Particulate Matter Contamination of Bee Pollen in an Industrial Area of the Po Valley (Italy)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
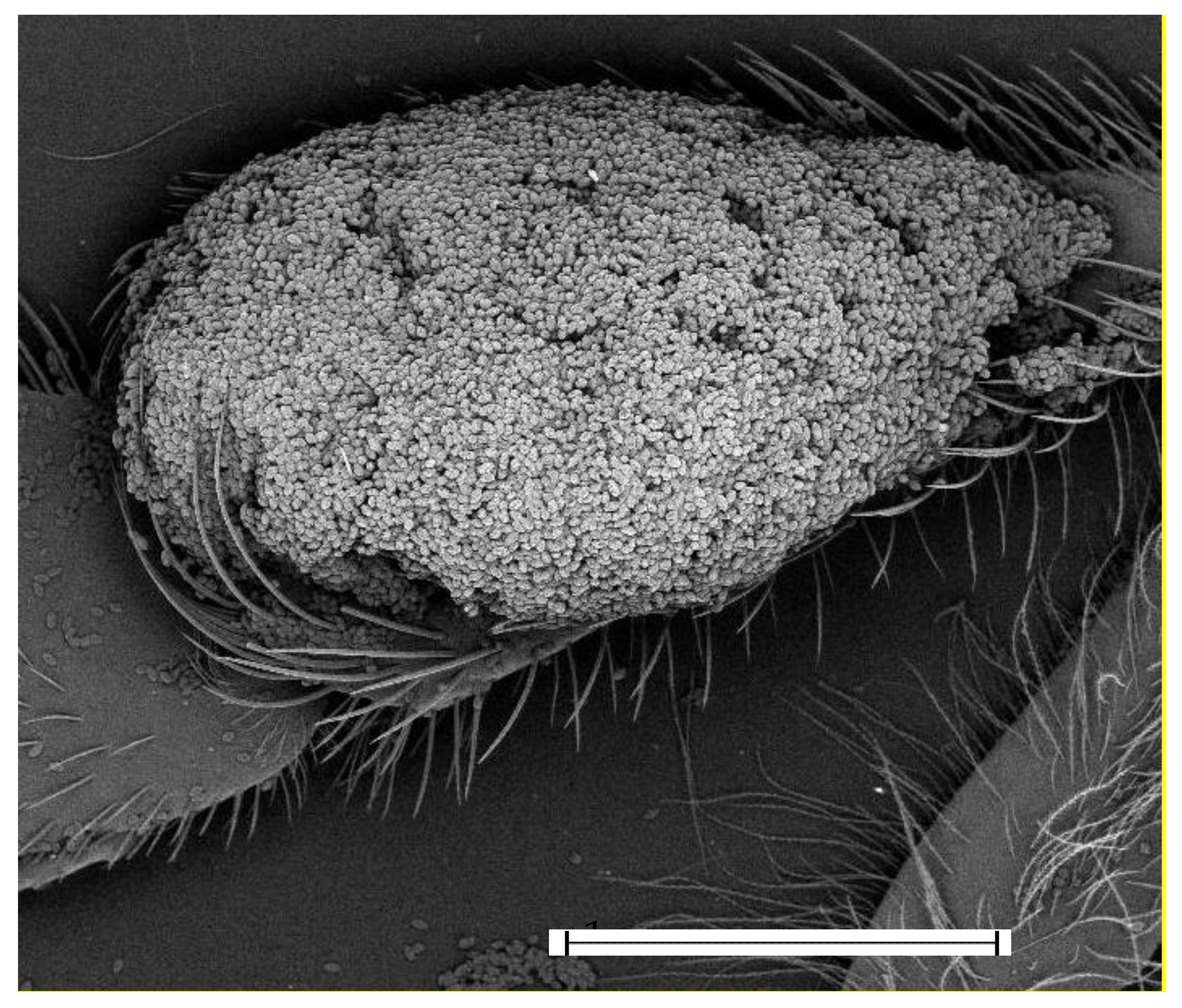
2.2. Scanning Electron Microscopy with Energy Dispersive X-ray Spectrometry Investigation
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thakur, M.; Nanda, V. Composition and functionality of bee pollen: A review. Trends Food Sci. Technol. 2020, 98, 82–106. [Google Scholar] [CrossRef]
- Denisow, B.; Denisow-Pietrzyk, M. Biological and therapeutic properties of bee pollen: A review. J. Sci. Food Agric. 2016, 96, 4303–4309. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elashal, M.H.; Yosri, N.; Du, M.; Musharraf, S.G.; Nahar, L.; Sarker, S.D.; Guo, Z.; Cao, W.; Zou, X.; et al. Bee Pollen: Current Status and Therapeutic Potential. Nutrients 2021, 13, 1876. [Google Scholar] [CrossRef]
- Mărgăoan, R.; Stranț, M.; Varadi, A.; Topal, E.; Yücel, B.; Cornea-Cipcigan, M.; Campos, M.G.; Vodnar, D.C. Bee Collected Pollen and Bee Bread: Bioactive Constituents and Health Benefits. Antioxidants 2019, 8, 568. [Google Scholar] [CrossRef] [Green Version]
- Papa, G.; Capitani, G.; Capri, E.; Pellecchia, M.; Negri, I. Vehicle-derived ultrafine particulate contaminating bees and bee products. Sci. Total Environ. 2021, 750, 141700. [Google Scholar] [CrossRef]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A.; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the american heart association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lomer, M.C.E.; Thompson, R.P.H.; Powell, J.J. Fine and ultrafine particles of the diet: Influence on the mucosal immune response and association with Crohn’s disease. Proc. Nutr. Soc. 2002, 61, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salim, S.Y.; Jovel, J.; Wine, E.; Kaplan, G.G.; Vincent, R.; Thiesen, A.; Barkema, H.W.; Madsen, K.L. Exposure to Ingested Airborne Pollutant Particulate Matter Increases Mucosal Exposure to Bacteria and Induces Early Onset of Inflammation in Neonatal IL-10–Deficient Mice. Inflamm. Bowel Dis. 2014, 20, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Kish, L.; Hotte, N.; Kaplan, G.G.; Vincent, R.; Tso, R.; Gänzle, M.; Rioux, K.P.; Thiesen, A.; Barkema, H.W.; Wine, E.; et al. Environmental Particulate Matter Induces Murine Intestinal Inflammatory Responses and Alters the Gut Microbiome. PLoS ONE 2013, 8, e62220. [Google Scholar] [CrossRef] [Green Version]
- Woodby, B.; Schiavone, M.L.; Pambianchi, E.; Mastaloudis, A.; Shelly, N.H.; Wood, S.M.; Pecorelli, A.; Valacchi, G. Particulate Matter Decreases Intestinal Barrier-Associated Proteins Levels in 3D Human Intestinal Model. Int. J. Environ. Res. Public Health 2020, 17, 3234. [Google Scholar] [CrossRef]
- Papa, G.; Di Prisco, G.; Spini, G.; Puglisi, E.; Negri, I. Acute and chronic effects of Titanium dioxide (TiO2) PM1 on honey bee gut microbiota under laboratory conditions. Sci. Rep. 2021, 11, 5946. [Google Scholar] [CrossRef]
- AL Naggar, Y.; Dabour, K.; Masry, S.; Sadek, A.; Naiem, E.; Giesy, J.P. Sublethal effects of chronic exposure to CdO or PbO nanoparticles or their binary mixture on the honey bee (Apis millefera L.). Environ. Sci. Pollut. Res. 2020, 27, 19004–19015. [Google Scholar] [CrossRef]
- Marcazzan, G.M.; Vaccaro, S.; Valli, G.; Vecchi, R. Characterisation of PM10 and PM2.5 particulate matter in the ambient air of Milan (Italy). Atmos. Environ. 2001, 35, 4639–4650. [Google Scholar] [CrossRef]
- Pietrogrande, M.C.; Bacco, D.; Ferrari, S.; Ricciardelli, I.; Scotto, F.; Trentini, A.; Visentin, M. Characteristics and major sources of carbonaceous aerosols in PM2.5 in Emilia Romagna Region (Northern Italy) from four-year observations. Sci. Total Environ. 2016, 553, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Pozzer, A.; Bacer, S.; Sappadina, S.D.Z.; Predicatori, F.; Caleffi, A. Long-term concentrations of fine particulate matter and impact on human health in Verona, Italy. Atmos. Pollut. Res. 2019, 10, 731–738. [Google Scholar] [CrossRef]
- Capitani, G.; Papa, G.; Pellecchia, M.; Negri, I. Disentangling multiple PM emission sources in the Po Valley (Italy) using honey bees. Heliyon 2021, 7, e06194. [Google Scholar] [CrossRef]
- Satta, A.; Verdinelli, M.; Ruiu, L.; Buffa, F.; Salis, S.; Sassu, A.; Floris, I. Combination of beehive matrices analysis and ant biodiversity to study heavy metal pollution impact in a post-mining area (Sardinia, Italy). Environ. Sci. Pollut. Res. 2012, 19, 3977–3988. [Google Scholar] [CrossRef] [PubMed]
- Perugini, M.; Manera, M.; Grotta, L.; Abete, M.C.; Tarasco, R.; Amorena, M. Heavy Metal (Hg, Cr, Cd, and Pb) Contamination in Urban Areas and Wildlife Reserves: Honeybees as Bioindicators. Biol. Trace Elem. Res. 2011, 140, 170–176. [Google Scholar] [CrossRef]
- van der Steen, J.J.M.; de Kraker, J.; Grotenhuis, T. Spatial and temporal variation of metal concentrations in adult honeybees (Apis mellifera L.). Environ. Monit. Assess. 2012, 184, 4119–4126. [Google Scholar] [CrossRef] [Green Version]
- Zarić, N.M.; Ilijevic, K.; Stanisavljevic, L.; Grzetic, I. Use of honeybees (Apis mellifera L.) as bioindicators of spatial variations and origin determination of metal pollution in Serbia. J. Serb. Chem. Soc. 2018, 83, 773–784. [Google Scholar] [CrossRef]
- Negri, I.; Mavris, C.; Di Prisco, G.; Caprio, E.; Pellecchia, M. Honey bees (Apis mellifera, L.) as active samplers of airborne particulate matter. PLoS ONE 2015, 10, e0132491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellecchia, M.; Negri, I. Particulate matter collection by honey bees (Apis mellifera, L.) near to a cement factory in Italy. PeerJ 2018, 2018, e5322. [Google Scholar] [CrossRef] [Green Version]
- QGIS. A QGIS Geographic Information System. 2021. [Google Scholar]
- Edo, C.; Fernández-Alba, A.R.; Vejsnæs, F.; van der Steen, J.J.M.; Fernández-Piñas, F.; Rosal, R. Honeybees as active samplers for microplastics. Sci. Total Environ. 2021, 767, 144481. [Google Scholar] [CrossRef] [PubMed]
- Kutchko, B.G.; Kim, A.G. Fly ash characterization by SEM-EDS. Fuel 2006, 85, 2537–2544. [Google Scholar] [CrossRef]
- Alam, Q.; Schollbach, K.; van Hoek, C.; van der Laan, S.; de Wolf, T.; Brouwers, H.J.H. In-depth mineralogical quantification of MSWI bottom ash phases and their association with potentially toxic elements. Waste Manag. 2019, 87, 1–12. [Google Scholar] [CrossRef]
- Morf, L.S.; Gloor, R.; Haag, O.; Haupt, M.; Skutan, S.; Di Lorenzo, F.; Böni, D. Precious metals and rare earth elements in municipal solid waste—Sources and fate in a Swiss incineration plant. Waste Manag. 2013, 33, 634–644. [Google Scholar] [CrossRef]
- Muchova, L.; Bakker, E.; Rem, P. Precious metals in municipal solid waste incineration bottom ash. Water Air Soil Pollut. Focus 2009, 9, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Li, W.; Tam, V.W.Y.; Xue, C. Advanced progress in recycling municipal and construction solid wastes for manufacturing sustainable construction materials. Resour. Conserv. Recycl. X 2020, 6, 100036. [Google Scholar] [CrossRef]
- Carrero, J.A.; Arana, G.; Madariaga, J.M. Chapter 6. Use of Raman spectroscopy and scanning electron microscopy for the detection and analysis of road transport pollution. In Spectroscopic Properties of Inorganic and Organometallic Compounds; Royal Society of Chemistry: London, UK, 2014; Volume 45, pp. 178–210. ISBN 9781849739191. [Google Scholar]
- Gonet, T.; Maher, B.A. Airborne, Vehicle-Derived Fe-Bearing Nanoparticles in the Urban Environment: A Review. Environ. Sci. Technol. 2019, 53, 9970–9991. [Google Scholar] [CrossRef]
- Lee, W.K.; Rhee, T.H.; Kim, H.S.; Jang, H. Effects of antimony trisulfide (Sb2S3) on sliding friction of automotive brake friction materials. Met. Mater. Int. 2013, 19, 1101–1107. [Google Scholar] [CrossRef]
- Österle, W.; Griepentrog, M.; Gross, T.; Urban, I. Chemical and microstructural changes induced by friction and wear of brakes. Wear 2001, 251, 1469–1476. [Google Scholar] [CrossRef]
- Neis, P.D.; Ferreira, N.F.; Fekete, G.; Matozo, L.T.; Masotti, D. Towards a better understanding of the structures existing on the surface of brake pads. Tribol. Int. 2017, 105, 135–147. [Google Scholar] [CrossRef]
- Menapace, C.; Leonardi, M.; Matějka, V.; Gialanella, S.; Straffelini, G. Dry sliding behavior and friction layer formation in copper-free barite containing friction materials. Wear 2018, 398–399, 191–200. [Google Scholar] [CrossRef]
- Adamiec, E.; Jarosz-Krzemińska, E.; Wieszała, R. Heavy metals from non-exhaust vehicle emissions in urban and motorway road dusts. Environ. Monit. Assess. 2016, 188, 369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, K.E.; Weis, D.; Amini, M.; Shiel, A.E.; Lai, V.W.M.; Gordon, K. Honey as a biomonitor for a changing world. Nat. Sustain. 2019, 2, 223–232. [Google Scholar] [CrossRef]





| Analyzed PM | Natural Source | Anthropogenic Source | May | June | July | August |
|---|---|---|---|---|---|---|
| Clay minerals | + | agriculture | + | + | + | + |
| Calcite | + | incinerator, agriculture | + | + | + | + |
| Quartz | + | incinerator, agriculture | + | + | + | + |
| Iron oxides/Hydroxides | traffic | + | + | + | + | |
| Barite | traffic | + | + | + | + | |
| Feldspars | + | agriculture | + | + | ||
| Fe-alloy (Fe-Cr; Fe-Cr-Mn) | traffic | + | + | |||
| Spherical dioxide | incinerator | + | ||||
| Heavy metals (Zn, Pb, Sb, Sn) | traffic, incinerator | + | ||||
| Gold | incinerator | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papa, G.; Capitani, G.; Pellecchia, M.; Negri, I. Particulate Matter Contamination of Bee Pollen in an Industrial Area of the Po Valley (Italy). Appl. Sci. 2021, 11, 11390. https://doi.org/10.3390/app112311390
Papa G, Capitani G, Pellecchia M, Negri I. Particulate Matter Contamination of Bee Pollen in an Industrial Area of the Po Valley (Italy). Applied Sciences. 2021; 11(23):11390. https://doi.org/10.3390/app112311390
Chicago/Turabian StylePapa, Giulia, Giancarlo Capitani, Marco Pellecchia, and Ilaria Negri. 2021. "Particulate Matter Contamination of Bee Pollen in an Industrial Area of the Po Valley (Italy)" Applied Sciences 11, no. 23: 11390. https://doi.org/10.3390/app112311390
APA StylePapa, G., Capitani, G., Pellecchia, M., & Negri, I. (2021). Particulate Matter Contamination of Bee Pollen in an Industrial Area of the Po Valley (Italy). Applied Sciences, 11(23), 11390. https://doi.org/10.3390/app112311390







