Screening of Xanthium strumarium (IAPS) Growing on Abandoned Habitats in Khyber Pakhtunkhwa, Pakistan: Perspectives for Phytoremediation
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
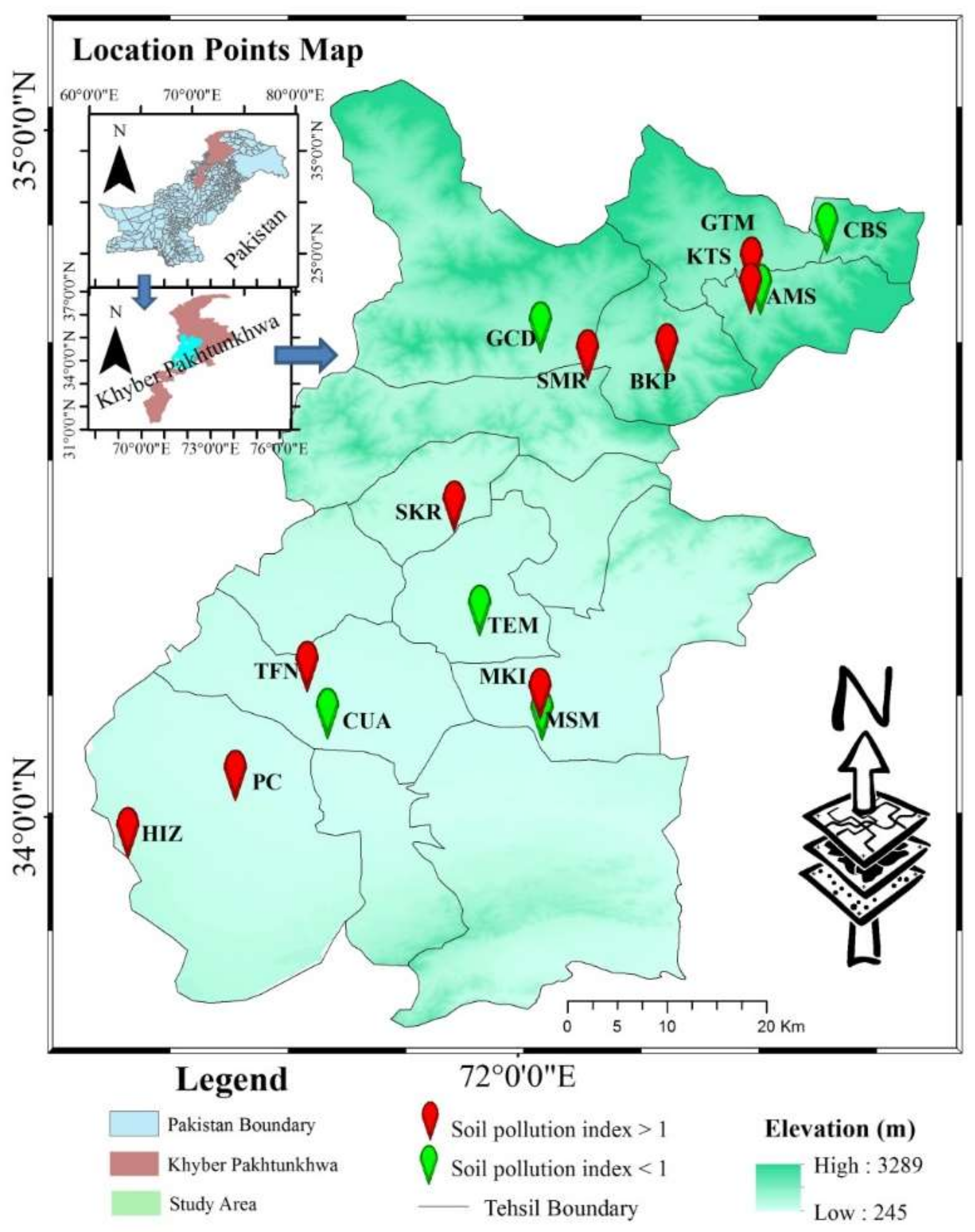
2.1. Experimental Materials
2.2. Soil–Plant Samples Preparation and Heavy Metal Analysis
2.3. Determination Phytoremediation Potential
2.4. Statistical Analysis
3. Results and Discussion
3.1. Soil–Plant Metal Concentration and Its Biomedical Safety
3.2. Bioaccumulation Factor
3.3. Translocation Factor
3.4. Soil–Plant Heavy-Metals Relationships
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumari, A.; Lal, B.; Rai, U.N. Assessment of native plant species for phytoremediation of heavy metals growing in the vicinity of NTPC sites, Kahalgaon, India. Int. J. Phytoremediation 2016, 18, 592–597. [Google Scholar] [CrossRef]
- Conesa, H.M.; Faz, Á.; Arnaldos, R. Heavy metal accumulation and tolerance in plants from mine tailings of the semiarid Cartagena–La Unión mining district (SE Spain). Sci. Total Environ. 2006, 366, 1–11. [Google Scholar] [CrossRef]
- Shu, W.S.; Ye, Z.H.; Lan, C.Y.; Zhang, Z.Q.; Wong, M.H. Lead, zinc and copper accumulation and tolerance in populations of Paspalum distichum and Cynodon dactylon. Environ. Pollut. 2002, 120, 445–453. [Google Scholar] [CrossRef]
- Khan, T.A.; Sharma, S.; Ali, I. Adsorption of Rhodamine B dye from aqueous solution onto acid activated mango (Magnifera indica) leaf powder: Equilibrium, kinetic and thermodynamic studies. Toxicol. Environ. Health Sci. 2011, 3, 286–297. [Google Scholar]
- Chary, N.S.; Kamala, C.T.; Raj, D.S.S. Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxicol. Environ. Saf. 2008, 69, 513–524. [Google Scholar] [CrossRef]
- Khan, S.; Cao, Q.; Zheng, Y.M.; Huang, Y.Z.; Zhu, Y.G. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ. Pollut. 2008, 152, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Noman, A.; Aqeel, M.; Javed, M.T.; Zafar, S.; Ali, Q.; Islam, W.; Rshad, M.K.; Buriro, M.; Kanwal, H.; Khalid, N.; et al. Histological changes in Hibiscus rosa-sinensis endorse acclimation and phytoremediation of industrially polluted sites. J. Anim. Plant. Sci. 2017, 27, 1637–1648. [Google Scholar]
- Ali, I.; Aboul-Enein, H.Y.; Gupta, V.K. Nano Chromatography and Capillary Electrophoresis: Pharmaceutical and Environmental Analyses; Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Ali, I.; Jain, C.K. Advances in arsenic speciation techniques. Int. J. Env. Anal. Chem. 2004, 84, 947–964. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.J.; Ali, A.; DeLaune, R.D. Heavy metals and metalloid contamination in Louisiana Lake Pontchartrain Estuary along I-10 Bridge. Transp. Res. D Transp. Environ. 2016, 44, 66–77. [Google Scholar] [CrossRef]
- Khalid, N.; Hussain, M.; Young, H.S.; Ashraf, M.; Hameed, M.; Ahmad, R. Lead concentrations in soils and some wild plant species along two busy roads in Pakistan. Bull. Environ. Contam. Toxicol. 2018, 100, 250–258. [Google Scholar] [CrossRef]
- Noman, A.; Ali, Q.; Maqsood, J.; Iqbal, N.; Javed, M.T.; Rasool, N.; Naseem, J. Deciphering physio-biochemical, yield, and nutritional quality attributes of water-stressed radish (Raphanus sativus L.) plants grown from Zn-Lys primed seeds. Chemosphere 2018, 195, 175–189. [Google Scholar] [CrossRef]
- Chen, X.; Xia, X.; Zhao, Y.; Zhang, P. Heavy metal concentrations in roadside soils and correlation with urban traffic in Beijing, China. J. Hazard. Mater. 2010, 181, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Ozaki, H.; Watanabe, I.; Kuno, K. As, Sb and Hg distribution and pollution sources in the roadside soil and dust around Kamikochi, Chubu Sangaku National Park, Japan. Geochem. J. 2004, 38, 473–484. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- Zhao, F.J.; Lombi, E.; McGrath, S.P. Assessing the potential for zinc and cadmium phytoremediation with the hyperaccumulator Thlaspi caerulescens. Plant Soil 2003, 249, 37–43. [Google Scholar] [CrossRef]
- Balabanova, B.; Stafilov, T.; Bačeva, K. Bioavailability and bioaccumulation characterisation of essential and heavy metals contents in R. acetosa, S. oleracea and U. dioica from copper polluted and referent areas. J. Environ. Health Sci. Eng. 2015, 13, 1–13. [Google Scholar]
- Zhuang, P.; Yang, Q.W.; Wang, H.B.; Shu, W.S. Phytoextraction of heavy metals by eight plant species in the field. Water Air Soil Pollut. 2007, 184, 235–242. [Google Scholar] [CrossRef]
- Khalid, N.; Noman, A.; Aqeel, M.; Masood, A.; Tufail, A. Phytoremediation potential of Xanthium strumarium for heavy metals contaminated soils at roadsides. Int. J. Environ. Sci. Technol. 2019, 16, 2091–2100. [Google Scholar] [CrossRef]
- Chinmayee, M.D.; Mahesh, B.; Pradesh, S.; Mini, I.; Swapna, T.S. The assessment of phytoremediation potential of invasive weed Amaranthus spinosus L. Appl. Biochem. Biotechnol. 2012, 167, 1550–1559. [Google Scholar] [CrossRef]
- Pandey, V.C. Invasive species based efficient green technology for phytoremediation of fly ash deposits. J. Geochem. Explor. 2012, 123, 13–18. [Google Scholar] [CrossRef]
- Trueman, R.J.; Erber, L. Invasive species may offer advanced phytoremediation of endocrine disrupting chemicals in aquatic ecosystems. Emir. J. Food. Agric. 2013, 2013, 648–656. [Google Scholar]
- Prabakaran, K.; Li, J.; Anandkumar, A.; Leng, Z.; Zou, C.B.; Du, D. Managing environmental contamination through phytoremediation by invasive plants: A review. Ecol. Eng. 2019, 138, 28–37. [Google Scholar] [CrossRef]
- Marwat, K.B.; Hashim, S.; Ali, H. Weed management: A case study from north-west Pakistan. Pak. J. Bot. 2010, 42, 341–353. [Google Scholar]
- Löve, D.; Dansereau, P. Biosystematic studies on Xanthium: Taxonomic appraisal and ecological status. Can. J. Bot. 1959, 37, 173–208. [Google Scholar] [CrossRef]
- Kamboj, A.; Saluja, A.K. Phytopharmacological review of Xanthium strumarium L. (Cocklebur). Int. J. Green Pharm. 2010, 4, 129–140. [Google Scholar] [CrossRef]
- Martin, R.B. Optimal control drug scheduling of cancer chemotherapy. Automatica 1992, 28, 1113–1123. [Google Scholar] [CrossRef]
- Khan, N.; Ali, K.; Saeed, S. How environmental variables can determine the Chir pine (Pinus roxburghii sarg.) Distribution in swat Hindukush range of Pakistan: Current and future prospective of the species. Appl. Ecol. Environ. Res. 2021, 19, 2405–2424. [Google Scholar] [CrossRef]
- Khan, W.; Ali, K.; Hussain, M.; Ali, M.; Nisar, M. Distribution and phenotypic variation in Juglans regia L. growing in Hindu Kush ranges of Pakistan. Acta Ecol. Sin. 2020, 40, 363–372. [Google Scholar] [CrossRef]
- Rahman, I.U.; Khan, N.; Ali, K.; Ahmad, S. Vegetation–environment relationship in Pinus wallichiana forests of the Swat Hindukush range of Pakistan. J. For. Res. 2018, 31, 185–195. [Google Scholar] [CrossRef]
- Yafa, C.; Farmer, J.G.; Graham, M.C.; Bacon, J.R.; Barbante, C.; Cairns, W.R.L.; Bindler, R.; Renberg, I.; Cheburkin, A.; Kylander, M.; et al. Development of an ombrotrophic peat bog (low ash) reference material for the determination of elemental concentrations. Environ. Monit. Assess. 2004, 6, 493–501. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Teng, Y.; Lu, S.; Wang, Y.; Jiao, X. Evaluation of soil contamination indices in a mining area of Jiangxi, China. PLoS ONE 2014, 9, e112917. [Google Scholar] [CrossRef]
- Kachenko, A.G.; Singh, B. Heavy metals contamination in vegetables grew in urban and metal smelter contaminated sites in Australia. Water Air Soil Pollut. 2006, 169, 101–123. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Sardar, K.H.A.N.; Shah, M.T.; Brusseau, M.L.; Khan, S.A.; Mainhagu, J. Transfer of heavy metals from soils to vegetables and associated human health risks at selected sites in Pakistan. Pedosphere 2018, 28, 666–679. [Google Scholar] [CrossRef]
- Day, P.R. Particle Fractionation and Particle-Size Analysis; No. methods of soil analysis; American Society of Agronomy Madison, Soil Science Society of America: Madison, WI, USA, 1965; pp. 545–567. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis. Part 3. Chemical Method; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Summer, M.E., Eds.; Soil Science Society of America and American Society of Agronomy: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Atayese, M.O.; Eigbadon, A.I.; Adesodun, J.K. Heavy metal contamination of Amaranthus grown along major highways in Lagos, Nigeria. Afr. Crop. Sci. J. 2008, 16, 225–235. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K.; Yabuki, T.; Ono, Y. Roadside Rhododendron pulchrum leaves as bioindicators of heavy metal pollution in traffic areas of Okayama, Japan. Environ. Monit. Assess. 2009, 149, 133–141. [Google Scholar] [CrossRef]
- Weckwerth, G. Verification of traffic emitted aerosol components in the ambient air of Cologne (Germany). Atmos. Environ. 2011, 35, 5525–5536. [Google Scholar] [CrossRef]
- Malinowska, E.; Jankowski, K.; Wiśniewska-Kadżajan, B.; Sosnowski, J.; Kolczarek, R.; Jankowska, J.; Ciepiela, G.A. Content of zinc and copper in selected plants growing along a motorway. Bull. Environ. Contam. Toxicol. 2015, 95, 638–643. [Google Scholar] [CrossRef] [Green Version]
- Kabata-Pendias, A. Behavioural properties of trace metals in soils. J. Appl. Geochem. 1993, 8, 3–9. [Google Scholar] [CrossRef]
- Ul Islam, E.; Yang, X.E.; He, Z.L.; Mahmood, Q. Assessing potential dietary toxicity of heavy metals in selected vegetables and food crops. J. Zhejiang Univ. Sci. 2007, 8, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macnicol, R.D.; Beckett, P.H.T. Critical tissue concentrations of potentially toxic elements. Plant. Soil 1985, 85, 107–129. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, R.K.; Agrawal, M.; Marshall, F.M. Health risk assessment of heavy metals via dietary intake of foodstuffs from the wastewater irrigated site of a dry tropical area of India. Food Chem. Toxicol. 2010, 48, 611–619. [Google Scholar] [CrossRef]
- Alloway, B.J. Cadmium. In Heavy Metals in Soils; Blackie and Son, Ltd.: Glasgow, UK, 1990. [Google Scholar]
- Kashem, M.A.; Singh, B.R.; Kawai, S. Mobility and distribution of cadmium, nickel and zinc in contaminated soil profiles from Bangladesh. Nutr. Cycling Agroecosyst. 2007, 77, 187–198. [Google Scholar] [CrossRef]
- Tyokumbur, E.T.; Okorie, T. Bioconcentration of trace metals in the tissues of two leafy vegetables widely consumed in South West Nigeria. Biol. Trace Elem. Res. 2011, 140, 215–224. [Google Scholar] [CrossRef]
- Khan, Z.I.; Ahmad, K.; Ashraf, M.; Shoaib, N.; Parveen, R.; Bibi, Z.; Cazzato, E. Assessment of toxicological health risk of trace metals in vegetables mostly consumed in Punjab, Pakistan. Environ. Earth Sci. 2016, 75, 433. [Google Scholar] [CrossRef]
- Noman, A.; Ali, Q.; Hameed, M.; Mehmood, T.; Iftikhar, T. Comparison of leaf anatomical characteristics of Hibiscus rosa-sinensis grown in Faisalabad region. Pak. J. Bot. 2014, 46, 199–206. [Google Scholar]
- Noman, A.; Aqeel, M. miRNA-based heavy metal homeostasis and plant growth. Environ. Sci. Pollut. Res. 2017, 24, 10068–10082. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A. On the distribution of heavy metals as compared to some other elements between grain size fractions in soils. Swed. J. Agric. Res. 1979, 9, 7–13. [Google Scholar]
- Qian, J.I.N.; Shan, X.Q.; Wang, Z.J.; Tu, Q. Distribution and plant availability of heavy metals in different particle-size fractions of soil. Sci. Total Environ. 1996, 187, 131–141. [Google Scholar] [CrossRef]
- Scokart, P.O.; Meeus-Verdinne, K.; De Borger, R. Mobility of heavy metals in polluted soils near zinc smelters. Water Air Soil Pollut. 1983, 20, 451–463. [Google Scholar] [CrossRef]
- Eriksson, J.E. The influence of pH, soil type and time on adsorbtion and uptake by plants of Cd added to the soil. Water Air Soil Pollut. 1989, 48, 317–335. [Google Scholar] [CrossRef]
- MacLean, A.J. Cadmium in different plant species and its availability in soils as influenced by organic matter and additions of lime, P, Cd and Zn. Can. J. Soil Sci. 1976, 56, 129–138. [Google Scholar] [CrossRef]
- Street, J.J.; Lindsay, W.L.; Sabey, B.R. Solubility and plant uptake of cadmium in soils amended with cadmium and sewage sludge. American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America. J. Environ. Qual. 1977, 6, 72–77. [Google Scholar] [CrossRef]
- Martin, M.H.; Duncan, E.M.; Coughtrey, P.J. The distribution of heavy metals in a contaminated woodland ecosystem. Environ. Pollut. Ser. B Chem. Phys. 1982, 3, 147–157. [Google Scholar] [CrossRef]
- Brallier, S.; Harrison, R.B.; Henry, C.L.; Dongsen, X. Liming effects on availability of Cd, Cu, Ni and Zn in a soil amended with sewage sludge 16 years previously. Water Air Soil Pollut. 1996, 86, 195–206. [Google Scholar] [CrossRef]
- Ma, J.F.; Goto, S.; Tamai, K.; Ichii, M. Role of root hairs and lateral roots in silicon uptake by rice. Plant. Physiol. 2001, 127, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Kachenko, A.; Singh, B. Heavy metals contamination of home grown vegetables near metal smelters in NSW. In Proceedings of the 3rd Australian New Zealand Soils Conference, Sydney, Australia, 5–9 December 2004. [Google Scholar]
- Salt, D.E.; Smith, R.D.; Raskin, I. Phytoremediation. Annu. Rev. Plant. Biol. 1998, 49, 643–668. [Google Scholar] [CrossRef]
- Eapen, S.; D’souza, S.F. Prospects of genetic engineering of plants for phytoremediation of toxic metals. Biotechnol. Adv. 2005, 23, 97–114. [Google Scholar] [CrossRef]
- Hani, A.; Pazira, E. Heavy metals assessment and identification of their sources in agricultural soils of Southern Tehran, Iran. Environ. Monit. Assess. 2011, 176, 677–691. [Google Scholar] [CrossRef]
- Borymski, S.; Cycoń, M.; Beckmann, M.; Mur, L.A.; Piotrowska-Seget, Z. Plant species and heavy metals affect biodiversity of microbial communities associated with metal-tolerant plants in metalliferous soils. Front. Microbiol. 2018, 9, 1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ireland, M.P. Effect of the earthworm Dendrobaena rubida on the solubility of lead, zinc, and calcium in heavy metal contaminated soil in Wales. J. Soil Sci. 1975, 26, 313–318. [Google Scholar] [CrossRef]
- Gee, C. Mineralogy and weathering processes in historical smelting slags and their effect on the mobilisation of lead. J. Geochem. Explor. 1979, 58, 249–257. [Google Scholar] [CrossRef]
- Alloway, B.J.; Thornton, I.; Smart, G.A.; Sherlock, J.C.; Quinn, M.J. Metal availability. Sci. Total Environ. 1988, 75, 41–69. [Google Scholar] [CrossRef]
- Banin, A.; Navrot, J.; Perl, A. Thin-horizon sampling reveals highly localised concentrations of atmophile heavy metals in a forest soil. Sci. Total Environ. 1987, 61, 145–152. [Google Scholar] [CrossRef]
- Cui, S.; Zhou, Q.; Chao, L. Potential hyperaccumulation of Pb, Zn, Cu and Cd in endurant plants distributed in an old smeltery, northeast China. Environ. Geol. 2007, 51, 1043–1048. [Google Scholar] [CrossRef]
- Yang, P.; Yang, M.; Mao, R.; Shao, H. Multivariate-statistical assessment of heavy metals for agricultural soils in northern China. Sci. World J. 2014, 2014, 517020. [Google Scholar] [CrossRef]


| M Type | Pb | Zn | Cu | Cd | OM% | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| St Code | BAF (S/R) | BAF (S/P) | BAF (S/R) | BAF (S/P) | BAF (S/R) | BAF (S/P) | BAF (S/R) | BAF (S/P) | STC | |
| KTH | 0.71 a | 0.33 b | 0.45 a | 0.30 b | 0.38 a | 0.24 b | 2.13 a | 1.30 b | SL | 0.67 |
| GTM | 0.36 a | 0.33 a | 0.56 a | 0.29 b | 0.47 a | 0.23 b | 2.79 a | 1.18 b | SL | 0.82 |
| SKR | 1.38 a | 0.62 b | 0.48 a | 0.28 b | 0.39 a | 0.24 b | 2.11 a | 1.31 b | SL | 1.45 |
| SMR | 1.531 a | 0.794 b | 0.50 a | 0.27 b | 0.51 a | 0.27 b | 2.58 a | 1.11 b | SiL | 0.38 |
| PC | 1.528 a | 0.622 b | 0.52 a | 0.27 b | 0.39 a | 0.20 b | 2.54 a | 1.08 b | SL | 0.89 |
| HIZ | 0.294 a | 0.154 b | 0.42 a | 0.22 b | 0.27 a | 0.14 b | 1.05 a | 0.63 b | SiL | 1.21 |
| MKI | 0.83 a | 0.39 b | 0.57 a | 0.30 b | 0.32 a | 0.15 b | 1.13 a | 0.48 b | CL | 0.45 |
| BKP | 1.33 a | 0.69 b | 0.49 a | 0.24 b | 0.36 a | 0.18 b | 0.15 a | 0.16 b | SL | 1.38 |
| CUA | 0.62 a | 0.31 b | 0.97 a | 0.50 b | 0.45 a | 0.21 b | 0.97 a | 0.48 b | SiL | 1.32 |
| MSM | 0.73 a | 0.33 b | 0.65 a | 0.30 b | 0.27 a | 0.13 b | 1.44 a | 0.63 b | SiL | 1.92 |
| TFN | 0.52 a | 0.34 b | 0.11 a | 0.05 b | 0.14 a | 0.07 b | 0.32 a | 0.18 b | CL | 1.21 |
| TBM | 2.10 a | 1.06 b | 0.20 a | 0.09 b | 0.80 a | 0.38 b | 0.33 a | 0.18 b | SL | 0.89 |
| GCD | 2.20 a | 0.90 b | 0.41 a | 0.17 b | 0.92 a | 0.40 b | 0.33 a | 0.18 b | SL | 0.73 |
| AMS | 1.86 a | 0.85 b | 4.94 a | 2.07 b | 0.37 a | 0.18 b | 0.39 a | 0.19 b | SL | 0.73 |
| CBS | 1.47 a | 0.65 b | 2.90 a | 1.33 b | 0.39 a | 0.19 b | 0.34 a | 0.18 b | SL | 0.41 |
| M Type | Pb | Zn | Cu | Cd | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| St Code | TF | TF | TF | TF | TF | TF | TF | TF | TF | TF | TF | TF |
| (R-S) | (S-L) | (L-F) | (R-S) | (S-L) | (L-F) | (R-S) | (S-L) | (L-F) | (R-S) | (S-L) | (L-F) | |
| KTH | 0.43 a | 0.53 b | 0.88 b | 0.67 a | 0.83 b | 0.76 c | 0.38 a | 0.63 b | 0.64 b | 0.59 a | 1.00 b | 0.45 c |
| GTM | 0.47 a | 0.72 b | 0.70 b | 0.55 a | 0.59 b | 0.77 c | 0.47 a | 0.45 a | 0.74 b | 0.44 a | 0.39 b | 0.43 a |
| SKR | 0.42 a | 0.52 a | 0.73 b | 0.64 a | 0.67 a | 0.68 a | 0.39 a | 0.61 b | 0.66 c | 0.61 a | 1.02 b | 0.40 c |
| SMR | 0.55 a | 0.64 b | 0.50 c | 0.54 a | 0.60 b | 0.75 c | 0.51 a | 0.53 a | 0.64 b | 0.46 a | 0.40 b | 0.39 b |
| PC | 0.35 a | 0.57 b | 1.27 c | 0.54 a | 0.60 b | 0.77 c | 0.39 a | 0.57 b | 0.60 b | 0.44 a | 0.43 a | 0.39 b |
| HIZ | 0.51 a | 0.62 b | 0.86 c | 0.47 a | 0.80 b | 0.70 c | 0.27 a | 0.43 b | 1.02 c | 0.42 a | 0.56 b | 0.48 c |
| MKI | 0.51 a | 0.33 b | 1.12 c | 0.50 a | 0.71 b | 0.71 b | 0.32 a | 0.44 b | 0.20 c | 0.39 a | 0.49 b | 0.54 c |
| BKP | 0.56 a | 0.54 a | 0.70 b | 0.46 a | 0.66 b | 0.69 b | 0.36 a | 0.44 b | 1.15 c | 0.42 a | 0.51 b | 1.06 c |
| CUA | 0.58 a | 0.35 b | 0.95 c | 0.49 a | 0.72 b | 0.65 c | 0.45 a | 0.42 a | 0.35 b | 0.42 a | 0.59 b | 1.22 c |
| MSM | 0.44 a | 0.40 b | 1.00 c | 0.42 a | 0.68 a | 0.55 a | 0.27 a | 0.43 b | 0.46 b | 0.41 a | 0.51 b | 0.59 c |
| TFN | 0.60 a | 0.80 b | 1.16 c | 0.48 a | 0.52 a | 0.75 b | 0.14 a | 0.54 b | 0.51 b | 0.61 a | 0.73 b | 0.61 a |
| TBM | 0.63 a | 0.67 a | 1.17 b | 0.60 a | 0.40 b | 0.38 b | 0.8 a | 0.51 b | 0.46 c | 0.59 a | 0.48 b | 0.98 c |
| GCD | 0.62 a | 0.65 a | 0.53 b | 0.48 a | 0.33 b | 0.52 c | 0.92 a | 0.43 b | 0.49 c | 0.54 a | 0.81 b | 0.49 c |
| AMS | 0.70 a | 0.57 b | 0.68 c | 0.42 a | 0.43 a | 0.39 a | 0.37 a | 0.44 b | 0.56 c | 0.45 a | 0.70 b | 0.56 c |
| CBS | 0.48 a | 0.78 b | 1.20 c | 0.46 a | 0.54 b | 0.50 c | 0.39 a | 0.52 b | 0.52 b | 0.55 a | 0.71 b | 0.58 a |
| Axis | Eigenvalues | % Variance | % Cum Var. | Broken-Stick Eigenvalues |
|---|---|---|---|---|
| 1 | 9.14 | 38.1 | 38.10 | 3.776 |
| 2 | 4.97 | 20.74 | 58.84 | 2.776 |
| 3 | 2.58 | 10.78 | 69.62 | 2.276 |
| 4 | 2.15 | 8.96 | 78.59 | 1.943 |
| 5 | 1.57 | 6.56 | 85.15 | 1.693 |
| 6 | 1.29 | 5.37 | 90.53 | 1.493 |
| 7 | 0.84 | 3.51 | 94.05 | 1.326 |
| 8 | 0.53 | 2.23 | 96.28 | 1.183 |
| 9 | 0.44 | 1.86 | 98.15 | 1.058 |
| 10 | 0.19 | 0.82 | 98.97 | 0.947 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, R.; Khan, N.; Ali, K.; Khan, M.E.H.; Jones, D.A. Screening of Xanthium strumarium (IAPS) Growing on Abandoned Habitats in Khyber Pakhtunkhwa, Pakistan: Perspectives for Phytoremediation. Appl. Sci. 2021, 11, 11704. https://doi.org/10.3390/app112411704
Ullah R, Khan N, Ali K, Khan MEH, Jones DA. Screening of Xanthium strumarium (IAPS) Growing on Abandoned Habitats in Khyber Pakhtunkhwa, Pakistan: Perspectives for Phytoremediation. Applied Sciences. 2021; 11(24):11704. https://doi.org/10.3390/app112411704
Chicago/Turabian StyleUllah, Rafi, Nasrullah Khan, Kishwar Ali, Muhammad Ezaz Hasan Khan, and David Aaron Jones. 2021. "Screening of Xanthium strumarium (IAPS) Growing on Abandoned Habitats in Khyber Pakhtunkhwa, Pakistan: Perspectives for Phytoremediation" Applied Sciences 11, no. 24: 11704. https://doi.org/10.3390/app112411704
APA StyleUllah, R., Khan, N., Ali, K., Khan, M. E. H., & Jones, D. A. (2021). Screening of Xanthium strumarium (IAPS) Growing on Abandoned Habitats in Khyber Pakhtunkhwa, Pakistan: Perspectives for Phytoremediation. Applied Sciences, 11(24), 11704. https://doi.org/10.3390/app112411704








