The Pattern of Affective Responses to Dance-Based Group Exercise Differs According to Physical Fitness, as Measured by a Smartwatch
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Materials
2.2.1. Physical Fitness Measures
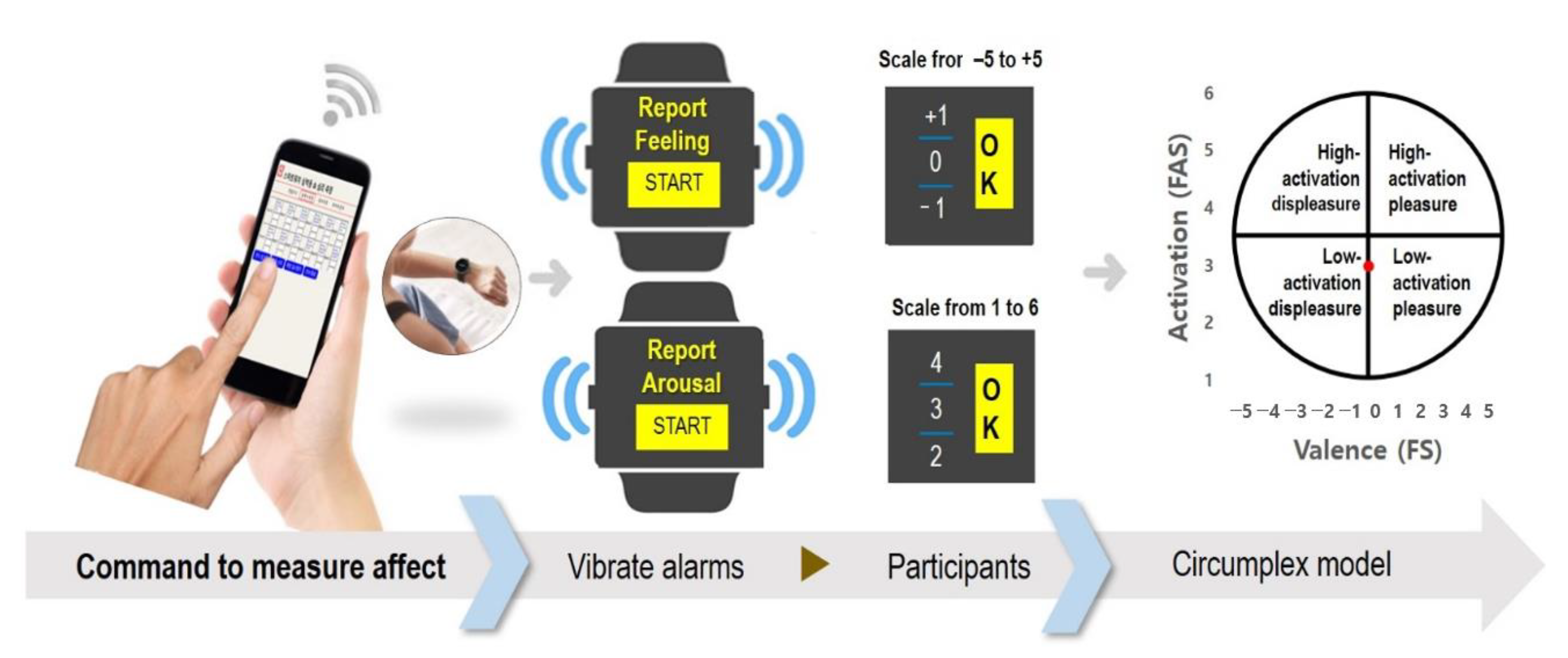
2.2.2. Smartphone
2.2.3. Smartwatch
2.2.4. Application Development and Smartwatch Control
2.3. Affective Measures
2.3.1. Positive and Negative Affect Schedule-Expanded Form (PANAS-X)
2.3.2. Two-Dimensional Circumplex Model of Affect
2.3.3. The Feeling Scale (FS)
2.3.4. The Felt Arousal Scale (FAS)
2.4. Dance Fitness Program
2.5. Procedures
2.6. Data Collection and Processing
2.7. Statistical Analysis
3. Results
3.1. HR and Exercise Intensity (%HRmax)
3.2. PANAS-X
3.2.1. Positive Affect
3.2.2. Negative Affect
3.3. The Feeling Scale and Felt Arousal Scale
3.3.1. The Feeling Scale
3.3.2. The Felt Arousal Scale
3.4. The Two-Dimensional Circumplex Model
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- White, K.; Kendrick, T.; Yardley, L. Change in self-esteem, self-efficacy and the mood dimensions of depression as potential mediators of the physical activity and depression relationship: Exploring the temporal relation of change. Ment. Health Phys. Act. 2009, 2, 44–52. [Google Scholar] [CrossRef]
- Dimeo, F.; Bauer, M.; Varahram, I.; Proest, G.; Halter, U. Benefits from aerobic exercise in patients with major depression: A pilot study. Br. J. Sports Med. 2001, 35, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Sigwalt, A.R.; Budde, H.; Helmich, I.; Glaser, V.; Ghisoni, K.; Lanza, S.; Cadore, E.L.; Lhullier, F.L.; de Bem, A.F.; Hohl, A.; et al. Molecular aspects involved in swimming exercise training reducing anhedonia in a rat model of depression. Neuroscience 2011, 192, 661–674. [Google Scholar] [CrossRef]
- Buckworth, J.; Dishman, R. Determinants of exercise and physical activity. In Exercise Psychology; Human Kinetics: Champaign, IL, USA, 2002; pp. 191–209. [Google Scholar]
- Mun, C. The Effects of Preference Mode and Intensity of Exercise on Participants’ Psychological and Physiological Responses. Korean J. Sport Psychol. 2011, 22, 149–169. [Google Scholar]
- Bixby, W.R.; Lochbaum, M.R. Affect Responses to Acute Bouts of Aerobic Exercise in Fit and Unfit Participants: An Examination of Opponent-Process Theory. J. Sport Behav. 2006, 29, 111–125. [Google Scholar]
- Ekkekakis, P.; Hall, E.E.; Petruzzello, S.J. The relationship between exercise intensity and affective responses demystified: To crack the 40-year-old nut, replace the 40-year-old nutcracker! Ann. Behav. Med. 2008, 35, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, M.; Kraemer, R.; Bartholomew, J.; Acevedo, E.; Jarreau, D. Affective responses to exercise are dependent on intensity rather than total work. Med. Sci. Sports Exerc. 2007, 39, 1417–1422. [Google Scholar] [CrossRef]
- Sheppard, K.E.; Parfitt, G. Acute Affective Responses to Prescribed and Self-Selected Exercise Intensities in Young Adolescent Boys and Girls. Pediatr. Exerc. Sci. 2008, 20, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Rose, E.A.; Parfitt, G. A quantitative analysis and qualitative explanation of the individual differences in affective responses to prescribed and self-selected exercise intensities. J. Sport Exerc. Psychol. 2007, 29, 281–309. [Google Scholar] [CrossRef]
- Williams, D.M.; Rhodes, R.E.; Conner, M.T. (Eds.) Psychological hedonism, hedonic motivation, and health behavior. In Affective Determinants of Health Behavior; Oxford University Press: New York, NY, USA, 2018; pp. 204–234. [Google Scholar]
- Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report; Department of Health and Human Services: Washington, DC, USA, 2008. [Google Scholar]
- Box, A.G.; Petruzzello, S.J. Why do they do it? Differences in high-intensity exercise-affect between those with higher and lower intensity preference and tolerance. Psychol. Sport Exerc. 2020, 47, 101521. [Google Scholar] [CrossRef]
- Schneider, M.; Graham, D. Personality, physical fitness, and affective response to exercise among adolescents. Med. Sci. Sports Exerc. 2009, 41, 947. [Google Scholar] [CrossRef]
- Turner, J.H. A Theory of Social Interaction; Stanford University Press: Stanford, CA, USA, 1988. [Google Scholar]
- Burke, P.J. Contemporary Social Psychological Theories; Stanford University Press: Stanford, CA, USA, 2020. [Google Scholar]
- Mikkelsen, K.; Stojanovska, L.; Polenakovic, M.; Bosevski, M.; Apostolopoulos, V. Exercise and mental health. Maturitas 2017, 106, 48–56. [Google Scholar] [CrossRef]
- Campion, M.; Levita, L. Enhancing positive affect and divergent thinking abilities: Play some music and dance. J. Posit. Psychol. 2014, 9, 137–145. [Google Scholar] [CrossRef]
- Koch, S.; Kunz, T.; Lykou, S.; Cruz, R. Effects of dance movement therapy and dance on health-related psychological outcomes: A meta-analysis. Arts Psychother 2014, 41, 46–64. [Google Scholar] [CrossRef]
- Thompson, M. Cultural Theory; Routledge: New York, NY, USA, 2018. [Google Scholar]
- Zhang, Z.; Pi, Z.; Liu, B. TROIKA: A general framework for heart rate monitoring using wrist-type photoplethysmographic signals during intensive physical exercise. IEEE. Trans. Biomed. 2015, 62, 522–531. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Woo, J. Is heart rate measured by smartwatch during exercise reliable? Analysis of correlation and agreement between heart rates of Polar and smartwatch. J. Korea Converg. Soc. 2020, 11, 331–339. [Google Scholar]
- Nieri, T.; Hughes, E. All about having fun: Women’s experience of Zumba fitness. Sociol. Sport J. 2016, 33, 135–145. [Google Scholar] [CrossRef]
- Luettgen, M.; Foster, C.; Doberstein, S.; Mikat, R.; Porcari, J. ZUMBA®: Is the “fitness-party” a good workout? J. Sci. Med. Sport 2012, 11, 357–358. [Google Scholar]
- Riebe, D.; Ehrman, J.K.; Linguori, G.; Magal, M. (Eds.) American College of Sports Medicine’s Guidelines for Exercise Testing and Prescription, 10th ed.; Kluwer: Philadelphia, PA, USA, 2017. [Google Scholar]
- Donath, L.; Roth, R.; Hohn, Y.; Zahner, L.; Faude, O. The effects of Zumba training on cardiovascular and neuromuscular function in female college students. Eur. J. Sport Sci. 2014, 14, 569–577. [Google Scholar] [CrossRef]
- Haghjoo, M.; Zar, A.; Hoseini, S.A. The Effect of 8 weeks Zumba Training on Women’s Body Composition with Overweight. Pars Jahrom Univ. Med Sci. 2016, 14, 21–30. [Google Scholar] [CrossRef][Green Version]
- Lee, J.; Park, J.; Kim, Y.; Woo, M. Affective Change with Variations in Zumba Fitness Intensity as Measured by a Smartwatch. Percept. Mot. Skills 2021, 128, 2255–2278. [Google Scholar] [CrossRef]
- Cugusi, L.; Wilson, B.; Serpe, R.; Medda, A.; Deidda, M.; Gabba, S.; Satta, G.; Chiappori, P.; Mercuro, G. Cardiovascular effects, body composition, quality of life and pain after a Zumba fitness program in Italian overweight women. J. Sports Med. Phys. Fit. 2016, 56, 328–335. [Google Scholar]
- Norouzi, E.; Hosseini, F.; Vaezmosavi, M.; Gerber, M.; Puhse, U.; Brand, S. Zumba dancing and aerobic exercise can improve working memory, motor function, and depressive symptoms in female patients with Fibromyalgia. Eur. J. Sport Sci. 2020, 20, 981–991. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Ministry of Culture, Sports and Tourism. Development of Standards for National Fitness in Adulthood; Ministry of Culture, Sports and Tourism: Sejong, Korea, 2010. [Google Scholar]
- Ministry of Culture, Sports and Tourism. Improvement of National Fitness 100 Evaluation Criteria; Ministry of Culture, Sports and Tourism: Sejong, Korea, 2015. [Google Scholar]
- Henriksen, A.; Haugen Mikalsen, M.; Woldaregay, A.Z.; Muzny, M.; Hartvigsen, G.; Hopstock, L.A.; Grimsgaard, S. Using Fitness Trackers and Smartwatches to Measure Physical Activity in Research: Analysis of Consumer Wrist-Worn Wearables. J. Med. Internet Res. 2018, 20, e110. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.; Clark, L.A. The PANAS-X: Manual for the Positive and Negative Affect Schedule-Expanded Form; University of Iowa: Iowa City, IA, USA, 1994. [Google Scholar]
- Russell, J.A. A circumplex model of affect. J. Pers. Soc. Psychol. 1980, 39, 1161–1178. [Google Scholar] [CrossRef]
- Russell, J.A. Core affect and the psychological construction of emotion. Psychol. Rev. 2003, 110, 145–172. [Google Scholar] [CrossRef] [PubMed]
- Hardy, C.J.; Rejeski, W.J. Not what, but how one feels: The measurement of affect during exercise. J. Psychol Sport Exerc. 1989, 11, 304–317. [Google Scholar] [CrossRef]
- Svebak, S.; Murgatroyd, S. Metamotivational dominance: A multimethod validation of reversal theory constructs. J. Pers. Soc. Psychol. 1985, 48, 107–116. [Google Scholar] [CrossRef]
- Fox, S.M., III. Physical activity and the prevention of coronary heart disease. Ann. Med. Res. 1971, 3, 404–432. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Perez, B.; Greenwood-Robinson, M. Zumba: Ditch the Workout, Join the Party! The Zumba Weight Loss Program; Grand Central Life & Style: New York, NY, USA, 2014. [Google Scholar]
- Yu, J.; Park, S. K-Pop Girl Group Dance Movement Classification Systems and Visualization Implementation. Korean J. Danc. 2019, 19, 25–36. [Google Scholar]
- Ekkekakis, P.; Petruzzello, S.J. Acute aerobic exercise and affect. Sports Med. 1999, 28, 337–347. [Google Scholar] [CrossRef]
- Busing, K.; West, C. Determining the relationship between physical fitness, gender, and life satisfaction. SAGE Open. 2016, 6, 1–5. [Google Scholar] [CrossRef]
- Rezazadeh, A.; Talebi, N. Relationship Between Emotion Regulation and Health-Related of Physical Fitness in Tehran Firefighters. Clin. Psychol. Personal. 2021. [Google Scholar] [CrossRef]
- Lin, T.-W.; Kuo, Y.-M. Exercise Benefits Brain Function: The Monoamine Connection. Brain Sci. 2013, 3, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Melancon, M.; Lorrain, D.; Dionne, I. Exercise and sleep in aging: Emphasis on serotonin. Pathol. Biol. 2014, 62, 276–283. [Google Scholar] [CrossRef]
- Klaperski, S.; von Dawans, B.; Heinrichs, M.; Fuchs, R. Effects of a 12-week endurance training program on the physiological response to psychosocial stress in men: A randomized controlled trial. J. Behav. Med. 2014, 37, 1118–1133. [Google Scholar] [CrossRef]
- Childs, E.; de Wit, H. Regular exercise is associated with emotional resilience to acute stress in healthy adults. Front. Physiol. 2014, 5, 161. [Google Scholar] [CrossRef] [PubMed]
- Strahler, J.; Fuchs, R.; Nater, U.M.; Klaperski, S. Impact of physical fitness on salivary stress markers in sedentary to low-active young to middle-aged men. Psychoneuroendocrinology 2016, 68, 14–19. [Google Scholar] [CrossRef]
- Wood, C.J.; Clow, A.; Hucklebridge, F.; Law, R.; Smyth, N. Physical fitness and prior physical activity are both associated with less cortisol secretion during psychosocial stress. Anxiety Stress Coping 2018, 31, 135–145. [Google Scholar] [CrossRef] [PubMed]







| Item | High-Fit Group | Low-Fit Group | ||
|---|---|---|---|---|
| M | SD | M | SD | |
| Age (yrs.) | 23.33 | 1.88 | 20.59 | 1.67 |
| BMI (kg/m2) | 23.56 | 3.96 | 22.63 | 3.84 |
| Body fat (%) | 25.09 | 6.94 | 31.87 | 7.94 |
| Fitness (Z-score) | 0.36 | 0.69 | −0.52 | 0.56 |
| Rhythm | Basic Steps | Music | bpm | |||
|---|---|---|---|---|---|---|
| Aerobics | March, step touch, V-step, lunge, heel-jack, knee up, grapevine, mambo, back-up, box, tap | Top Ten 24 #1 | 130 | |||
| Merengue | March | 2 Step | 6 Step | Beto Shuffle | Basic 1 review music | 124 |
| Fiesta | 124 | |||||
| Reggaeton | Stomp | Knee-lift | Destroza | Step bounce | Basic 1 review music | 94 |
| Toma reggaeton | 96 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Kim, J.; Woo, M. The Pattern of Affective Responses to Dance-Based Group Exercise Differs According to Physical Fitness, as Measured by a Smartwatch. Appl. Sci. 2021, 11, 11540. https://doi.org/10.3390/app112311540
Kim Y, Kim J, Woo M. The Pattern of Affective Responses to Dance-Based Group Exercise Differs According to Physical Fitness, as Measured by a Smartwatch. Applied Sciences. 2021; 11(23):11540. https://doi.org/10.3390/app112311540
Chicago/Turabian StyleKim, Yujin, Jihye Kim, and Minjung Woo. 2021. "The Pattern of Affective Responses to Dance-Based Group Exercise Differs According to Physical Fitness, as Measured by a Smartwatch" Applied Sciences 11, no. 23: 11540. https://doi.org/10.3390/app112311540
APA StyleKim, Y., Kim, J., & Woo, M. (2021). The Pattern of Affective Responses to Dance-Based Group Exercise Differs According to Physical Fitness, as Measured by a Smartwatch. Applied Sciences, 11(23), 11540. https://doi.org/10.3390/app112311540





