Design and In Vivo Evaluation of a Novel Transdermal Hydrogen/Oxygen-Generating Patch
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Hydrogen/Oxygen-Generating Granules
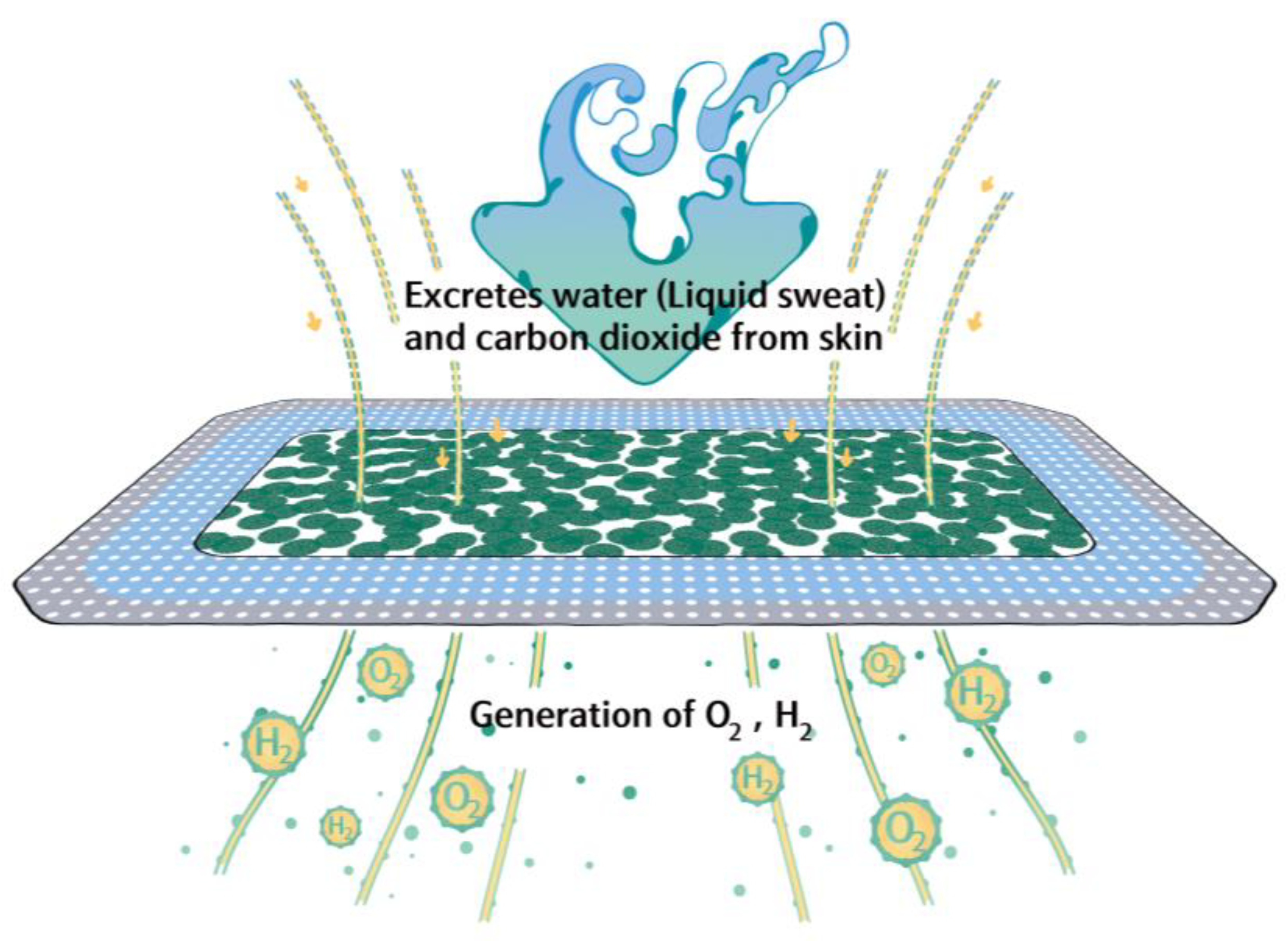
2.3. Preparation of the Hydrogen/Oxygen-Generating Patch (HOGP)
2.4. Physicochemical Evaluation of Hydrogen- and Oxygen-Generation Efficiency
2.5. In Vivo Permeation Study
2.5.1. Animals
2.5.2. Blood Gas Analysis
2.5.3. Stress Hormone and Inflammation Assays
2.6. Statistical Analysis
3. Results
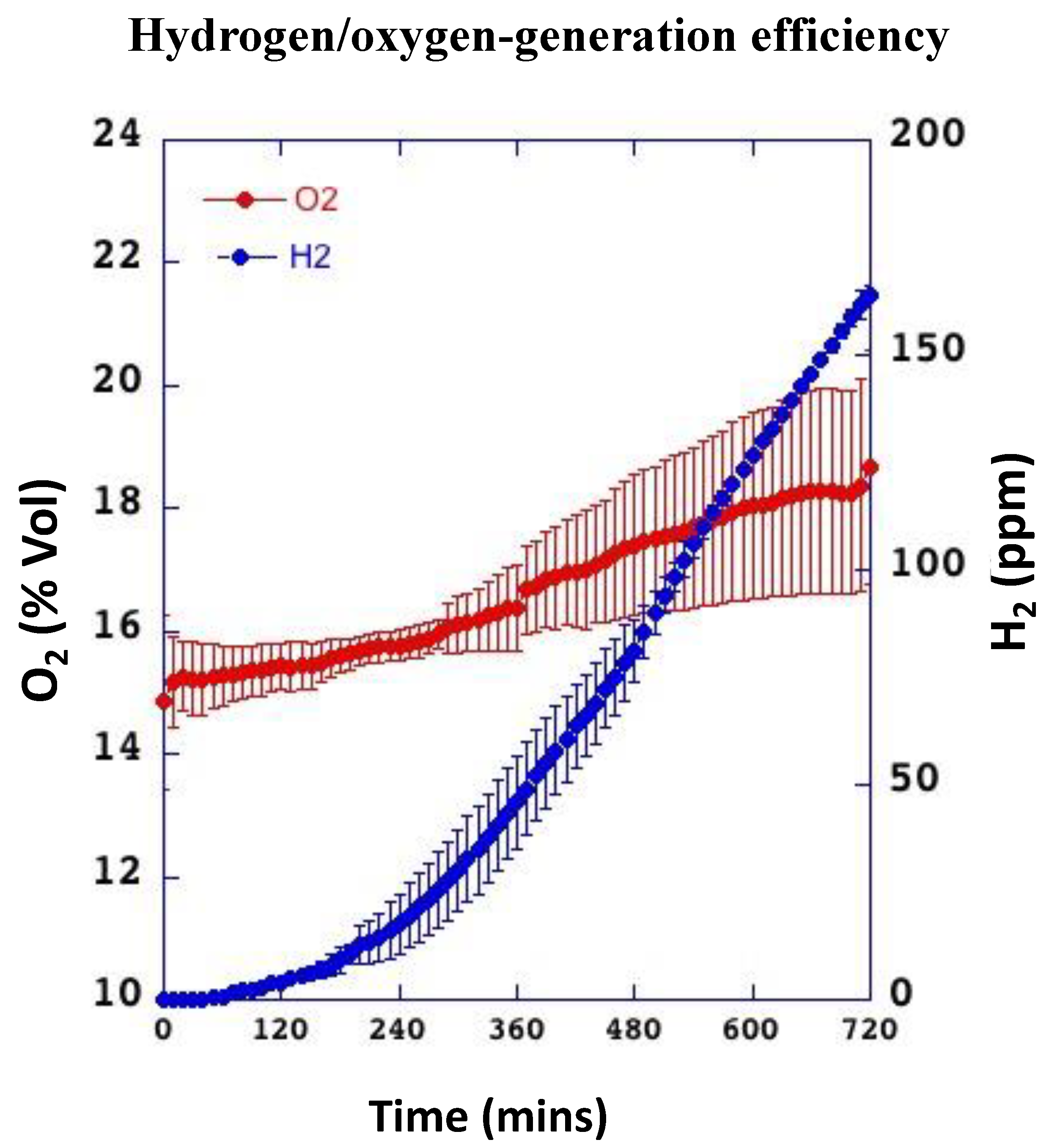
3.1. The Patches Continuously Release Hydrogen and Oxygen for Up to 12 h
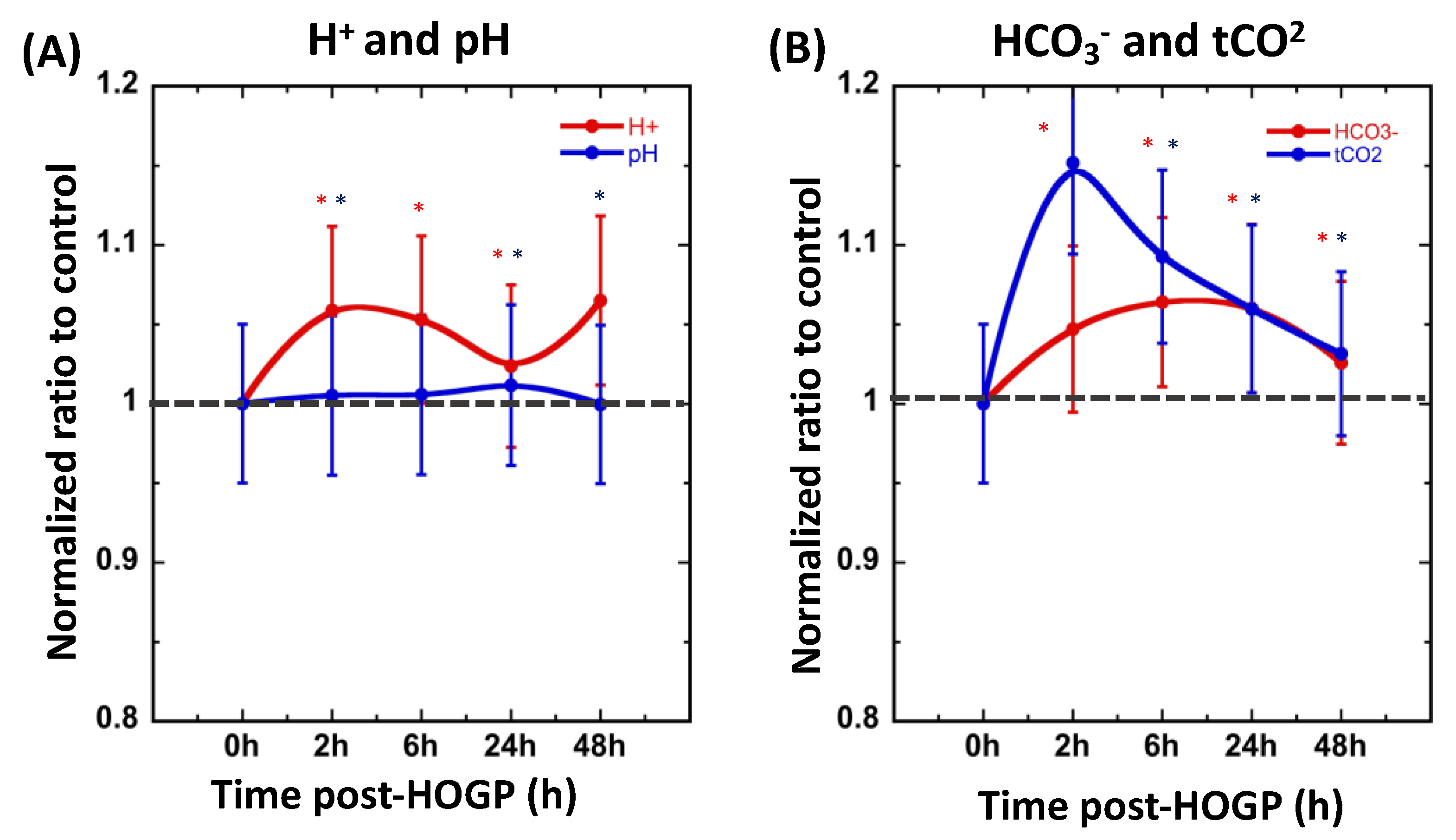
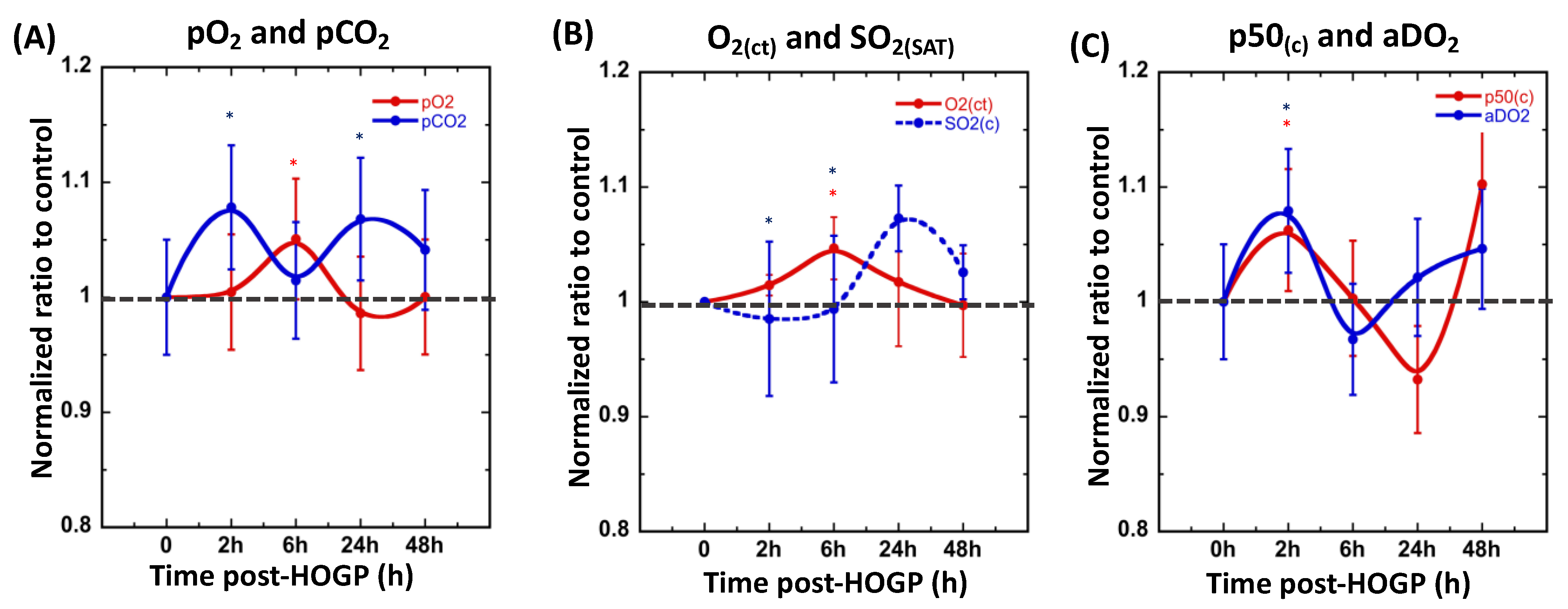
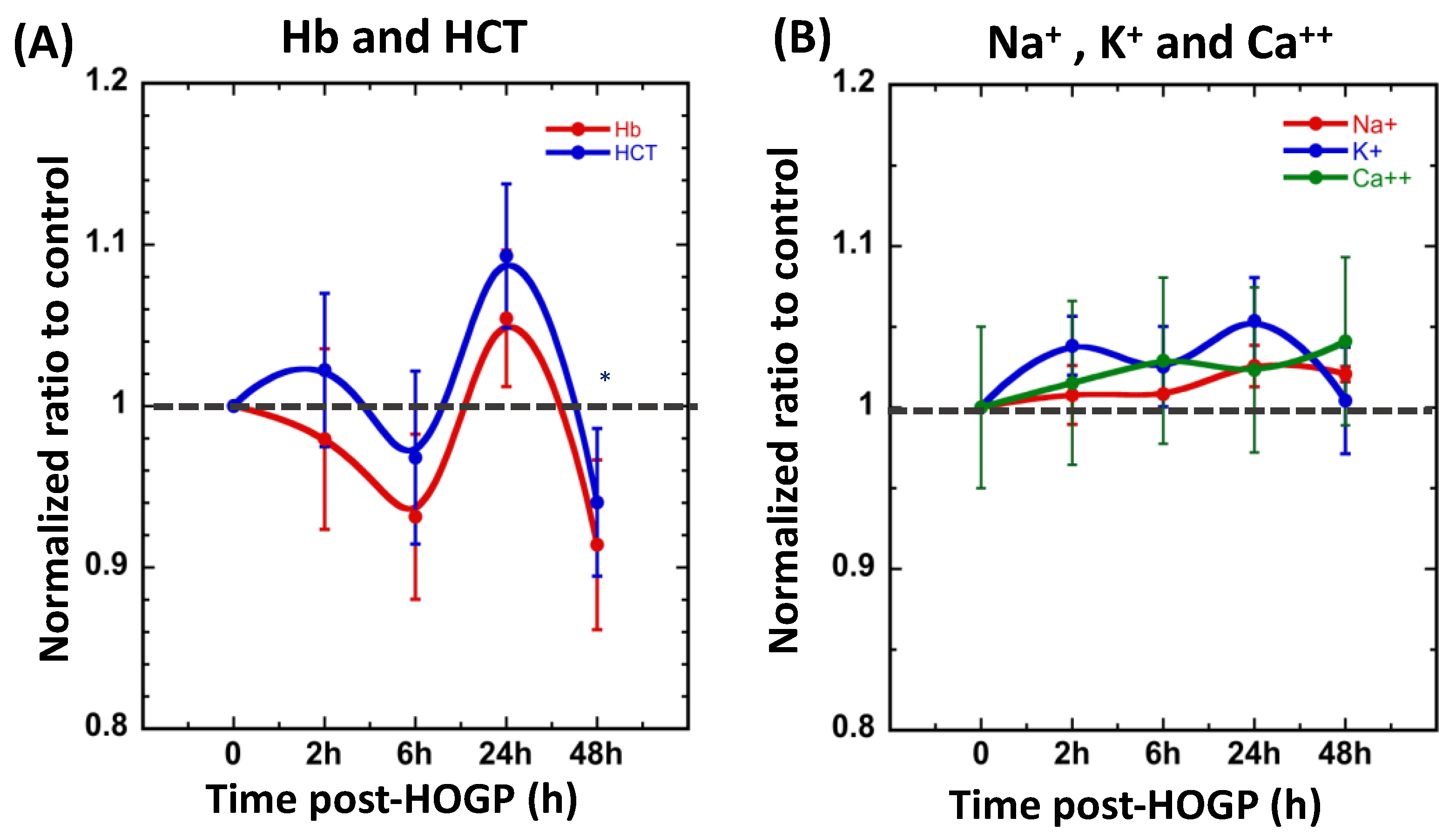
3.2. Wearing the Patch Significantly Increases Blood H+ and O2 Levels In Vivo
3.3. Effect of Acute Restraint Stress While Wearing Stretch Fabric Patch
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohta, S. Molecular hydrogen as a novel antioxidant: Overview of the advantages of hydrogen for medical applications. Methods Enzymol. 2015, 555, 289–317. [Google Scholar] [CrossRef]
- Sim, M.; Kim, C.S.; Shon, W.J.; Lee, Y.K.; Choi, E.Y.; Shin, D.M. Hydrogen-rich water reduces inflammatory responses and prevents apoptosis of peripheral blood cells in healthy adults: A randomized, double-blind, controlled trial. Sci. Rep. 2020, 10, 12130. [Google Scholar] [CrossRef] [PubMed]
- Sivarajah, A.; Collino, M.; Yasin, M.; Benetti, E.; Gallicchio, M.; Mazzon, E.; Cuzzocrea, S.; Fantozzi, R.; Thiemermann, C. Anti-apoptotic and anti-inflammatory effects of hydrogen sulfide in a rat model of regional myocardial I/R. Shock 2009, 31, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Moller, A.L.; Kristiansen, K.A.; Schulz, A.; Moller, I.M.; Schjoerring, J.K.; Jahn, T.P. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 2007, 282, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Lenahan, C.; Boling, W.; Tang, J.; Zhang, J.H. Molecular Hydrogen Application in Stroke: Bench to Bedside. Curr. Pharm. Des. 2021, 27, 703–712. [Google Scholar] [CrossRef]
- Kajiyama, S.; Hasegawa, G.; Asano, M.; Hosoda, H.; Fukui, M.; Nakamura, N.; Kitawaki, J.; Imai, S.; Nakano, K.; Ohta, M.; et al. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr. Res. 2008, 28, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Zong, C.; Zhang, Z.; Yu, Y.; Yao, S.; Jiao, P.; Tian, H.; Zhai, L.; Zhao, H.; Tian, S.; et al. Molecular hydrogen stabilizes atherosclerotic plaque in low-density lipoprotein receptor-knockout mice. Free Radic. Biol. Med. 2015, 87, 58–68. [Google Scholar] [CrossRef]
- Yoritaka, A.; Ohtsuka, C.; Maeda, T.; Hirayama, M.; Abe, T.; Watanabe, H.; Saiki, H.; Oyama, G.; Fukae, J.; Shimo, Y.; et al. Randomized, double-blind, multicenter trial of hydrogen water for Parkinson’s disease. Mov. Disord. 2018, 33, 1505–1507. [Google Scholar] [CrossRef]
- Zhai, X.; Chen, X.; Ohta, S.; Sun, X. Review and prospect of the biomedical effects of hydrogen. Med. Gas. Res. 2014, 4, 19. [Google Scholar] [CrossRef]
- LeBaron, T.W.; Laher, I.; Kura, B.; Slezak, J. Hydrogen gas: From clinical medicine to an emerging ergogenic molecule for sports athletes (1). Can. J. Physiol. Pharmacol. 2019, 97, 797–807. [Google Scholar] [CrossRef]
- Guan, P.; Sun, Z.M.; Luo, L.F.; Zhou, J.; Yang, S.; Zhao, Y.S.; Yu, F.Y.; An, J.R.; Wang, N.; Ji, E.S. Hydrogen protects against chronic intermittent hypoxia induced renal dysfunction by promoting autophagy and alleviating apoptosis. Life Sci. 2019, 225, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Deng, C.; Zhang, X.; Zhang, J.; Bai, C. Inhalation of hydrogen gas attenuates airway inflammation and oxidative stress in allergic asthmatic mice. Asthma Res. Pract. 2018, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.T.; Bao, C.; He, Y.; Tian, X.; Yang, Y.; Zhang, T.; Xu, K.F. Hydrogen gas (XEN) inhalation ameliorates airway inflammation in asthma and COPD patients. QJM 2020, 113, 870–875. [Google Scholar] [CrossRef]
- Yuichi Sugai, Y.O.; Kyuro, S. Simulation Study on Reservoir Souring Induced by Injection of Reservoir Brine Containing Sulfate-Reducing Bacteria. Sustainability 2020, 12, 4603. [Google Scholar] [CrossRef]
- Hayashida, K.; Sano, M.; Kamimura, N.; Yokota, T.; Suzuki, M.; Ohta, S.; Fukuda, K.; Hori, S. Hydrogen inhalation during normoxic resuscitation improves neurological outcome in a rat model of cardiac arrest independently of targeted temperature management. Circulation 2014, 130, 2173–2180. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Ding, X.; Rong, A.; Zheng, M.; Li, Z.; Pan, S.; Yang, W. Hydrogen gas inhalation alleviates myocardial ischemia-reperfusion injury by the inhibition of oxidative stress and NLRP3-mediated pyroptosis in rats. Life Sci. 2021, 272, 119248. [Google Scholar] [CrossRef]
- Tamura, T.; Hayashida, K.; Sano, M.; Onuki, S.; Suzuki, M. Efficacy of inhaled HYdrogen on neurological outcome following BRain Ischemia During post-cardiac arrest care (HYBRID II trial): Study protocol for a randomized controlled trial. Trials 2017, 18, 488. [Google Scholar] [CrossRef]
- Korovljev, D.; Stajer, V.; Ostojic, J.; LeBaron, T.W.; Ostojic, S.M. Hydrogen-rich water reduces liver fat accumulation and improves liver enzyme profiles in patients with non-alcoholic fatty liver disease: A randomized controlled pilot trial. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 688–693. [Google Scholar] [CrossRef]
- Yoon, Y.S.; Sajo, M.E.; Ignacio, R.M.; Kim, S.K.; Kim, C.S.; Lee, K.J. Positive Effects of hydrogen water on 2,4-dinitrochlorobenzene-induced atopic dermatitis in NC/Nga mice. Biol. Pharm. Bull. 2014, 37, 1480–1485. [Google Scholar] [CrossRef]
- Opasanon, S.; Pongsapich, W.; Taweepraditpol, S.; Suktitipat, B.; Chuangsuwanich, A. Clinical Effectiveness of Hyperbaric Oxygen Therapy in Complex Wounds. J. Am. Coll. Clin. Wound Spec. 2014, 6, 9–13. [Google Scholar] [CrossRef][Green Version]
- Runtuwene, J.; Amitani, H.; Amitani, M.; Asakawa, A.; Cheng, K.C.; Inui, A. Hydrogen-water enhances 5-fluorouracil-induced inhibition of colon cancer. PeerJ 2015, 3, e859. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.M.; Kang, Y.N.; Choi, I.B.; Gu, Y.; Kawamura, T.; Toyoda, Y.; Nakao, A. Effects of drinking hydrogen-rich water on the quality of life of patients treated with radiotherapy for liver tumors. Med. Gas. Res. 2011, 1, 11. [Google Scholar] [CrossRef]
- Li, S.; Liao, R.; Sheng, X.; Luo, X.; Zhang, X.; Wen, X.; Zhou, J.; Peng, K. Hydrogen Gas in Cancer Treatment. Front. Oncol. 2019, 9, 696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Liu, C.; Zhou, L.; Qu, K.; Wang, R.; Tai, M.H.; Lei Lei, J.C.; Wu, Q.F.; Wang, Z.X. A review of hydrogen as a new medical therapy. Hepatogastroenterology 2012, 59, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S. Molecular hydrogen as a preventive and therapeutic medical gas: Initiation, development and potential of hydrogen medicine. Pharmacol. Ther. 2014, 144, 1–11. [Google Scholar] [CrossRef]
- Ohta, S. Recent progress toward hydrogen medicine: Potential of molecular hydrogen for preventive and therapeutic applications. Curr. Pharm. Des. 2011, 17, 2241–2252. [Google Scholar] [CrossRef] [PubMed]
- Dixon, B.J.; Tang, J.; Zhang, J.H. The evolution of molecular hydrogen: A noteworthy potential therapy with clinical significance. Med. Gas. Res. 2013, 3, 10. [Google Scholar] [CrossRef]
- Zuttel, A. Hydrogen storage methods. Naturwissenschaften 2004, 91, 157–172. [Google Scholar] [CrossRef]
- Ueno, C.; Hunt, T.K.; Hopf, H.W. Using physiology to improve surgical wound outcomes. Plast. Reconstr. Surg. 2006, 117, 59S–71S. [Google Scholar] [CrossRef]
- Trabold, O.; Wagner, S.; Wicke, C.; Scheuenstuhl, H.; Hussain, M.Z.; Rosen, N.; Seremetiev, A.; Becker, H.D.; Hunt, T.K. Lactate and oxygen constitute a fundamental regulatory mechanism in wound healing. Wound Repair Regen. 2003, 11, 504–509. [Google Scholar] [CrossRef]
- Hunt, T.K.; Ellison, E.C.; Sen, C.K. Oxygen: At the foundation of wound healing—Introduction. World J. Surg. 2004, 28, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Camci-Unal, G.; Alemdar, N.; Annabi, N.; Khademhosseini, A. Oxygen Releasing Biomaterials for Tissue Engineering. Polym. Int. 2013, 62, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Pedraza, E.; Coronel, M.M.; Fraker, C.A.; Ricordi, C.; Stabler, C.L. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proc. Natl. Acad. Sci. USA 2012, 109, 4245–4250. [Google Scholar] [CrossRef]
- Akhtar, N.; Singh, V.; Yusuf, M.; Khan, R.A. Non-invasive drug delivery technology: Development and current status of transdermal drug delivery devices, techniques and biomedical applications. Biomed. Technol. 2020, 65, 243–272. [Google Scholar] [CrossRef]
- Ramadan, E.; Borg, T.; Abdelghani, G.M.; Saleh, N.M. Design and in vivo pharmacokinetic study of a newly developed lamivudine transdermal patch. Future J. Pharm. Sci. 2018, 4, 166–174. [Google Scholar] [CrossRef]
- Dong, S.; Zhu, P.; Zhang, S. Expression of collagen type 1 alpha 1 indicates lymph node metastasis and poor outcomes in squamous cell carcinomas of the lung. PeerJ 2020, 8, e10089. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cheng, Y.; Tian, J.; Yang, P.; Zhang, X.; Chen, Y.; Hu, Y.; Wu, J. Dissolved oxygen from microalgae-gel patch promotes chronic wound healing in diabetes. Sci. Adv. 2020, 6, eaba4311. [Google Scholar] [CrossRef] [PubMed]
- Winter, G.D. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 1962, 193, 293–294. [Google Scholar] [CrossRef]
- Chandra, P.K.; Ross, C.L.; Smith, L.C.; Jeong, S.S.; Kim, J.; Yoo, J.J.; Harrison, B.S. Peroxide-based oxygen generating topical wound dressing for enhancing healing of dermal wounds. Wound Repair Regen. 2015, 23, 830–841. [Google Scholar] [CrossRef]
- Marina Safonov, J.Y.; Jihyung, L.; Vladimir, L.; Safonov, D.B.; Donghui, Z. Hydrogen generating patch improves skin cell viability, migration activity, and collagen expression. Eng. Regen. 2020, 1, 1–5. [Google Scholar] [CrossRef]
- Chen, Y.; Zong, C.; Guo, Y.; Tian, L. Hydrogen-rich saline may be an effective and specific novel treatment for osteoradionecrosis of the jaw. Ther. Clin. Risk Manag. 2015, 11, 1581–1585. [Google Scholar] [CrossRef] [PubMed]
- Heyboer, M., 3rd; Sharma, D.; Santiago, W.; McCulloch, N. Hyperbaric Oxygen Therapy: Side Effects Defined and Quantified. Adv. Wound Care 2017, 6, 210–224. [Google Scholar] [CrossRef] [PubMed]
- Hopf, H.W.; Gibson, J.J.; Angeles, A.P.; Constant, J.S.; Feng, J.J.; Rollins, M.D.; Zamirul Hussain, M.; Hunt, T.K. Hyperoxia and angiogenesis. Wound Repair Regen. 2005, 13, 558–564. [Google Scholar] [CrossRef]
- Hodges, A.N.; Delaney, S.; Lecomte, J.M.; Lacroix, V.J.; Montgomery, D.L. Effect of hyperbaric oxygen on oxygen uptake and measurements in the blood and tissues in a normobaric environment. Br. J. Sports Med. 2003, 37, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Roe, D.F.; Gibbins, B.L.; Ladizinsky, D.A. Topical dissolved oxygen penetrates skin: Model and method. J. Surg. Res. 2010, 159, e29–e36. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, W.-T.; Yu, T.-H.; Chao, W.-H.; Wang, B.-Y.; Kuo, Y.-Y.; Lin, M.-H.; Yeh, S.H.-H. Design and In Vivo Evaluation of a Novel Transdermal Hydrogen/Oxygen-Generating Patch. Appl. Sci. 2021, 11, 11680. https://doi.org/10.3390/app112411680
Ho W-T, Yu T-H, Chao W-H, Wang B-Y, Kuo Y-Y, Lin M-H, Yeh SH-H. Design and In Vivo Evaluation of a Novel Transdermal Hydrogen/Oxygen-Generating Patch. Applied Sciences. 2021; 11(24):11680. https://doi.org/10.3390/app112411680
Chicago/Turabian StyleHo, Wen-Tsung, Tsung-Hsun Yu, Wen-Hung Chao, Bao-Yen Wang, Yu-Yeh Kuo, Ming-Hsien Lin, and Skye Hsin-Hsien Yeh. 2021. "Design and In Vivo Evaluation of a Novel Transdermal Hydrogen/Oxygen-Generating Patch" Applied Sciences 11, no. 24: 11680. https://doi.org/10.3390/app112411680
APA StyleHo, W.-T., Yu, T.-H., Chao, W.-H., Wang, B.-Y., Kuo, Y.-Y., Lin, M.-H., & Yeh, S. H.-H. (2021). Design and In Vivo Evaluation of a Novel Transdermal Hydrogen/Oxygen-Generating Patch. Applied Sciences, 11(24), 11680. https://doi.org/10.3390/app112411680






