [CrossRef]
- Zhu, L.; Luo, D.; Liu, Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int. J. Oral Sci. 2020, 12, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Quinlan, E.; Thompson, E.M.; Matsiko, A.; O’Brien, F.J.; López-Noriega, A. Functionalization of a Collagen-Hydroxyapatite Scaffold with Osteostatin to Facilitate Enhanced Bone Regeneration. Adv. Healthc. Mater. 2015, 4, 2649–2656. [Google Scholar] [CrossRef]
- Pashneh-Tala, S.; MacNeil, S.; Claeyssens, F. The tissue-engineered vascular graft-Past, present, and future. Tissue Eng. Part B Rev. 2016, 22, 68–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.; Li, Z.; Bai, Y.; Liao, G.; Pan, J.; Zhang, C. Collagen/nano-sized β-tricalcium phosphate conduits combined with collagen filaments and nerve growth factor promote facial nerve regeneration in miniature swine: An in vivo study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 128, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Amiri, F.Z.; Pashandi, Z.; Lotfibakhshaiesh, N.; Parsa, M.J.M.; Ghanbari, H.; Faridi-Majidi, R. Cell attachment effects of collagen nanoparticles on crosslinked electrospun nanofibers. Int. J. Artif. Organs 2020, 44, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Langasco, R.; Cadeddu, B.; Formato, M.; Lepedda, A.J.; Cossu, M.; Giunchedi, P.; Pronzato, R.; Rassu, G.; Manconi, R.; Gavini, E. Natural collagenic skeleton of marine sponges in pharmaceutics: Innovative biomaterial for topical drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Peng, X.-L.; Li, H.-R.; Liu, J.-X.; Cheng, J.-S.; Qi, X.-Y.; Ye, S.-J.; Gong, H.-L.; Zhao, X.-H.; Yu, J.; et al. Marine-Derived Collagen as Biomaterials for Human Health. Front. Nutr. 2021, 8, 493. [Google Scholar] [CrossRef]
- Sadasivuni, K.K.; Ponnamma, D.; Rajan, M.; Ahamed, M.B.; Al-Maadeed, M.A.S.A. Correction to: Polymer Nanocomposites in Biomedical Engineering. In Polymer Nanocomposites in Biomedical Engineering; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.S.; Todo, M. Effects of sintering temperature on the compressive mechanical properties of collagen/hydroxyapatite composite scaffolds for bone tissue engineering. Mater. Lett. 2016, 173, 231–234. [Google Scholar] [CrossRef]
- Albu, M.G.; Titorencu, I.; Ghica, M.V. Collagen-Based Drug Delivery Systems for Tissue Engineering. In Biomaterials Applications for Nanomedicine; InTechOpen: London, UK, 2011; Volume 17, pp. 333–358. [Google Scholar]
- Yadav, P.; Yadav, H.; Shah, V.G.; Shah, G.; Dhaka, G. Biomedical Biopolymers, their Origin and Evolution in Biomedical Sciences: A Systematic Review. J. Clin. Diagn. Res. 2015, 9, ZE21. [Google Scholar] [CrossRef]
- Kaul, G.; Amiji, M. Protein Nanoparticles for Gene Delivery. In Polymeric Gene Delivery; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Hamdan, S.; Pastar, I.; Drakulich, S.; Dikici, E.; Tomic-Canic, M.; Deo, S.; Daunert, S. Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications. ACS Cent. Sci. 2017, 3, 163–175. [Google Scholar] [CrossRef]
- Senturk, B.; Uzunalli, G.; Mammadov, R.; Guler, M.O.; Tekinay, A.B. Wound Healing Applications of Nanomaterials; Wiley: Hoboken, NJ, USA, 2016; pp. 87–117. [Google Scholar] [CrossRef] [Green Version]







| Preparation Method | Advantages | Limitations | References |
|---|---|---|---|
| Emulsification/Solvent Extraction | Process is simple, equipment required is simple, recovery can be controlled, high flexibility and selectivity | Require stabilizer and surfactant because of unstable thermodynamic nature, need to add organic solvent and then remove it, residues of organic solvent may be toxic | [10,34] |
| Complex Coacervation/Polyelectrolyte Complexation | Particles formed are very stable, NPs of smaller size, by guiding process conditions, nanoparticle size and shape can be controlled, can be combined with sensitive drugs | Hard process to scale up | [36,65] |
| Phase Separation | Specialized apparatus is not necessary, particle size controllable by altering polymer solution concentration, uniform particles are formed | Limited particle size diameter, small-scale production, organic solvent requirement | [43,44] |
| Nano Spray drying | Economical process, simple to carry out experimentally, encapsulation of hydrophilic drugs takes place easily, beneficial for heat-sensitive samples as it helps maintain temperature of the nanoparticle droplets | Small scale production, difficult to integrate hydrophobic drugs, reduced encapsulation efficiency, great energy consumption | [45,46] |
| Electrospraying | Can be scaled for industrial use, good drug loading efficiency, ease of particle synthesis due to single-step processing, formation of dry particles | Reduced flow, could produce degradation of macromolecules | [49,50] |
| Self-Assembly | Highly stable process, small NPs can be formed with high encapsulation efficiency | Hard to control NP size, shape and the potential of protein strain exists | [27,54] |
| Desolvation/Simple Coacervation | Increased encapsulation efficiency, Size and shape of NPs can be controlled using reaction conditions. | Can be carried out only for proteins influenced by dissolution or diluted by carrier proteins | [31,55] |
| Milling | Economical, easy experimentation, controllable NP size, large scale prodcution | Chamber has to be cooled due to heat release, uncontrollable NP shape, can be carried out only for coarse NPs | [57] |
| Interfacial Polymerization | Easy to carry out, inessential monomer purity | Expensive polymer monomer, takes a lot of time to be carried out | [59,60] |
| Polymer Chain Collapse | Controllable NP properties, high stability, enhanced spherical shape particle production | Limited particle diameter, hard to control side reaction occurrence | [62,63] |
| Agent (Crosslinking/Stabilising/Optimising) | Effect of Collagen NPs | Application | Reference |
|---|---|---|---|
| Malondialdehyde(MDA); 3-ethyl carbodiimide-hydrochloride (EDC-Hcl) | Enhanced bioavailability and improved therapeutic effect of silymarin drug | Drug for Neuronal injury | [69] |
| Enables identification of the particular cell surface receptors which allows transcytosis | Drug delivery to the brain | [70] | |
| Enhancement of motor functions in PD model and cognitive functions in AD model | Nerve Growth Factor (NGF) delivered to the brain | [73] | |
| Gold NPs (Au) on hydroxyapatite (HAp) surface | Enhanced Au–HAp–Col NPs with bioactive and biocompatible characteristics for loading and releasing of the doxorubicin drug | Drug delivery, scaffold materials, cell growth, proliferation and adhesion | [74] |
| Silver NPs (DdAgNPs) with doxycycline (DO) | Stronger antibacterial action against all the test organisms compared to the DdAgNPs alone | Bactericidal agent | [75] |
| 95-D, U87 and HCT116 cells | More precise therapeutic results and dynamics of the drug transport agents in vivo | Tumor infiltration for anti-cancer drug delivery | [77] |
| Agent (Crosslinking/Stabilising/Optimising) | Effect of Collagen NPs | Application | Reference |
|---|---|---|---|
| Gold NPs | Reduction of inflammation and formation of granulation tissue with no problems of rejection | Wound healing | [31] |
| Gold NPs and cryopreserved human fibroblasts | Increased rate of healing and enhanced collagen deposition | Burn and wound healing | [80] |
| Silver and pectin | Greater antibacterial activity and improved viability of the cell toward keratinocytes. | Dermal TE | [81] |
| Hydroxyapatite and silver | Burn and wound healing, skin repair, bone graft materials | [82] | |
| α-Fe2O3 NPs capped with starch | Enhanced mechanical properties, better crosslinking, better viability to the cell, greater super paramagnetic behaviour | Imaging, bio implants | [83] |
| Enhanced contractile force generation | Tissue constructs based on skeletal muscles with acellular tissue scaffolds | [84] | |
| Greater alveolar bone mineral density | Protection of residual ridge after tooth extraction, artificial collagen nanoparticle bone. | [85] | |
| Stem cells derived from adipose and allogene | Enhanced rate of solid fusion and bone mineral density | Repairing defects in the human ulna | [86] |
| Collagen glycosaminoglycan (CG) and bioactive glass | Bone TE | [87] | |
| Acted as template for growth into the vascular graft and cell recognition | For implants in vascular medicine, scaffold in Tissue Engineered Vascular Grafts | ||
| Nano-sized β-tricalcium phosphate and nerve growth agents | Regeneration of facial nerves | [92] | |
| Glutaraldehyde | Enhance cell viability, adhesion and more spreading on the scaffold | Mimicking extracellular matrix (ECM) | [93] |
| Prolonged release time of estradiol and improved absorption of estradiol | Osmotic accelerator for hormone replacement therapy for transdermal delivery of 17β-estradiol-hemihydrate, carriers for transdermal drug delivery |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arun, A.; Malrautu, P.; Laha, A.; Luo, H.; Ramakrishna, S. Collagen Nanoparticles in Drug Delivery Systems and Tissue Engineering. Appl. Sci. 2021, 11, 11369. https://doi.org/10.3390/app112311369
Arun A, Malrautu P, Laha A, Luo H, Ramakrishna S. Collagen Nanoparticles in Drug Delivery Systems and Tissue Engineering. Applied Sciences. 2021; 11(23):11369. https://doi.org/10.3390/app112311369
Chicago/Turabian StyleArun, Ashni, Pratyusha Malrautu, Anindita Laha, Hongrong Luo, and Seeram Ramakrishna. 2021. "Collagen Nanoparticles in Drug Delivery Systems and Tissue Engineering" Applied Sciences 11, no. 23: 11369. https://doi.org/10.3390/app112311369
APA StyleArun, A., Malrautu, P., Laha, A., Luo, H., & Ramakrishna, S. (2021). Collagen Nanoparticles in Drug Delivery Systems and Tissue Engineering. Applied Sciences, 11(23), 11369. https://doi.org/10.3390/app112311369







