Is the Treatment of the Tear Trough Deformity with Hyaluronic Acid Injections a Safe Procedure? A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
2.4. Study Selection
2.5. Data Collection Process
2.6. Data Items
2.7. Study Risk of Bias Assessment
2.8. Summary Measures
2.9. Additional Analyses
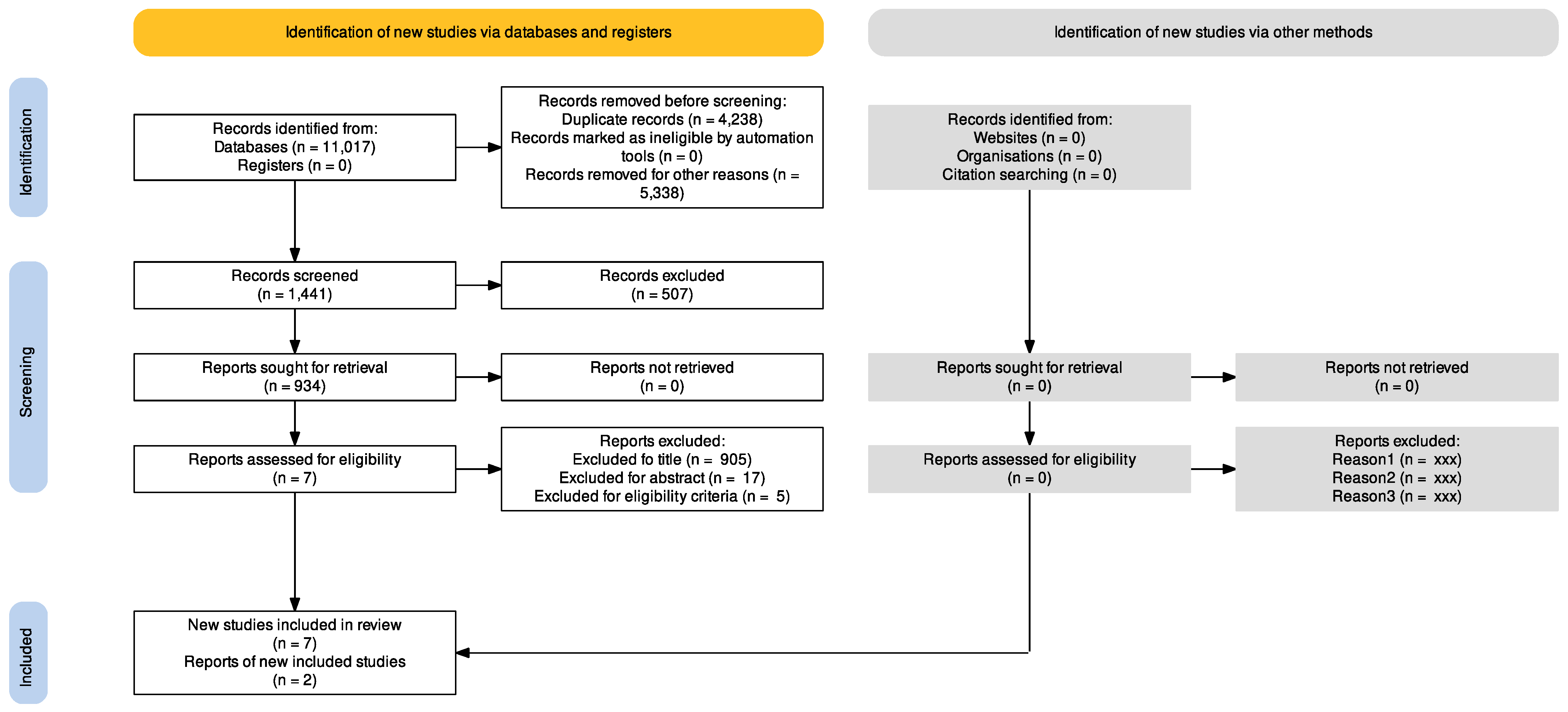
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias within Studies
3.4. Results of Individual Studies
3.5. Results of Synthesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldberg, R.A.; Fiaschetti, D. Filling the periorbital hollows with hyaluronic acid gel: Initial experience with 244 injections. Ophthalmic Plast. Reconstr. Surg. 2006, 22, 335–341. [Google Scholar] [CrossRef]
- Lim, H.K.; Suh, D.H.; Lee, S.J.; Shin, M.K. Rejuvenation effects of hyaluronic acid injection on nasojugal groove: Prospective randomized split face clinical controlled study. J. Cosmet. Laser Ther. 2014, 16, 32–36. [Google Scholar] [CrossRef]
- Flowers, R.S. Periorbital aesthetic surgery for men. Eyelids and related structures. Clin. Plast. Surg. 1991, 18, 689–729. [Google Scholar] [CrossRef]
- Hirmand, H. Anatomy and nonsurgical correction of the tear trough deformity. Plast. Reconstr. Surg. 2010, 125, 699–708. [Google Scholar] [CrossRef] [Green Version]
- Lambros, V.S. Hyaluronic acid injections for correction of the tear trough deformity. Plast. Reconstr. Surg. 2007, 120 (Suppl. S6), 74–80. [Google Scholar] [CrossRef] [Green Version]
- Pieres Viana, G.A.; Hentona Osaki, M.; Cariello, A.J.; Wendell Damasceno, R.; Hentona Osaki, T. Treatment of the tear trough deformity with hyaluronic acid. Aesthet. Surg. J. 2011, 31, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Rauso, R.; Zerbinati, N.; Fragola, R.; Nicoletti, G.F.; Tartaro, G. Transvascular hydrolisis oh hyaluronic acid filler with hyalu-ronidase. An ex vivo study. Dermatol. Surg. 2021, 47, 370–372. [Google Scholar]
- Rauso, R.; Colella, G.; Franco, R.; Chirico, F.; Ronchi, A.; Federico, F.; Volpicelli, A.; Tartaro, G. Is hyaluronidase able to reverse embolism associated with hyaluronic acid filler? An anatomical case study. J. Biol. Regul. Homeost. Agents 2019, 33, 1927–1930. [Google Scholar]
- Berguiga, M.; Galatoire, O. Tear trough rejuvenation: A safety evaluation of the treatment by a semi-cross-linked hyaluronic acid filler. Orbit 2017, 36, 22–26. [Google Scholar] [CrossRef]
- Pascali, M.; Quarato, D.; Pagnoni, M.; Carinci, F. Tear trough deformity: Study of filling procedures for its correction. J. Craniofac. Surg. 2017, 28, 2012–2015. [Google Scholar] [CrossRef]
- Bektas, G.; Cinpolat, A.; Rizvanovic, Z. Nasal filling in plastic surgery practice: Primary nasal filling, nasal filling for post-rhinoplasty defects, rhinoplasty after hyaluronidase injection in dissatisfied nasal filling patients. Aesthet. Plast. Surg. 2020, 44, 2208–2218. [Google Scholar] [CrossRef]
- Mochizuki, M.; Aoi, N.; Gonda, K.; Hirabayashi, S.; Komuro, Y. Evaluation of the in vivo kinetics and biostimulatory effects of subcutaneously injected hyaluronic acid filler. Plast. Reconstr. Surg. 2018, 142, 112–121. [Google Scholar] [CrossRef]
- Hexsel, D.; Soirefmann, M.; Porto, M.D.; Siega, C.; Schilling-Souza, J.; Brum, C. Double-blind, randomized, controlled clinical trial to compare safety and efficacy of a metallic cannula with that of a standard needle for soft tissue augmentation of the nasolabial folds. Dermatol. Surg. 2012, 38, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Rauso, R.; Gherardini, G.; Parlato, V.; Amore, R.; Tartaro, G. Polyacrylamide gel for facial wasting rehabilitation: How many milliliters per session? Aesthet. Plast. Surg. 2012, 36, 174–179. [Google Scholar] [CrossRef]
- Rauso, R.; Curinga, G.; Rusciani, A.; Colella, G.; Amore, R.; Tartaro, G. Safety and efficacy of one-step rehabilitation of human immunodeficiency virus-related facial lipoatrophy using an injectable calcium hydroxylapatite dermal filler. Dermatol. Surg. 2013, 39, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Rauso, R.; Zerbinati, N.; Franco, R.; Chirico, F.; Ronchi, A.; Sesenna, E.; Colella, G.; Tartaro, G. Cross-linked hyaluronic acid filler hydrolisis with hyaluronidase. Different setting to reproduce different clinical scenarios. Dermatol. Ther. 2020, 33, e13269. [Google Scholar] [CrossRef] [PubMed]
- Chatrath, V.; Banerjee, P.S.; Goodman, G.J.; Rahman, E. Soft-tissue filler–associated blindness: A systematic review of case reports and case series. Plast. Reconstr. Surg.-Glob. Open 2019, 7, e2173. [Google Scholar] [CrossRef] [PubMed]
- Rauso, R.; Sesenna, E.; Fragola, R.; Zerbinati, N.; Nicoletti, G.F.; Tartaro, G. Skin necrosis and vision loss or impairment after facial filler injection. J. Craniofac. Surg. 2020, 31, 2289–2293. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Berros, P. Periorbital contour abnormalities: Hollow eye ring management with hyalurostructure. Orbit 2010, 29, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Berros, P.; Lax, L.; Bétis, F. Hyalurostructure treatment: Superior clinical outcome through a new protocol-a 4-year comparative study of two methods for tear trough treatment. Plast. Reconstr. Surg. 2013, 132, 924–931. [Google Scholar] [CrossRef]
- Mustak, H.; Fiaschetti, D.; Goldberg, R.A. Filling the periorbital hollows with hyaluronic acid gel: Long-term review of outcomes and complications. J. Cosmet. Dermatol. 2018, 17, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.B.; Roy, S.; Buckingham, E.D. Novel use of a volumizing hyaluronic acid filler for treatment of infraorbital hollows. JAMA Facial Plast. Surg. 2018, 20, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.N.; Mangal, S.; Goodman, G.J. The Tick technique: A method to simplify and quantify treatment of the tear trough region. J. Cosmet. Dermatol. 2019, 18, 1642–1647. [Google Scholar] [CrossRef]
- Diwan, Z.; Trikha, S.; Etemad-Shahidi, S.; Alli, Z.; Rennie, C.; Penny, A. A prospective study on safety, complications and satisfaction analysis for tear trough rejuvenation using hyaluronic acid dermal fillers. Plast. Reconstr. Surg.-Glob. Open 2020, 8, 1–7. [Google Scholar] [CrossRef]
- Shah-Desai, S.; Joganathan, V. Novel technique of non-surgical rejuvenation of infraorbital dark circles. J. Cosmet. Dermatol. 2021, 20, 1214–1220. [Google Scholar] [CrossRef]
- Rauso, R.; Nicoletti, G.F.; Zerbinati, N.; Giudice, G.L.; Fragola, R.; Tartaro, G. Complications following self-administration of hyaluronic acid fillers: Literature review. Clin. Cosmet. Investig. Dermatol. 2020, 13, 767–771. [Google Scholar] [CrossRef]
- Chirico, F.; Colella, G.; Cortese, A.; Bove, P.; Fragola, R.; Rugge, L.; Audino, G.; Sgaramella, N.; Tartaro, G. Non-surgical touch-up with hyaluronic acid fillers following facial reconstructive surgery. Appl. Sci. 2021, 11, 7507. [Google Scholar] [CrossRef]
- Rauso, R.; Tartaro, G.; Chirico, F.; Zerbinati, N.; Albani, G.; Rugge, L. Rhinofilling with hyaluronic acid thought as a cartilage graft. J. Cranio-Maxillofac. Surg. 2020, 48, 223–228. [Google Scholar] [CrossRef]
- Rauso, R.; Federico, F.; Zerbinati, N.; De Cicco, D.; Nicoletti, G.F.; Tartaro, G. Hyaluronic acid injections to correct lips deformity following surgical removal of permanent implant. J. Craniofac. Surg. 2020, 31, e604–e606. [Google Scholar] [CrossRef] [PubMed]
- Graf, R.; Pace, D. Tear trough treatment with orbicularis oculi muscle suspension. Aesthet. Plast. Surg. 2021, 45, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Terino, E.O. Alloplastic midface augmentation. Aesthet. Surg. J. 2005, 25, 512–520. [Google Scholar] [CrossRef] [Green Version]
- Rusciani Scorza, A.; Rusciani Scorza, L.; Troccola, A.; Micci, D.M.; Rauso, R.; Curinga, G. Autologous fat transfer for face rejuvenation with tumescent technique fat harvesting and saline washing: A report of 215 cases. Dermatology 2012, 224, 244–250. [Google Scholar] [CrossRef]
- Bernardini, F.P.; Cetinkaya, A.; Devoto, M.H.; Zambelli, A. Calcium hydroxyl-apatite (Radiesse) for the correction of periorbital hollows, dark circles, and lower eyelid bags. Ophthalmic Plast. Reconstr. Surg. 2014, 30, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Di Girolamo, M.; Mattei, M.; Signore, A.; Grippaudo, F.R. MRI in the evaluation of facial dermal fillers in normal and complicated cases. Eur. Radiol. 2015, 25, 1431–1442. [Google Scholar] [CrossRef]
- Rauso, R.; Bove, R.; Rugge, L.; Chirico, F. Unusual intraoral necrosis after hyaluronic acid injection. Dermatol. Surg. 2021, 47, 1158–1160. [Google Scholar] [CrossRef]
- Sharad, J. Dermal fillers for the treatment of tear trough deformity: A review of anatomy, treatment techniques, and their outcomes. J. Cutan. Aesthet. Surg. 2012, 5, 229–238. [Google Scholar] [CrossRef]
- Kim, D.W.; Yoon, E.S.; Ji, Y.H.; Park, S.H.; Lee, B.I.; Dhong, E.S. Vascular complications of hyaluronic acid fillers and the role of hyaluronidase in management. J. Plast. Reconstr. Aesthet. Surg. 2011, 64, 1590–1595. [Google Scholar] [CrossRef]
- Nanda, S.; Bansal, S.; Lakhani, R. Use of Hyaluronic acid fillers in treatment of periorbital melanosis induced by tear trough deformity: Anatomical considerations, patient satisfaction, and management of complications. J. Cosmet. Dermatol. 2021, 1–9. [Google Scholar] [CrossRef]
- Murthy, R.; Roos, J.C.P.; Goldberg, R.A. Periocular hyaluronic acid fillers: Applications, implications, complications. Curr. Opin. Ophthalmol. 2019, 30, 395–400. [Google Scholar] [CrossRef]
- Jitaree, B.; Phumyoo, T.; Uruwan, S.; Sawatwong, W.; McCormick, L.; Tansatit, T. The feasibility determination of risky severe complications of arterial vasculature regarding the filler injection sites at the tear trough. Plast. Reconstr. Surg. 2018, 142, 1153–1163. [Google Scholar] [CrossRef]
- Vedamurthy, M. Beware what you inject: Complications of injectables—Dermal fillers. J. Cutan. Aesthet. Surg. 2018, 11, 60–66. [Google Scholar] [CrossRef]
- Hill, R.H.; Czyz, C.N.; Kandapalli, S.; Zhang-Nunes, S.X.; Cahill, K.V.; Wulc, A.E.; Foster, J.A. Evolving minimally invasive techniques for tear trough enhancement. Ophthalmic Plast. Reconstr. Surg. 2015, 31, 306–309. [Google Scholar] [CrossRef]
- Lee, J.H.; Hong, G. Definitions of groove and hollowness of the infraorbital region and clinical treatment using soft-tissue filler. Arch. Plast. Surg. 2018, 45, 214–221. [Google Scholar] [CrossRef] [Green Version]
- Scheuer, J.F.; Sieber, D.A.; Pezeshk, R.A.; Campbell, C.F.; Gassman, A.A.; Rohrich, R.J. Anatomy of the facial danger zones: Maximizing safety during soft-tissue filler injections. Plast. Reconstr. Surg. 2017, 139, 50e–58e. [Google Scholar] [CrossRef]
- Edsman, K.; Nord, L.I.; Öhrlund, Å.; Lärkner, H.; Kenne, A.H. Gel properties of hyaluronic acid dermal fillers. Dermatol. Surg. 2012, 38, 1170–1179. [Google Scholar] [CrossRef]
- Kablik, J.; Monheit, G.D.; Yu, L.P.; Chang, G.; Gershkovich, J. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol. Surg. 2009, 35 (Suppl. 1), 302–312. [Google Scholar] [CrossRef]
- Lafaille, P.; Benedetto, A. Fillers: Contraindications, side effects and precautions. J. Cutan. Aesthet. Surg. 2010, 3, 16–19. [Google Scholar] [CrossRef]
- Niamtu, J. Complications in fillers and botox. Oral Maxillofac. Surg. Clin. N. Am. 2009, 21, 13–21. [Google Scholar] [CrossRef]
- King, M. Management of tyndall effect. J. Clin. Aesthetic Dermatol. 2016, 9, E6–E8. [Google Scholar]
- Delorenzi, C. Complications of injectable fillers, part I. Aesthet. Surg. J. 2013, 33, 561–575. [Google Scholar] [CrossRef] [Green Version]
- Kane, M.A.C. Treatment of tear trough deformity and lower lid bowing with injectable hyaluronic acid. Aesthet. Plast. Surg. 2005, 29, 363–367. [Google Scholar] [CrossRef] [PubMed]


| Title | Authors | Type of Study | Numbers of Patients | Type of Filler Applied | Volume | Injection Layer | Complications |
|---|---|---|---|---|---|---|---|
| Treatment of the Tear Trough Deformity With Hyaluronic Acid | Giovanni Andrè Pires Viana et al., 2010 | Prospective clinical trial | 25 patients | Restylane (Galderma, Fort Worth, TX, USA) | Total injection volume per side (baseline and touch-ups) was 0.1 to 1.1 mL on the right side and 0.2 to 1.2 mL on the left side. | Pre-periosteal tissues immediately inferior to the orbital rim, with 30-gauge needle. |
|
| Tear trough rejuvenation: A safety evaluation of the treatment by a semi-cross-linked hyaluronic acid filler | Berguiga et al., 2017 | Prospective multicenter clinical trial | 151 patients | Teosyal® PureSense Redensity 2 (TEOXANE SA, Geneva, Switzerland) | Mean volume of 0.48 mL for side (range, 0.1–1.0 mL) |
| At the first visit post-treatment on 151 patients:
|
| A Prospective Study on Safety, Complications and Satisfaction Analysis for Tear Trough Rejuvenation Using Hyaluronic Acid Dermal Fillers | Diwan et al., 2020 | Prospective study | 24 patients | Teosyal Puresense Redensity 2 (TEOXANE SA, Geneva, Switzerland) | 0.2 to 0.6 mL for side | Supra-periosteal injection using cannula with microdroplet +/− linear threading technique |
|
| Filling the periorbital hollows with hyaluronic acid gel: Long-term review of outcomes and complications | Mustak et al., 2017 | Retrospective case review | 147 patients | Restylane (Galderma, Fort Worth, TX, USA) | Mean 3.19 mL Min 1.2 mL Max 9.7 mL | Fanning technique using a needle 30-gauge in the suborbicularis plane |
|
| The Tick technique: A method to simplify and quantify treatment of the tear trough region | Hussain et al., 2019 | Interventional non-randomized observational study | 150 patients | Juvederm Ultra plus XC (Allergan Inc., Dublin, Ireland) | The volume injected was different according to their grade of depression: 0.3 mL for Hirmand grade 1, 0.4 mL for grade 2, and 0.5 mL for grade 3 | Tick technique based on just three bolus injections at the supraperiosteal level, with 31 gauge needle. | Immediately after injection: −12/150 patients (8.0%) had swelling,
|
| Novel Use of a Volumizing Hyaluronic Acid Filler for Treatment of Infraorbital Hollows | Hall et al., 2018 | Retrospective observational study | 101 patients | Juvederm Voluma XC (Allergan Inc., Dublin, Ireland) | The volume injected was 1.0 mL, 0.5 mL for each side. Touch-up in 18 patients, with 0.9 mL in total (range 0.5–1.0 mL) | Microcannula 27-gauge in the supraperiosteal or submuscular plane | Immediately after injection:
|
| Novel technique of non-surgical rejuvenation of infraorbital dark circles. | Desai et al., 2021 | Retrospective case note review | 165 patients | Restylane Vital light (Galderma, Watford, UK) | Amount of product used range (0.1–0.2 mL for each side) | Needle 31-gauge 4 mm, sub dermal layer, using a serial puncture injection technique |
|
| Periorbital Contour Abnormalities: Hollow EyeRing Management with Hyalurostructure | P. Berros, 2010 | Prospective study | 26 patients | Restylane (Galderma, Fort Worth, TX, USA) | Amount of product used 0.8–1.0 mL. | Microcannula injection 40mm long, parallel to periosteum layer. |
|
| Hyalurostructure Treatment: Superior ClinicalOutcome through a New Protocol—A 4-YearComparative Study of Two Methods for TearTrough Treatment | Berros et al., 2013 | Retrospective study | Group A 41 patients | Restylane (Galderma, Fort Worth, TX, USA) | Gentle injection of 0.6 to 1.0 mLof hyaluronic acid per side. | 25-gauge periorbital cannulapenetration until bone contact, followed bypositioning the cannula parallel to the periosteum.Group A: Injection point in rim. | Group A:
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amato, S.; Fragola, R.; Bove, P.; Lo Giudice, G.; Gennaro, P.; Vitagliano, R.; Staglianò, S. Is the Treatment of the Tear Trough Deformity with Hyaluronic Acid Injections a Safe Procedure? A Systematic Review. Appl. Sci. 2021, 11, 11489. https://doi.org/10.3390/app112311489
D’Amato S, Fragola R, Bove P, Lo Giudice G, Gennaro P, Vitagliano R, Staglianò S. Is the Treatment of the Tear Trough Deformity with Hyaluronic Acid Injections a Safe Procedure? A Systematic Review. Applied Sciences. 2021; 11(23):11489. https://doi.org/10.3390/app112311489
Chicago/Turabian StyleD’Amato, Salvatore, Romolo Fragola, Pierfrancesco Bove, Giorgio Lo Giudice, Paolo Gennaro, Rita Vitagliano, and Samuel Staglianò. 2021. "Is the Treatment of the Tear Trough Deformity with Hyaluronic Acid Injections a Safe Procedure? A Systematic Review" Applied Sciences 11, no. 23: 11489. https://doi.org/10.3390/app112311489







