Influence of Probiotic Supplementation on Health Status of the Dogs: A Review
Abstract
:Featured Application
Abstract
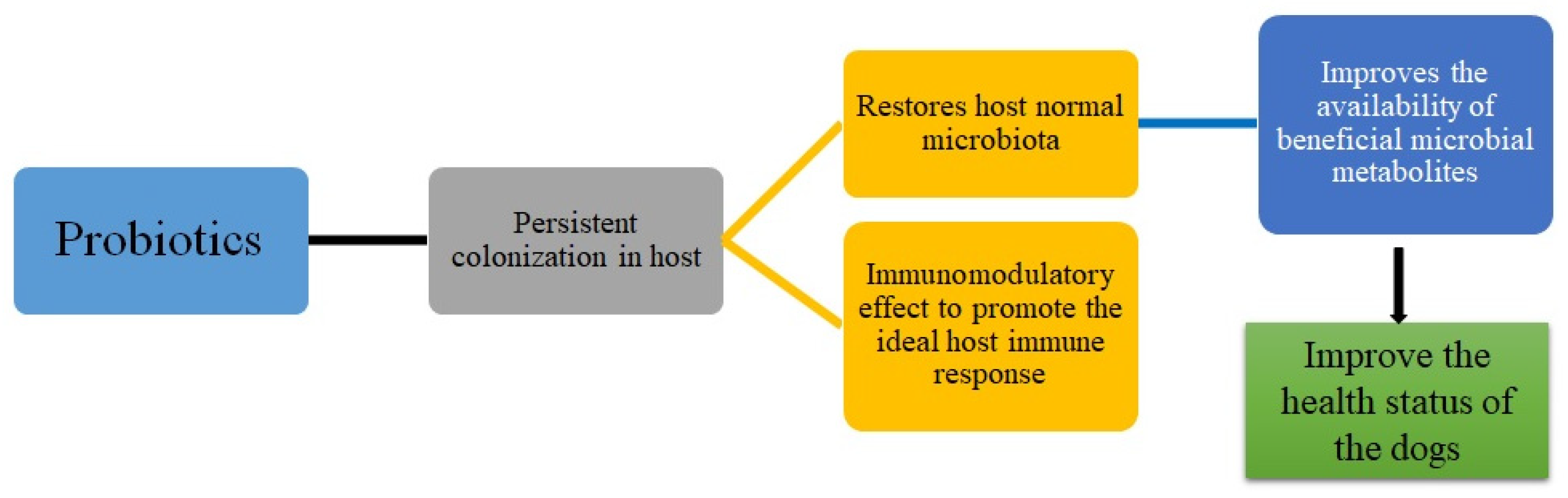
1. Introduction
2. Characterization of the Microbiota in Dogs
3. Health Benefits of Probiotic Supplementation in Healthy Dogs
3.1. Microbiota Changes and Other Benefits in Healthy Dogs upon Probiotic Supplementation
| Ref. | Model | Probiotic Used | Dose per Animal | Duration | Effect of Probiotic Supplementation |
|---|---|---|---|---|---|
| [41] | Healthy dogs (Probiotic group, n = 12) | Enterococcus faecium NCIB 10415 | 9.2 × 109 CFU/day | 18 days | Reduced the fecal count of Clostridium spp. Not effective in inhibiting the growth of Campylobacter spp. and Salmonella spp. |
| [42] | Healthy dogs (Probiotic group, n = 9) | Lactobacillusanimalis LA4 | 109 CFU/g; 0.5 g per day | 10 days | LA4 could withstand the gastrointestinal environment and proliferate in the dog intestines |
| [43] | Healthy dogs (Probiotic group, n = 8; Control group, n = 8) | Bacillus amyloliquefaciens CECT 5940 and E. faecium CECT 4515 | 0.2 g of a probiotic blend = 1 × 108 CFU of each probiotic strain per day | 39 days (probiotic supplementation period); 6 days adaptation period before the supplementation period | Reduced fecal clostridia count |
| [44] | Healthy dogs (Probiotic group 1, n = 23; Probiotic group 2, n = 12) | Lactobacillus, Bifidobacterium, and Bacillus species | Daily dose: 1 g of probiotic supplement/10 pounds of body weight; Lactobacillus (>64 × 109 CFU/g), Bifidobacterium (30 × 109 CFU/g), and Bacillus species (24 × 109 CFU/g) | 14 days (probiotic group 1); 28 days (probiotic group 2) | No increase in the prevalence of vaginal lactic acid bacteria |
| [45] | Healthy dogs (Probiotic group, n = 5; Placebo group, n = 5; Control group, n = 5) | Queso Blanco cheese containing Bifidobacterium longum KACC 91563 | 5 × 108 CFU/10 g cheese/kg of body weight/day | 8 weeks | Reduced the fecal Enterobacteriaceae and Clostridium count Increased the fecal Bifidobacterium count Increased the short-chain fatty acids such as acetic and propionic acid levels |
| [46] | Healthy dogs (Probiotic group, n = 4; Placebo group, n = 4; Control group, n = 4) | Queso Blanco cheese containing Bifidobacterium longum KACC 91563 | 5 × 108 CFU/10 g cheese/day | 8 weeks | Increased the fecal Bifidobacterium Decreased the fecal C. perfringens, Enterobacteriaceae, Collinesella, Blautia, and Fusobacterium |
| [47] | Healthy dogs (Probiotic group, n = 4; Control group, n = 4) | Bacillus amyloliquefaciens CECT 5940 | 1 × 109 CFU/g dry matter of probiotic product/day | 30 days (probiotic supplementation period); 6 days adaptation period before the supplementation period | Increased the fecal bacillus count Decreased the fecal coliform count |
| [48] | Healthy dogs (Elderly probiotic group, n = 15; Elderly control group, n = 15; Young probiotic group, n = 12; Young control group, n = 12; Training probiotic group, n = 18; Training control group, n = 18) | Lactobacillus casei Zhang, Lactobacillus plantarum P-8, and Bifidobacterium animalis subsp. lactis V9 | Equal proportions of 3 probiotic strains in a final concentration of 2 × 109 CFU/g; Dosage: 10 g/day (Elderly group); 2 g/day (Young group); 4 g/day (Training group) | 60 days | Increased the fecal load of Lactobacillus spp. and Faecalibacterium prausnitzii and decreased the abundance of Sutterella stercoricanisin and Escherichia coli in the elderly dogs. |
| [51] | Healthy dogs (Probiotic group, n = 11) | E. faecium EE3 | 109 CFU/mL | 1 week | Reduced the fecal staphylococci and Pseudomonas load Reduced total lipid levels in most of the dogs |
| [52] | Healthy dogs (Probiotic group, n = 5; Placebo group, n = 5; Control group, n = 5) | Lactobacillus johnsonii CPN23 or L. acidophilus NCDC15 | 108 CFU per mL; 0.1 mL per kg of body weight | 13 weeks (9 weeks probiotic supplementation period) (next 4 weeks to study the withdrawal effects) | Reduced the plasma glucose levels in the dogs of both probiotic groups Superoxide dismutase and glutathione peroxidase activity were higher in L. johnsonii CPN23-treated dogs |
| [53] | Healthy dogs (Probiotic group, n = 18; Control group, n = 18) | E. faecium SF68 | 5 × 108 CFU/g/day | 14 days | An effective cobalamin reduction was observed during the 28th day (after a 14-day follow-up) |
| [54] | Healthy dogs (Probiotic group, n = 18; Control group, n = 18) | E. faecium SF68 | 5 × 108 CFU/g/day | 14 days | No changes in serum alanine transferase and alkaline phosphatase activity |
| [55] | Healthy dogs (Probiotic group supplemented with two different concentrations of probiotic, n = each 5; Control group, n = 5) | Weissella cibaria JW15 | 50 g per day; 3 × 108 CFU/g or 3 × 109 CFU/g | 14 days | Decreased the serum TG levels and fecal ammonia emissions in the probiotic group. Improvement of fecal lactobacilli load and serum HDL-C levels |
| [56] | Healthy dogs (Probiotic group, n = 8; Control group, n = 8) | Bacillus subtilis and Bacillus licheniformis | 3.66 × 107 CFU of each bacterial strain/kg of the diet | 20 days | Improved the fecal consistency and reduced the occurrence of fetid feces Reduced the fecal biogenic amine content, thereby reducing the fecal odor |
| [57] | Healthy dogs (Probiotic group, n = 14; Control group, n = 16) | L. acidophilus D2/CSL | 5 × 109 CFU/g of feed additive; Pre-mixture contains 50 g of feed additive + 9950 g of maltodextrin; 20 g of pre-mixture per day | 35 days (probiotic supplementation period); 7 days adaptation period before the supplementation period | Maintained a perfect body condition score throughout the study Improved the fecal moisture content |
| [58] | Healthy dogs (n = 15) | Lactobacillus fermentum AD1 | 109 CFU | 7 days | Increased the fecal count of lactobacilli and enterococci and, also increased serum level of total lipid and total protein Reduced the serum glucose level |
| [59] | Healthy dogs (Probiotic group 1, n = 12; Probiotic group 2, n = 11) | Lactobacillus fermentum AD1-CCM7421 | Probiotic group 1 (2 g per day; 108 CFU/g of milk powder); Probiotic group 2 (1 g per day; 107 CFU/g of milk powder) | 14 days (Probiotic group 1); 7 days (Probiotic group 2) | Reduced the fecal count of clostridia and increased the total fecal concentration of SCFA such as butyric, formic, succinic, and valeric acid in probiotic group 1 Reduced the fecal count of Aeromonas sp., E. coli and Pseudomonas sp. in probiotic group 2 |
3.2. Immune Immunomodulatory Properties
4. Health Benefits of Probiotic Supplementation in Diseased Dogs
4.1. Chronic Kidney Disease
4.2. Atopic Dermatitis
4.3. Gastrointestinal Diseases and Abnormalities
4.3.1. Chronic Enteropathy
4.3.2. Acute Diarrhea
4.3.3. Nonspecific Diarrhea
4.3.4. Other Diseases Associated with Diarrhea or Prevention of Gastrointestinal Infections
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- National Research Council. Nutrient Requirements of Dogs and Cats; The National Academies Press: Washington, DC, USA, 2006. [Google Scholar] [CrossRef]
- Thatcher, C.D.; Hand, M.S.; Remillard, R.L. Small animal clinical nutrition: An interactive process. In Small Animal Clinical Nutrition, 5th ed.; Hand, M.S., Thatcher, C.D., Remillard, R.L., Roudebush, P., Novotny, B.J., Eds.; Mark Morris Institute: Topeka, Kansas, 2010; pp. 1–18. [Google Scholar]
- Grześkowiak, Ł.; Endo, A.; Beasley, S.; Salminen, S. Microbiota and probiotics in canine and feline welfare. Anaerobe 2015, 34, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Pilla, R.; Suchodolski, J.S. The Gut Microbiome of Dogs and Cats, and the Influence of Diet. Vet. Clin. Small Anim. 2021, 51, 605–621. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A.; et al. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdellatif, B.; McVeigh, C.; Bendriss, G.; Chaari, A. The Promising Role of Probiotics in Managing the Altered Gut in Autism Spectrum Disorders. Int. J. Mol. Sci. 2020, 21, 4159. [Google Scholar] [CrossRef]
- Arora, K.; Green, M.; Prakash, S. The microbiome and Alzheimer’s Disease: Potential and limitations of prebiotic, synbiotic, and probiotic formulations. Front. Bioeng. Biotechnol. 2020, 8, 537847. [Google Scholar] [CrossRef]
- FAO/WHO. Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria; Food and Agriculture Organization of the United Nations: Cordoba, Argentina, 2001. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Collins, J.; Thornton, G.; Sullivan, G. Selection of probiotic strains for human applications. Int. Dairy J. 1998, 8, 487–490. [Google Scholar] [CrossRef]
- Bajagai, Y.S.; Klieve, A.V.; Dart, P.J.; Bryden, W.L. Probiotics in Animal Nutrition–Production, Impact and Regulationby FAO Animal Production and Health Paper No. 179; Harinder, P.S., Ed.; FAO: Rome, Italy, 2016; ISBN 978-92-5-109333-7. [Google Scholar]
- Kothari, D.; Patel, S.; Kim, S.-K. Probiotic supplements might not be universally-effective and safe: A review. Biomed. Pharmacother. 2019, 111, 537–547. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. A review of the role of probiotic supplementation in dental caries. Probiotics Antimicrob. Proteins 2020, 12, 1300–1309. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. The role of probiotics in colorectal cancer management. Evid. Based Complement. Alternat. Med. 2020, 2020, 3535982. [Google Scholar] [CrossRef] [Green Version]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. A mini-review of human studies on cholesterol-lowering properties of probiotics. Sci. Pharm. 2019, 87, 26. [Google Scholar] [CrossRef] [Green Version]
- Kesika, P.; Sivamaruthi, B.S.; Chaiyasut, C. Do probiotics improve the health status of individuals with diabetes mellitus? A review on outcomes of clinical trials. Bio. Med. Res. Int. 2019, 2019, 1531567. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, R.; Shah, N.P. Immune system stimulation by probiotic microorganisms. Crit. Rev. Food Sci. Nutr. 2014, 54, 938–956. [Google Scholar] [CrossRef]
- Kelley, R.L.; Park, J.S.; O’Mahony, L.; Minikhiem, D.; Fix, A. Safety and tolerance of dietary supplementation with a canine-derived probiotic (Bifidobacterium animalis strain AHC7) fed to growing dogs. Vet. Ther. 2010, 11, E1–E14. [Google Scholar] [PubMed]
- Marsella, R. Evaluation of Lactobacillus rhamnosus strain GG for the prevention of atopic dermatitis in dogs. Am. J. Vet. Res. 2009, 70, 735–740. [Google Scholar] [CrossRef]
- Marsella, R.; Santoro, D.; Ahrens, K. Early exposure to probiotics in a canine model of atopic dermatitis has long-term clinical and immunological effects. Vet. Immunol. Immunopathol. 2012, 146, 185–189. [Google Scholar] [CrossRef]
- Sauter, S.N.; Benyacoub, J.; Allenspach, K.; Gaschen, F.; Ontsouka, E.; Reuteler, G.; Cavadini, C.; Knorr, R.; Blum, J.W. Effects of probiotic bacteria in dogs with food responsive diarrhea treated with an elimination diet. J. Anim. Physiol. Anim. Nutr. 2006, 90, 269–277. [Google Scholar] [CrossRef]
- Gómez-Gallego, C.; Junnila, J.; Männikkö, S.; Hämeenoja, P.; Valtonen, E.; Salminen, S.; Beasley, S. A canine-specific probiotic product in treating acute or intermittent diarrhea in dogs: A double-blind placebo-controlled efficacy study. Vet. Microbiol. 2016, 197, 122–128. [Google Scholar] [CrossRef]
- Nixon, S.L.; Rose, L.; Muller, A.T. Efficacy of an orally administered anti-diarrheal probiotic paste (Pro-Kolin Advanced) in dogs with acute diarrhea: A randomized, placebo-controlled, double-blinded clinical study. J. Vet. Intern. Med. 2019, 33, 1286–1294. [Google Scholar] [CrossRef] [Green Version]
- Shmalberg, J.; Montalbano, C.; Morelli, G.; Buckley, G.J. A randomized double blinded placebo-controlled clinical trial of a probiotic or metronidazole for acute canine diarrhea. Front. Vet. Sci. 2019, 6, 163. [Google Scholar] [CrossRef] [Green Version]
- Fenimore, A.; Martin, L.; Lappin, M.R. Evaluation of Metronidazole with and without Enterococcus faecium SF68 in shelter dogs with diarrhea. Top. Companion Anim. Med. 2017, 32, 100–103. [Google Scholar] [CrossRef]
- Coelho, L.P.; Kultima, J.R.; Costea, P.I.; Fournier, C.; Pan, Y.; Czarnecki-Maulden, G.; Hayward, M.R.; Forslund, S.K.; Schmidt, T.S.B.; Descombes, P.; et al. Similarity of the dog and human gut microbiomes in gene content and response to diet. Microbiome 2018, 6, 72. [Google Scholar] [CrossRef]
- Hooda, S.; Minamoto, Y.; Suchodolski, J.S.; Swanson, K.S. Current state of knowledge: The canine gastrointestinal microbiome. Anim. Health Res. Rev. 2012, 13, 78–88. [Google Scholar] [CrossRef]
- Redfern, A.; Suchodolski, J.; Jergens, A. Role of the gastrointestinal microbiota in small animal health and disease. Vet. Rec. 2017, 181, 370. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.M.; Staley, C.; Wang, P.; Dalzell, B.; Chun, C.L.; Sadowsky, M.J. A High-Throughput DNA-Sequencing Approach for Determining Sources of Fecal Bacteria in a Lake Superior Estuary. Environ. Sci. Technol. 2017, 51, 8263–8271. [Google Scholar] [CrossRef] [PubMed]
- German, A.J.; Day, M.J.; Ruaux, C.G.; Steiner, J.M.; Williams, D.A.; Hall, E.J. Comparison of direct and indirect tests for small intestinal bacterial overgrowth and antibiotic-responsive diarrhea in dogs. J. Vet. Intern. Med. 2003, 17, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Mentula, S.; Harmoinen, J.; Heikkilä, M.; Westermarck, E.; Rautio, M.; Huovinen, P.; Könönen, E. Comparison between cultured small-intestinal and fecal microbiotas in beagle dogs. Appl. Environ. Microbiol. 2005, 71, 4169–4175. [Google Scholar] [CrossRef] [Green Version]
- Suchodolski, J.S. Intestinal microbiota of dogs and cats: A bigger world than we thought. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mazcorro, J.F.; Dowd, S.E.; Poulsen, J.; Steiner, J.M.; Suchodolski, J.S. Abundance and short-term temporal variability of fecal microbiota in healthy dogs. Microbiologyopen 2012, 1, 340–347. [Google Scholar] [CrossRef]
- Jha, A.R.; Shmalberg, J.; Tanprasertsuk, J.; Perry, L.; Massey, D.; Honaker, R.W. Characterization of gut microbiomes of household pets in the United States using a direct-to consumer approach. PLoS ONE 2020, 15, e0227289. [Google Scholar] [CrossRef] [Green Version]
- Pilla, R.; Suchodolski, J.S. The Role of the Canine Gut Microbiome and Metabolome in Health and Gastrointestinal Disease. Front. Vet. Sci. 2020, 6, 498. [Google Scholar] [CrossRef] [Green Version]
- Suchodolski, J.S.; Ruaux, C.G.; Steiner, J.M.; Fetz, K.; Williams, D.A. Assessment of the qualitative variation in bacterial microflora among compartments of the intestinal tract of dogs by use of a molecular fingerprinting technique. Am. J. Vet. Res. 2005, 66, 1556–1562. [Google Scholar] [CrossRef]
- Honneffer, J.B.; Steiner, J.M.; Lidbury, J.A.; Suchodolski, J.S. Variation of the microbiota and metabolome along the canine gastrointestinal tract. Metabolomics 2017, 13, 26. [Google Scholar] [CrossRef]
- Suchodolski, J.S.; Camacho, J.; Steiner, J.M. Analysis of bacterial diversity in the canine duodenum, jejunum, ileum, and colon by comparative 16S rRNA gene analysis. FEMS Microbiol. Ecol. 2008, 66, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Handl, S.; Dowd, S.E.; Garcia-Mazcorro, J.F.; Steiner, J.M.; Suchodolski, J.S. Massive parallel 16S rRNA gene pyrosequencing reveals highly diverse fecal bacterial and fungal communities in healthy dogs and cats. FEMS Microbiol. Ecol. 2011, 76, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Hand, D.; Wallis, C.; Colyer, A.; Penn, C.W. Pyrosequencing the canine faecal microbiota: Breadth and depth of biodiversity. PLoS ONE 2013, 8, e53115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vahjen, W.; Männer, K. The effect of a probiotic Enterococcus faecium product in diets of healthy dogs on bacteriological counts of Salmonella spp., Campylobacter spp. and Clostridium spp. in feces. Arch. Anim. Nutr. 2003, 57, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Biagi, G.; Cipollini, I.; Pompei, A.; Zaghini, G.; Matteuzzi, D. Effect of a Lactobacillus animalis strain on composition and metabolism of the intestinal microflora in adult dogs. Vet. Microbiol. 2007, 124, 160–165. [Google Scholar] [CrossRef]
- González-Ortiz, G.; Castillejos, L.; Mallo, J.J.; Àngels Calvo-Torras, M.; Dolores Baucells, M. Effects of dietary supplementation of Bacillus amyloliquefaciens CECT 5940 and Enterococcus faecium CECT 4515 in adult healthy dogs. Arch. Anim. Nutr. 2013, 67, 406–415. [Google Scholar] [CrossRef]
- Hutchins, R.G.; Bailey, C.S.; Jacob, M.E.; Harris, T.L.; Wood, M.W.; Saker, K.E.; Vaden, S.L. The effect of an oral probiotic containing Lactobacillus, Bifidobacterium, and Bacillus species on the vaginal microbiota of spayed female dogs. J. Vet. Intern. Med. 2013, 27, 1368–1371. [Google Scholar] [CrossRef]
- Park, H.E.; Kim, Y.J.; Do, K.H.; Kim, J.K.; Ham, J.S.; Lee, W.K. Effects of Queso Blanco cheese containing Bifidobacterium longum KACC 91563 on the intestinal microbiota and short chain fatty acid in healthy companion dogs. Korean J. Food Sci. Anim. Resour. 2018, 38, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Park, H.E.; Kim, Y.J.; Kim, M.; Kim, H.; Do, K.H.; Kim, J.K.; Ham, J.S.; Lee, W.K. Effects of Queso Blanco cheese containing Bifidobacterium longum KACC 91563 on fecal microbiota, metabolite and serum cytokine in healthy beagle dogs. Anaerobe 2020, 64, 102234. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Mendoza, P.; Elghandour, M.M.M.; Alaba, P.A.; Sánchez-Aparicio, P.; Alonso-Fresán, M.U.; Barbabosa-Pliego, A.; Salem, A.Z.M. Antimicrobial and bactericidal impacts of Bacillus amyloliquefaciens CECT 5940 on fecal shedding of pathogenic bacteria in dairy calves and adult dogs. Microb. Pathog. 2018, 114, 458–463. [Google Scholar] [CrossRef]
- Xu, H.; Huang, W.; Hou, Q.; Kwok, L.Y.; Laga, W.; Wang, Y.; Ma, H.; Sun, Z.; Zhang, H. Oral administration of compound probiotics improved canine feed intake, weight gain, immunity and intestinal microbiota. Front. Immunol. 2019, 10, 666. [Google Scholar] [CrossRef]
- Kim, D.H.; Jeong, D.; Kang, I.B.; Lim, H.W.; Cho, Y.; Seo, K.H. Modulation of the intestinal microbiota of dogs by kefir as a functional dairy product. J. Dairy Sci. 2019, 102, 3903–3911. [Google Scholar] [CrossRef]
- Gaspardo, A.; Zannoni, A.; Turroni, S.; Barone, M.; Sabetti, M.C.; Zanoni, R.G.; Forni, M.; Brigidi, P.; Pietra, M. Influence of Lactobacillus kefiri on intestinal microbiota and fecal IgA content of healthy dogs. Front. Vet. Sci. 2020, 7, 146. [Google Scholar] [CrossRef]
- Marciňáková, M.; Simonová, M.; Strompfová, V.; Lauková, A. Oral application of Enterococcus faecium strain EE3 in healthy dogs. Folia Microbiol. 2006, 51, 239–242. [Google Scholar] [CrossRef]
- Kumar, S.; Pattanaik, A.K.; Sharma, S.; Jadhav, S.E. Species-specific probiotic Lactobacillus johnsonii CPN23 supplementation modulates blood biochemical profile and erythrocytic antioxidant indices in Labrador dogs. Indian J. Anim. Sci. 2016, 86, 918–924. [Google Scholar]
- Lucena, R.; Olmedilla, A.B.; Blanco, B.; Novales, M.; Ginel, P.J. Effect of Enterococcus faecium SF68 on serum cobalamin and folate concentrations in healthy dogs. J. Small Anim. Pract. 2018, 59, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Lucena, R.; Novales, M.; Blanco, B.; Hernández, E.; Ginel, P.J. Effect of probiotic Enterococcus faecium SF68 on liver function in healthy dogs. J. Vet. Intern. Med. 2019, 33, 2628–2634. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.Y.; Kim, K.P.; Bae, C.H.; Choi, A.J.; Paik, H.D.; Kim, I.H. Evaluation of Weissella cibaria JW15 probiotic derived from fermented Korean vegetable product supplementation in diet on performance characteristics in adult beagle dog. Animals 2019, 9, 581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastos, T.S.; De Lima, D.C.; Souza, C.M.M.; Maiorka, A.; De Oliveira, S.G.; Bittencourt, L.C.; Félix, A.P. Bacillus subtilis and Bacillus licheniformis reduce faecal protein catabolites concentration and odour in dogs. BMC Vet. Res. 2020, 16, 116. [Google Scholar] [CrossRef] [PubMed]
- Bruni, N.; Martello, E.; Fusi, E.; Meineri, G.; Giardini, A. Study of faecal parameters and body condition in dogs with a diet supplemented with Lactobacillus acidophilus D2/CSL (CECT 4529). Ital. J. Anim. Sci. 2020, 19, 704–711. [Google Scholar] [CrossRef]
- Strompfová, V.; Marcináková, M.; Simonová, M.; Bogovic-Matijasić, B.; Lauková, A. Application of potential probiotic Lactobacillus fermentum AD1 strain in healthy dogs. Anaerobe 2006, 12, 75–79. [Google Scholar] [CrossRef]
- Strompfová, V.; Lauková, A.; Gancarčíková, S. Effectivity of freeze-dried form of Lactobacillus fermentum AD1-CCM7421 in dogs. Folia Microbiol. 2012, 57, 347–350. [Google Scholar] [CrossRef]
- Strompfová, V.; Kubašová, I.; Lauková, A. Health benefits observed after probiotic Lactobacillus fermentum CCM 7421 application in dogs. Appl. Microbiol. Biotechnol. 2017, 101, 6309–6319. [Google Scholar] [CrossRef]
- Benyacoub, J.; Czarnecki-Maulden, G.L.; Cavadini, C.; Sauthier, T.; Anderson, R.E.; Schiffrin, E.J.; Von der Weid, T. Supplementation of food with Enterococcus faecium (SF68) stimulates immune functions in young dogs. J. Nutr. 2003, 133, 1158–1162. [Google Scholar] [CrossRef] [Green Version]
- Delucchi, L.; Fraga, M.; Perelmuter, K.; Cella, C.D.; Zunino, P. Effect of native Lactobacillus murinus LbP2 administration on total fecal IgA in healthy dogs. Can. J. Vet. Res. 2014, 78, 153–155. [Google Scholar]
- Kumar, S.; Pattanaik, A.K.; Sharma, S.; Jadhav, S.E.; Dutta, N.; Kumar, A. Probiotic potential of a Lactobacillus bacterium of canine faecal-origin and its impact on select gut health indices and immune response of dogs. Probiotics Antimicrob. Proteins 2017, 9, 262–277. [Google Scholar] [CrossRef]
- Lippi, I.; Perondi, F.; Ceccherini, G.; Marchetti, V.; Guidi, G. Effects of probiotic VSL#3 on glomerular filtration rate in dogs affected by chronic kidney disease: A pilot study. Can. Vet. J. 2017, 58, 1301–1305. [Google Scholar]
- Kim, H.; Rather, I.A.; Kim, H.; Kim, S.; Kim, T.; Jang, J.; Seo, J.; Lim, J.; Park, Y.H. A double-blind, placebo controlled-trial of a probiotic strain Lactobacillus sakei probio-65 for the prevention of canine atopic dermatitis. J. Microbiol. Biotechnol. 2015, 25, 1966–1969. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Yun, T.; Ham, J.; Lee, W.; Kang, J.; Yang, M.; Kang, B. Clinical trial of oral administration of Bifidobacterium longum in dogs with atopic dermatitis. Korean J. Vet. Res. 2020, 60, 19–24. [Google Scholar] [CrossRef]
- D’Angelo, S.; Fracassi, F.; Bresciani, F.; Galuppi, R.; Diana, A.; Linta, N.; Bettini, G.; Morini, M.; Pietra, M. Effect of Saccharomyces boulardii in dogs with chronic enteropathies: Double-blinded, placebo-controlled study. Vet. Rec. 2018, 182, 258. [Google Scholar] [CrossRef]
- Schmitz, S.; Werling, D.; Allenspach, K. Effects of ex-vivo and in-vivo treatment with probiotics on the inflammasome in dogs with chronic enteropathy. PLoS ONE 2015, 10, e0120779. [Google Scholar] [CrossRef]
- Rossi, G.; Pengo, G.; Caldin, M.; Piccionello, A.P.; Steiner, J.M.; Cohen, N.D.; Jergens, A.E.; Suchodolski, J.S. Comparison of microbiological, histological, and immunomodulatory parameters in response to treatment with either combination therapy with prednisone and metronidazole or probiotic VSL#3 strains in dogs with idiopathic inflammatory bowel disease. PLoS ONE 2014, 9, e94699. [Google Scholar]
- Xu, H.; Zhao, F.; Hou, Q.; Huang, W.; Liu, Y.; Zhang, H.; Sun, Z. Metagenomic analysis revealed beneficial effects of probiotics in improving the composition and function of the gut microbiota in dogs with diarrhea. Food Funct. 2019, 10, 2618–2629. [Google Scholar] [CrossRef] [PubMed]
- Delucchi, L.; Fraga, M.; Zunino, P. Effect of the probiotic Lactobacillus murinus lbP2 on clinical parameters of dogs with distemper-associated diarrhea. Can. J. Vet. Res. 2017, 81, 118–121. [Google Scholar] [PubMed]
- Fernández, L.; Martínez, R.; Pérez, M.; Arroyo, R.; Rodríguez, J.M. Characterization of Lactobacillus rhamnosus MP01 and Lactobacillus plantarum MP02 and assessment of their potential for the prevention of gastrointestinal infections in an experimental canine model. Front. Microbiol. 2019, 10, 1117. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Razik, K.A.; Ibrahim, E.S.; Younes, A.M.; Arafa, A.A.; Abuelnaga, A.S.M.; Hedia, R.H. Enterococcus faecium isolated from healthy dogs for potential use as probiotics. Bulg. J. Vet. Med. 2020, 23, 197–205. [Google Scholar] [CrossRef]
- Kubašová, I.; Lauková, A.; Hamarová, Ľ.; Pristaš, P.; Strompfová, V. Evaluation of enterococci for potential probiotic utilization in dogs. Folia Microbiol. 2019, 64, 177–187. [Google Scholar] [CrossRef]
- Ibs, K.H.; Rink, L. Zinc-altered immune function. J. Nutr. 2003, 133, 1452S–1456S. [Google Scholar] [CrossRef] [PubMed]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.G.W.; Pires, E.; et al. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity 2019, 50, 432–445.e7. [Google Scholar] [CrossRef] [Green Version]
- Bobeck, E.A. Nutrition and health: Companion animal applications: Functional nutrition in livestock and companion animals to modulate the immune response. J. Anim. Sci. 2020, 98, skaa035. [Google Scholar] [CrossRef]
- Kandil, O.M.; Abou-Zeina, H.A. Effect of parenteral vitamin E and selenium supplementation on immune status of dogs vaccinated with subunit and somatic antigens against Taenia hydatigena. J. Egypt. Soc. Parasitol. 2005, 35, 537–550. [Google Scholar]
- Singh, S.K.; Dimri, U.; Saxena, S.K.; Jadhav, R.K. Therapeutic management of canine atopic dermatitis by combination of pentoxifylline and PUFAs. J. Vet. Pharmacol. Ther. 2010, 33, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Amaretti, A.; Raimondi, S. Folate production by probiotic bacteria. Nutrients 2011, 3, 118–134. [Google Scholar] [CrossRef] [Green Version]
- Hosomi, K.; Kunisawa, J. The specific roles of vitamins in the regulation of immunosurveillance and maintenance of immunologic homeostasis in the gut. Immune Netw. 2017, 17, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Ruaux, C.G. Cobalamin in companion animals: Diagnostic marker, deficiency states and therapeutic implications. Vet. J. 2013, 196, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Rutgers, H.C.; Batt, R.M.; Elwood, C.M.; Lamport, A. Small intestinal bacterial overgrowth in dogs with chronic intestinal disease. J. Am. Vet. Med. Assoc. 1995, 206, 187–193. [Google Scholar]
- Allenspach, K.; Wieland, B.; Gröne, A.; Gaschen, F. Chronic enteropathies in dogs: Evaluation of risk factors for negative outcome. J. Vet. Intern. Med. 2007, 21, 700–708. [Google Scholar] [CrossRef]
- Toresson, L.; Steiner, J.M.; Suchodolski, J.S.; Spillmann, T. Oral Cobalamin Supplementation in Dogs with Chronic Enteropathies and Hypocobalaminemia. J. Vet. Intern. Med. 2016, 30, 101–107. [Google Scholar] [CrossRef]
- Berghoff, N.; Parnell, N.K.; Hill, S.L.; Suchodolski, J.S.; Steiner, J.M. Serum cobalamin and methylmalonic acid concentrations in dogs with chronic gastrointestinal disease. Am. J. Vet. Res. 2013, 74, 84–89. [Google Scholar] [CrossRef]
- Taranto, M.P.; Vera, J.L.; Hugenholtz, J.; De Valdez, G.F.; Sesma, F. Lactobacillus reuteri CRL1098 produces cobalamin. J. Bacteriol. 2003, 185, 5643–5647. [Google Scholar] [CrossRef] [Green Version]
- Schauf, S.; de la Fuente, G.; Newbold, C.J.; Salas-Mani, A.; Torre, C.; Abecia, L.; Castrillo, C. Effect of dietary fat to starch content on fecal microbiota composition and activity in dogs. J. Anim. Sci. 2018, 96, 3684–3698. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Grieshop, C.M.; Flickinger, E.A.; Bauer, L.L.; Chow, J.; Wolf, B.W.; Garleb, K.A.; Fahey, G.C., Jr. Fructooligosaccharides and Lactobacillus acidophilus modify gut microbial populations, total tract nutrient digestibilities and fecal protein catabolite concentrations in healthy adult dogs. J. Nutr. 2002, 132, 3721–3731. [Google Scholar] [CrossRef] [Green Version]
- Kamath, P.S.; Hoepfner, M.T.; Phillips, S.F. Short-chain fatty acids stimulate motility of the canine ileum. Am. J. Physiol. 1987, 253 Pt 1, 427–433. [Google Scholar] [CrossRef]
- Suchodolski, J.S. Diagnosis and interpretation of intestinal dysbiosis in dogs and cats. Vet. J. 2016, 215, 30–37. [Google Scholar] [CrossRef]
- Suchodolski, J.S.; Markel, M.E.; Garcia-Mazcorro, J.F.; Unterer, S.; Heilmann, R.M.; Dowd, S.E.; Kachroo, P.; Ivanov, I.; Minamoto, Y.; Dillman, E.M.; et al. The fecal microbiome in dogs with acute diarrhea and idiopathic inflammatory bowel disease. PLoS ONE 2012, 7, e51907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minamoto, Y.; Minamoto, T.; Isaiah, A.; Sattasathuchana, P.; Buono, A.; Rangachari, V.R.; McNeely, I.H.; Lidbury, J.; Steiner, J.M.; Suchodolski, J.S. Fecal short-chain fatty acid concentrations and dysbiosis in dogs with chronic enteropathy. J. Vet. Intern. Med. 2019, 33, 1608–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guard, B.C.; Barr, J.W.; Reddivari, L.; Klemashevich, C.; Jayaraman, A.; Steiner, J.M.; Vanamala, J.; Suchodolski, J.S. Characterization of microbial dysbiosis and metabolomic changes in dogs with acute diarrhea. PLoS ONE 2015, 10, e0127259. [Google Scholar] [CrossRef] [Green Version]
- Gagné, J.W.; Wakshlag, J.J.; Simpson, K.W.; Dowd, S.E.; Latchman, S.; Brown, D.A.; Brown, K.; Swanson, K.S.; Fahey, G.C., Jr. Effects of a synbiotic on fecal quality, short-chain fatty acid concentrations, and the microbiome of healthy sled dogs. BMC Vet. Res. 2013, 9, 246. [Google Scholar] [CrossRef] [Green Version]
- Jensen, A.P.; Bjørnvad, C.R. Clinical effect of probiotics in prevention or treatment of gastrointestinal disease in dogs: A systematic review. J. Vet. Intern. Med. 2019, 33, 1849–1864. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, S.S. Value of probiotics in canine and feline gastroenterology. Vet. Clin. N. Am. Small Anim. Pract. 2021, 51, 171–217. [Google Scholar] [CrossRef] [PubMed]
- Wernimont, S.M.; Radosevich, J.; Jackson, M.I.; Ephraim, E.; Badri, D.V.; MacLeay, J.M.; Jewell, D.E.; Suchodolski, J.S. The effects of nutrition on the gastrointestinal microbiome of cats and dogs: Impact on health and disease. Front. Microbiol. 2020, 11, 1266. [Google Scholar] [CrossRef] [PubMed]
| Ref. | Model | Probiotic Used | Dose per Animal | Duration | Effect of Probiotic Supplementation |
|---|---|---|---|---|---|
| [46] | Healthy dogs (Probiotic group, n = 4; Placebo group, n = 4; Control group, n = 4) | Queso Blanco cheese containing Bifidobacterium longum KACC 91563 | 5 × 108 CFU/10 g cheese/day | 8 weeks | Increased the serum levels of IL-6 and TNF-α |
| [48] | Healthy dogs (Elderly probiotic group, n = 15; Elderly control group, n = 15; Young probiotic group, n = 12; Young control group, n = 12; Training probiotic group, n = 18; Training control group, n = 18) | Lactobacillus casei Zhang, Lactobacillus plantarum P-8, and Bifidobacterium animalis subsp. lactis V9 | Equal proportions of 3 probiotic strains in a final concentration of 2 × 109 CFU/g; Dosage: 10 g/day (Elderly group); 2 g/day (Young group); 4 g/day (Training group) | 60 days | Increased serum IgG, and IFNα levels, fecal SIgA levels in the probiotic-treated group |
| [61] | Healthy young dogs (Probiotic group, n = 7; Placebo group, n = 7) | Enterococcusfaecium | 5 × 108 CFU/day | 1 year | Increased the fecal IgA, and canine distemper virus vaccine-specific circulating IgG and IgA. Increased the proportion of mature B cells. |
| [62] | Healthy dogs (Probiotic group, n = 7; Placebo group, n = 6) | Lactobacillusmurinus LbP2 | 5 × 109 CFU/day; dosage on alternative days | 2 weeks | Increased the total fecal IgA concentration |
| [63] | Healthy dogs (Probiotic group, n = 5; Placebo group, n = 5) | Lactobacillusjohnsonii CPN23 | 2–3 × 108 CFU/day | 9 weeks | Improved the phytohaemagglutinin-P reaction and the concentration of acetate and butyrate. Reduced fecal ammonia. No change in antibody reaction to sheep erythrocytes. |
| Ref. | Model | Probiotic Used | Dose per Animal | Duration | Effect of Probiotic Supplementation |
|---|---|---|---|---|---|
| [19] | Dog puppies (Probiotic group, n = 9; Control group, n = 7) | Lactobacillusrhamnosus strain GG | 20 × 109 CFU per capsule; 10 capsules per day (mother); 5 capsules per day (puppy) | Prenatal and postnatal (mother dog at third week of gestation and continuing throughout lactation); postnatal (Starting at the age of 3 weeks until 6 months old) | Reduced the immunologic indicators of AD. No significant reduction in clinical signs of AD. |
| [65] | Dogs with AD (Probiotic group, n = 32; Placebo group, n = 10) | Lactobacillussakei probio-65 | 2 × 109 CFU/day | 2 months | Reduced the CADESI score |
| [66] | Dogs with AD (Probiotic group, n = 7; Placebo group, n = 4) | Bifidobacteriumlongum | 5 × 1010 CFU/day | 12 weeks | Reduced the CADESI score |
| Ref. | Model | Pro/Prebiotic Used | Dose per Animal | Duration | Effect of Probiotic Supplementation |
|---|---|---|---|---|---|
| [21] | Dogs with FRD (Probiotic group, n = 11; Placebo group, n = 10) | Lactobacillusacidophilus NCC2628, L. acidophilus NCC2766, Lactobacillus johnsonii NCC2767 | 1010 CFU of each probiotic strain/day | 4 weeks | Reduced Enterobacteriaceae count, Increased Lactobacillus spp. count, Improved clinical signs |
| [22] | Dogs with diarrhea (Probiotic group, n = 25; Placebo group, n = 19) | Lactobacillusfermentum VET 9A, Lactobacillus rhamnosus VET 16A, and Lactobacillus plantarum VET 14A | Daily dose: 2 × 109 CFU/mL of 3 bacterial strains | 7 days | Improved the stool consistency, normalized the appetite, decreased Clostridium perfringens and Enterococcus faecium count |
| [23] | Dogs with acute diarrhea (Probiotic group, n = 57; Placebo group, n = 61) | E.faecium 4b1707 | 2 × 108 CFU/gram paste; dosage according to body weight; 3 times per day (every 8 h) | 10 days | Reduced the duration of diarrhea and improved acute diarrhea |
| [24] | Dogs with ACD (Probiotic group, n = 19; Placebo group, n = 19; Metronidazole group, n = 19) | Bifidobacterium bifidum VPBB-6, Bifidobacterium longum VPBL-5, Bifidobacterium animalis VPBA-4, Bifidobacterium infantis VPBI-6, L. acidophilus VPLA-4, L. plantarum VPLP-5, Lactobacillus casei VPLC-1, Lactobacillus brevis VPLB-5, Lactobacillus reuteri VPLR-1, and Lactobacillus bulgaricus VPLB-7. | 30 × 109 CFU/425 mg/capsule *; 125 mg/4–10 kg body weight; 250 mg/10.1–20 kg body weight; 400 mg/20.1–45 kg body weight; dose twice a day | 10 days | Improved stool consistency |
| [25] | Dogs with diarrhea (Probiotic group, n = 16; Placebo group, n = 16) | Metronidazole and E. faecium SF68 | 5 × 108 CFU/day | 7 days | Eliminated Giardia cysts and cured diarrhea |
| [67] | Dogs with chronic enteropathies (Probiotic group, n = 6; Placebo group, n = 7) | Saccharomyces boulardii | 1 × 109 CFU/kg/12 h | 60 days | CCECAI, fecal consistency and frequency, and body condition score were improved. |
| [68] | Dogs with chronic enteropathy (n = 12; Diseased dogs during 1st visit (Control) and 2nd visit (Treatment) after 6 weeks of treatment | E.faecium and FOS | 1 × 109 CFU/day | 6 weeks | No change in the expression of the inflammasome or its components |
| [69] | Dogs with IBD (Probiotic group, n = 10; Control group, n = 10) | VSL#3 (L. casei, L. plantarum. L. acidophilus, and Lactobacillus delbrueckii subsp. bulgaricus, B. longum, Bifidobacterium breve, B. infantis, and Streptococcus salivarius subsp. thermophilus) | 112–225 × 109 CFU/10 kg/day | 60 days | Decreased the clinical and histological scores. Normalized dysbiosis. Enhanced regulatory T-cell markers. |
| [70] | Dogs with diarrhea (Probiotic group, n = 20; Placebo group, n = 20) | L.casei Zhang, L. plantarum P-8, and Bifidobacterium animalis subsp. lactis V9 | Probiotic mixture (equal proportion of 3 probiotic strain) containing 3 × 109 CFU/g (each Lactobacillus strain), 4 × 109 CFU/g (Bifidobacterium strain); Dosage: 10 g/day (elderly dogs); 4 g/day (young dogs); 2 g/day (adult dogs) | 60 days | Increased the abundance of L. johnsonii, L. reuteri, L. acidophilus, Butyricicoccus pullicaecorum Reduced the count of C. perfringens and Stenotrophomonas maltophilia |
| [71] | Dogs with DAD (Probiotic group, n = 13; Placebo group, n = 7) | Lactobacillusmurinus LbP2 | 5 × 109 CFU/day | 5 days | Improved the clinical score |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. Influence of Probiotic Supplementation on Health Status of the Dogs: A Review. Appl. Sci. 2021, 11, 11384. https://doi.org/10.3390/app112311384
Sivamaruthi BS, Kesika P, Chaiyasut C. Influence of Probiotic Supplementation on Health Status of the Dogs: A Review. Applied Sciences. 2021; 11(23):11384. https://doi.org/10.3390/app112311384
Chicago/Turabian StyleSivamaruthi, Bhagavathi Sundaram, Periyanaina Kesika, and Chaiyavat Chaiyasut. 2021. "Influence of Probiotic Supplementation on Health Status of the Dogs: A Review" Applied Sciences 11, no. 23: 11384. https://doi.org/10.3390/app112311384
APA StyleSivamaruthi, B. S., Kesika, P., & Chaiyasut, C. (2021). Influence of Probiotic Supplementation on Health Status of the Dogs: A Review. Applied Sciences, 11(23), 11384. https://doi.org/10.3390/app112311384








